
Usability Assessment of an Intraoperative Planning Software
Federico Sternini
1a
, Giuseppe Isu
2b
, Giada Iannizzi
2
, Diego Manfrin
2
, Noemi Stuppia
1,3
,
Federica Rusinà
1
and Alice Ravizza
1c
1
USE-ME-D srl, I3P Politecnico di Torino, C.so Castelfidardo 30/a, Torino, Italy
2
Medics srl, via Avogadro 19, Torino, Italy
3
Politecnico di Torino, C.so Duca degli Abruzzi 24, Torino, Italy
{noemi.stuppia, federica.rusina, alice.ravizza}@use-me-d.com
Keywords: Usability, Learning Curve, Touchless.
Abstract: Usability is a crucial aspect of medical device safety. The brand-new European Regulation requires the
manufacturer to assess the usability of the new medical devices. In this study, we evaluate the usability of a
new medical device intended to assist the intraoperative planning with the visualization of 3d patient-specific
organ models. The usability study started from the early stage of the device design and iterated through an
early formative, completed with desk-based activities, late formative, completed with a focus group, and
summative phase, that comprised a user test, and questionnaire filling. The identified usability issues are
mitigated, the safety of the device user interface is confirmed and the training contents are defined and
confirmed. Additional information regarding the user experience is collected and analyzed to identify further
improvements of the device.
1 INTRODUCTION
Usability assessment of medical devices is becoming
a widely diffused practice during device design. The
diffusion of this practice is partly eased by the
European regulatory framework for medical devices.
Medical device regulation 2017/745 (European
Parliament and of the Council, 2017) requires that
risk evaluation includes the evaluation of risks and
hazards related to human factors.
During the design of the medical device, object of
this study, the methodology for the assessment of the
human factors follows the relevant international
standards. The international standards define a
method designed to ensure a high-level quality of the
medical device interface in terms of safety for both
patients and operators. The method foresees an
iterative workflow, that requires different steps to be
completed. The first phases are so-called formative,
which are used to define the interface design and to
establish the details of the device design. In the later
phases, the confirmation of the user interface safety
(called “summative”) is completed.
a
https://orcid.org/0000-0002-5510-2296
b
https://orcid.org/0000-0002-8440-607X
c
https://orcid.org/0000-0003-2368-7258
2 MATERIALS
2.1 Device
The device assessed is a software as a medical device
(SaMD) intended to aid the surgeon for the
intraoperative planning thanks to the presentation of
3d reconstructed models of the patient-specific
anatomy. Briefly, the models are realized as based on
the radiological images of the patient (e.g. CT or
MRI) through the segmentation of the 2D medical
images. The obtained 3D models are then made
available to the physician through a proprietary
platform. In the platform, the physician can add notes,
information, and custom requests to the model. Once
the model is confirmed by the physician, it is made
available in the device ICON, which accesses the
platform. Thus, the model can be visualized in all its
parts. The visualization is aided by a touchless user
interface enabled by the LEAP MOTION sensor
(Ultraleap, US), that tracks and identifies the hands of
the users, without the need for additional sensors. The
user can modify the visualization of the organ model
Sternini, F., Isu, G., Iannizzi, G., Manfrin, D., Stuppia, N., Rusinà, F. and Ravizza, A.
Usability Assessment of an Intraoperative Planning Software.
DOI: 10.5220/0010252904830492
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 5: HEALTHINF, pages 483-492
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
483

Figure 1: Examples of device interaction. On the left, the user is rotating the model, as can be seen by the “pinching” gesture.
On the right, representation of menu opening.
in terms of positioning, zooming and orientation. In
addition, the user can select the visibility of model
specific parts selecting one of the three visibility
statuses: solid, transparent, and hidden. The
management of the visualization is completed with
three main hand gestures and with interaction with a
menu. The gestures are the following:
Gesture for the model rotation. The user shall
place the hand on the 3D model and
subsequently pinch point finger and thumb
together. Keeping the pinching, the user can
move the hand in any direction in the space and
the 3D model will start rotating following the
hand rotation around the center of mass of the
model.
Gesture for the model panning. The user shall
place the hand on the 3D model and
subsequently close the hand in a fist. The user
can translate the 3D model moving the hand in
any direction in the space while keeping the
fist.
Gesture for the model scaling. The user shall
place both hands open to surround the 3D
model. Subsequently, moving the hands away
from the 3D model the model will start scaling
up; moving the hands towards the 3D model,
the model will start scaling down.
The visibility status of all components of the
organ model can be managed using a floating menu.
The floating menu can be opened by the user rotating
the hand palm up, and the menu will be positioned in
the user's palm. The menu presents round buttons that
represent the main categories of the model elements,
i.e. bodies and vessels, and the button to access the
setting section. Once one category is opened, the
elements belonging to the selected category are
presented and available for selection. Interaction with
the buttons composing the menu is completed by
pressing the circles.
3 METHODS
As suggested by the international standard IEC 62366
(International Electrotechnical Commission, 2015),
the methodology for the usability assessment was
structured in two phases: first a formative evaluation
and then a summative evaluation.
3.1 Formative Evaluation
The formative evaluation is the phase intended to
iterate the device design until a satisfactory quality
level is reached. The formative evaluation of this
device was designed in two separate phases. As the
formative evaluation began during the early phase of
the development of the device, the first phase was
desk-based, while the second phase comprised the
participation of real users as participants to a focus
group.
In the first step, designers and usability experts
used techniques considered appropriate to the design
development stage in terms of outputs and resources
needed (Ravizza et al., 2019). The team used a quick
and dirty approach and used low resources techniques
listed in the IEC 62366, such as brainstorming, FTA,
cognitive walkthrough, and standard review. The
outputs of the first phase included the definition of a
set of primary operating functions, i.e. the functions
that the user shall be able to complete to achieve the
intended use, that have to be evaluated in the next
phases. Additionally, this first phase had as output the
definition of the position of the sensor and the screen
to allow correct ergonomics of the user.
The following phase was the focus group. This
technique was planned at this stage in the usability
evaluation to confirm the outputs of the previous stage
and to identify possible additional issues thanks to the
analysis of the end-user perspective. The focus group
was organized during the Covid-19 pandemic and
HEALTHINF 2021 - 14th International Conference on Health Informatics
484

therefore required the moderator to assist only one
participant for each session; the consensus statement of
the participants was obtained by virtual meetings. Each
focus group session was then structured into 4 brief
sections, for a duration of a maximum of 90 minutes.
The first section included a brief training for the device
use. This section lasted a maximum of 30 minutes and
provided the minimum information required to use
correctly the device to the users. The training session is
designed based on the previous outputs and is designed
to be consistent with the training that will be provided
to actual users. The focus group training was used as a
basis for the future commercial training required after
the distribution of ICON to the customers. After the
training session, the users were invited to complete a
set of tasks with the device. After the completion of the
tasks, the users were asked to provide an evaluation of
the primary operating functions and to provide
information regarding some crucial aspects with a
closed-ended questionnaire built on the base of a 5
point scale. The scale is designed to range from the
value zero, which is associated with the absence of
usability problems, to a value equal to four, which
represents the presence of usability problems that
could impact patient health. Finally, after the
completion of the questionnaire, the users were invited
to a discussion with the designers and the usability
experts to find additional usability problems and to
propose any suggestion for the user interface.
3.2 Summative Evaluation
The summative evaluation is the last phase of the
usability evaluation and is intended to confirm the
usability of the medical device. Therefore, the device
involved in the study shall be consistent with the final
version of the medical device and shall present all the
features of the medical device.
After the completion of the formative phase, the
device user interface received the following modifica-
tions, that impacted the primary operating functions
and the structure of the summative evaluation: the
positioning of the sensor, of the screen, and the para-
meters of the virtual view, are set by the manufacturer,
and a tutorial section is included in the device to allow
the users to familiarize with the gestures and the menu
structure. The tutorial section contents were obtained
from the training contents identified during the
formative desk-based phase and the analysis of the
issues presented by the users during the focus group.
The summative evaluation of the medical device
involved final users in sessions of simulated use of
the device. The simulations were completed in a
setting intended to represent the real setting of the
medical device inside the operating room. Therefore,
the simulated use setting included the provision of a
surgical column, consistent with the column that will
be provided by the manufacturer to users, equipped
with a medical-grade workstation for the software
proper execution, a medical-grade screen, and a
flexible arm for the sensor placement. The column
was placed on one side of a table covered with cloths
intended to mimic the sterile drapes usually placed on
the patient during surgical procedures. Also, as the
device allows for the visualization of the virtual
model combined with a video stream collected from
external video sources, a simulation of a patient
undergoing a laparoscopic procedure was realized by
a closed box containing the tip of the video
laparoscope and a 3d printed model of the liver. The
model was the physical print of the same model
presented to the user inside the medical device.
The user test is structured in different phases.
3.2.1 Training
Training: the design team presents the medical device
to the user, explaining all the relevant information for
the device use. This information included the gestures
required for the device interaction and the tips
intended to ease the first use of the medical device.
3.2.2 Task Analysis
Task analysis: the moderatos asked the user to
complete some complex actions while observing the
device use and annotating the performance of the user
for each task. The moderators classified each task
completed by the user in one of the following 4 classes:
Ok: the user completed the task correctly.
Ue: use error. It represents any task that the
user was unable to complete, that was
completed without awareness of its meaning,
that was completed by mistake, or that required
intervention of moderators.
Te: technical error, represents the cases when
the device presented some technical issue that
did not allow the user to complete the task.
C: critical, represents particular cases of use
errors that can be associated with an impact on
patient health
3.2.3 Heuristic Evaluation
After the completion of the simulated tasks, all users
were asked to compile a questionnaire for the
heuristic evaluation of the device. The heuristic
analysis is an inspective technique intended to
identify the elements that violate the usability
Usability Assessment of an Intraoperative Planning Software
485
heuristics (i.e. identify usability problems in the user
interface). After the identification of the violations, a
score is assigned to assess the severity of the violation
(Zhang et al., 2003).
The designer and the usability experts designed
the questions, analyzing the heuristic principles
proposed by Zhang et al (Zhang et al., 2003) and
proposing a set of questions designed to fit the user
interface features of the device under assessment. The
presentation of closed-ended questionnaires allowed
for the evaluation of the severity of heuristic
violations, even if the user is not an expert in this
technique. The user could answer each question using
the same scale proposed for the questionnaire
proposed during the focus group to maintain the
consistency of the test methods across the different
stages of the usability evaluation.
3.2.4 Primary Operating Functions and
Risk Questionnaire
Later the user was asked to fill a questionnaire
consistent with the one proposed during the focus
group, intended to exploit the crucial aspects of the
user interface of the device and its primary operating
functions. As the heuristic evaluation, the scale for the
answers is the same proposed during the focus group.
3.2.5 UEQ Questionnaire
After the completion of the two first questionnaires,
the user was asked to complete a third questionnaire,
that is not relevant for the evaluation of the risk
profile of the device, but that is intended to describe
the overall usability of the user interface. The UEQ
questionnaire is a standardized questionnaire and
presents a set of couples of terms, and the user has to
select the evaluation of the device for each couple of
terms, positioning the device evaluation in the scale
described by the terms (Laugwitz et al., 2008).
3.2.6 UEQ Questionnaire Stereoscopy
After the completion of the three questionnaires, the
moderators asked the participants to try a different
visualization mode provided by the medical device.
This mode is designed to allow the use of the model
in stereoscopic screens and displays. The simulation
was completed with a virtual reality visor. As the
setting of the simulated setting was not representative
of the real medical device use, the tasks are not
evaluated as in the previous stages, but an additional
questionnaire was proposed to the participants, asking
them to fill the questionnaire considering the
stereoscopic visualization only.
4 RESULTS
4.1 Formative Evaluation
4.1.1 Desk-based Phase
During the desk-based activity, the usability and
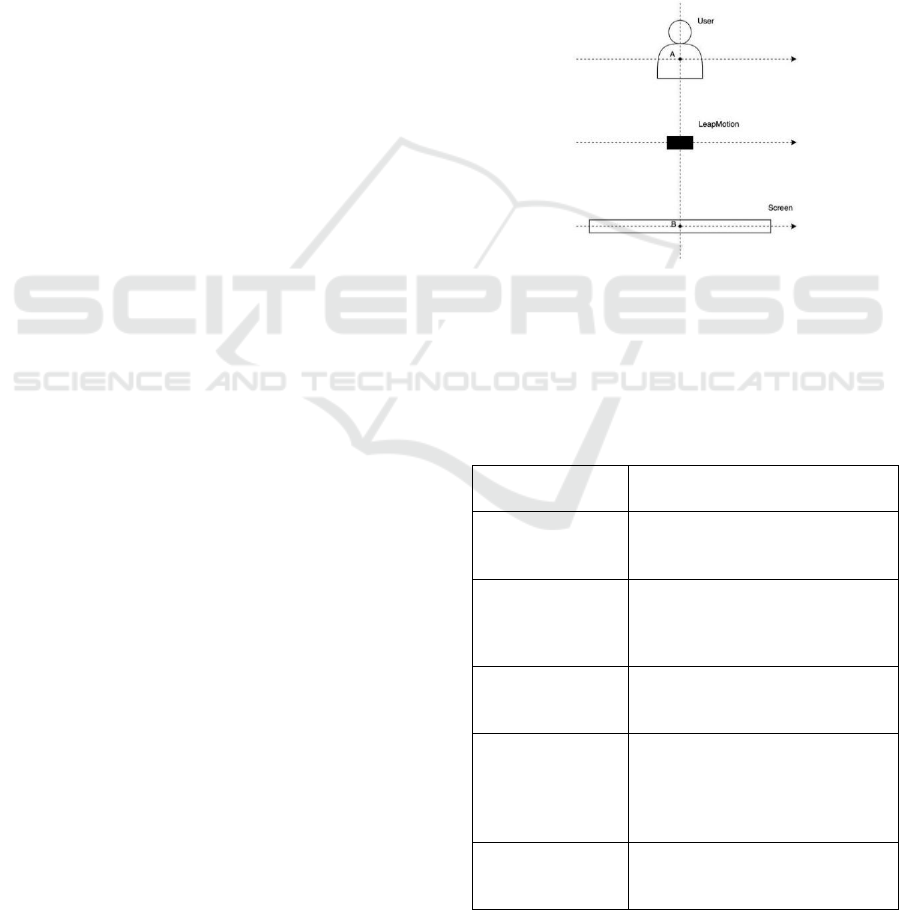
design team identified the positioning for the
LEAPMOTION sensor and the screen that allows the
user to have a comfortable organ model visualization.
The frontal positioning of the screen and sensor
allows the user to have a visualization consistent with
the placement of the hands.
Figure 2: Scheme of the positioning of the user, sensor, and
screen.
After the identification of the positioning, the
primary operating functions are identified, here listed
in Table 1.
Table 1: Primary operating functions.
Primary operating
functions
Interface testable technical
requirements
Choose the case
The user shall be able to select the
proper case use (organ model) for
the surgery
Set up of the
operator against
the virtual view
The information provided by the
system shall allow to set up
appropriately the user position
against the virtual view
Handling the
organ model
The hand gestures shall allow to
manipulate the organ model in an
intuitive manner
Management of
the parts belonging
to the organ model
The hand gestures and the menu
setting shall allow to isolate and
change the transparency of the
parts belonging to the organ model
in an intuitive manner
Management of
the scene
background
The user shall be able to switch
the scene background in an
intuitive and simple manner
HEALTHINF 2021 - 14th International Conference on Health Informatics
486
4.1.2 Focus Group
The focus group was completed with the participation
of four users, in line with standard recommendation,
which suggests at least 4 participants and a maximum
of 8 participants (International Electrotechnical
Commission, 2016). The four users were all surgeons,
two orthopedics, and two thoracic surgeons. During
the task completion, the following issues were
identified by users and moderators:
The gesture required to zoom the model is not
so intuitive.
The hands should be placed in the sensing
volume completing a predefined movement
that allows easy identification of the hands
(half-moon shape trajectory).
The users had some troubles when trying to
pinch and rotate the model.
Some users found the position of the menu
opening uncomfortable and would have
preferred a position that does not require taking
the hand backward to select the tiles
Some users had some difficulties to visualize
and read the menu elements due to the
transparency of the menu overlaid to the model.
One user could not use the gestures because
kept the second hand in the sensing volume.
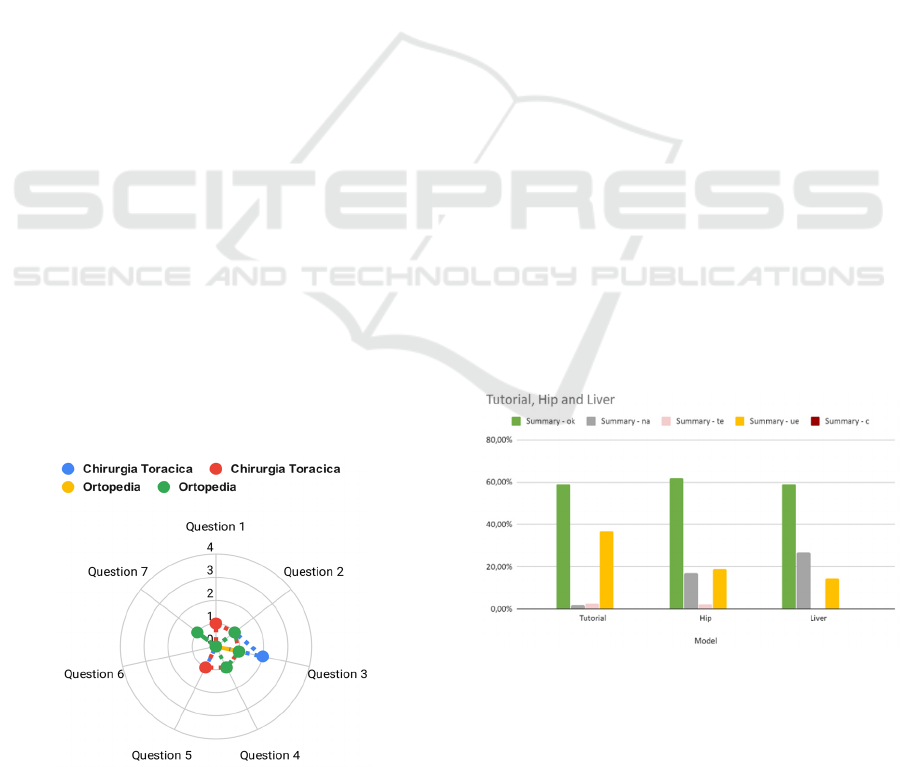
After the completion of the tasks, the users
compiled the questionnaire, results did not present
any value higher than two, which represents that the
device made the user nervous. We recall that the
higher is the score, the worse is the usability problem.
In particular, the highest score was obtained by one
single operator on the question regarding the
possibility to manage the transparency of the model
components. The details of the questionnaire answers
are presented in Figure 3.
Figure 3: Details of focus group questionnaire results.
4.2 Summative Evaluation
The participants in the user test were 16. The number
of participants is appropriate for the task of user
interface safety confirmation, as 15 users are
considered the minimum practical number for
usability validation purposes (Center for Devices and
Radiological Health, 2016). All of them were
professional users. Five of them were urologists,
seven were orthopedics, two were generic surgeons,
one was an emergency surgeon and one was a
thoracic surgeon, representing all the specialties that
the manufacturer can provide with 3D patient-
specific organ models.
4.2.1 Task Analysis
15 out of 16 users completed the list of requested
tasks. The users completed correctly 60% of the tasks,
while 20% of the tasks were classified as use errors.
The remaining 18,59% of tasks were not performed
by the users and technical errors occurred in 1,41% of
the tasks.
The performance of the users could be divided
into three macro tasks: first, the users are asked to
complete the tutorial section of the device, then the
users are asked to interact with the medical device
while visualizing a hip arthroplasty model, and
finally, the user interacted with a liver model.
Considering the division in macro tasks, during the
device use the user performed reducing the
prevalence of use errors and increasing the number of
not completed tasks, while the prevalence of the
correctly completed tasks remained quite stable for all
the phases of the test.
Figure 4: Performance of users divided per macro tasks.
4.2.2 Heuristic Analysis
All the participants filled the questionnaire for the
heuristic analysis of the device. The users were never
assigned a score higher than 2, which corresponds to
Usability Assessment of an Intraoperative Planning Software
487
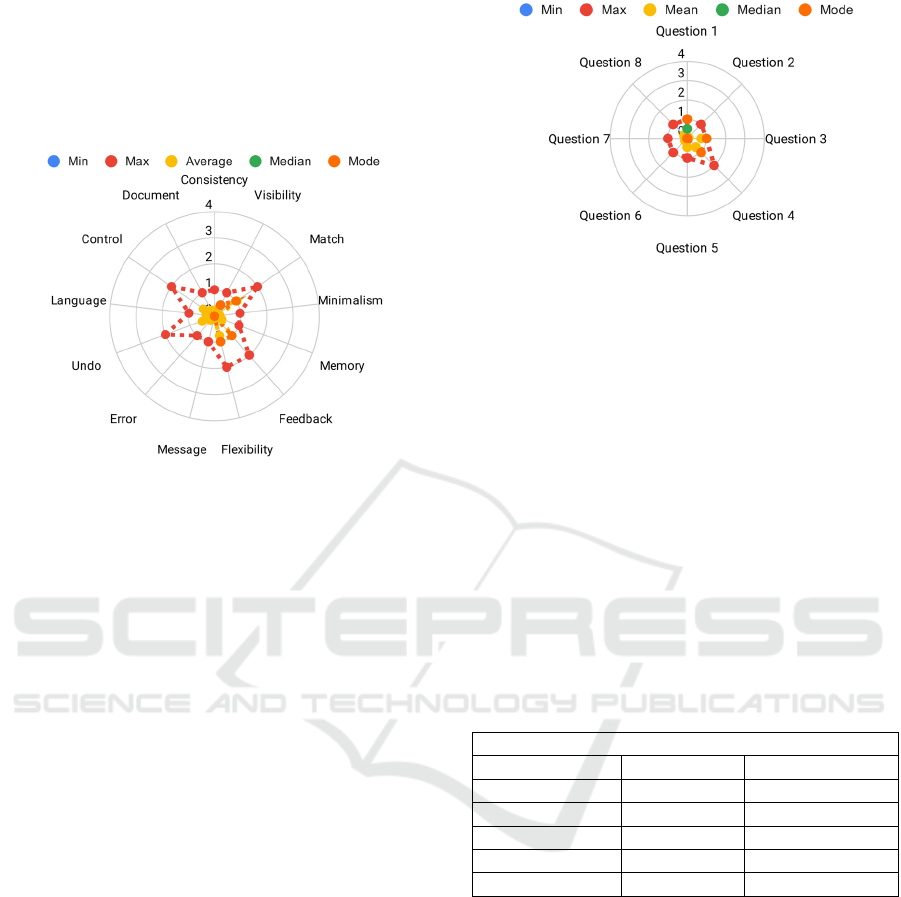
a violation of the heuristic principle that made the
user nervous during the device use. Therefore, the
user never answered that the user was impossible to
use or that the device use could lead to an impact on
patient health. The details of the aggregated answers
are presented in Figure 5.
Figure 5: Minimum, maximum, average, median, and mode
value of the scores assigned by the users to each relevant
heuristic principle.
4.2.3 Primary Operating Functions and
Risk Questionnaire
All the participants filed the questionnaire regarding
the primary operating functions and the specific
questions regarding the risks of the device. The
primary operating functions were modified from the
previous iteration of the usability testing due to the
modification of the device. Therefore, the primary
operating functions were defined as follows:
Completion of the tutorial
Choose the case
Handling the organ model
Management of the parts belonging to the
organ model
Management of the scene background
Also, three questions related to the completeness
of the user interface, the color-coding, and the clarity
of the notifications are asked. All participants
but one assigned scores lower than two (device use
made me nervous) to all the primary operating
functions and situations related to the main risks
associated with the device. The primary operating
function that received a score equal to two is the one
associated with the management of the parts
belonging to the organ model. Details of the
questionnaire results are presented in Figure 6.
Figure 6: Minimum, maximum, average, median, and mode
value of the scores assigned by the users to the five primary
operating functions and the three risk-related questions.
4.2.4 UEQ Questionnaire
All participants filled the UEQ questionnaire. The
questionnaire results were evaluated according to the
dedicated data analysis tool, the results are cleaned
removing the inconsistencies of the answers provided
by the users, and the results detailed in Table 2 in the
usability areas of the device are obtained (User
Experience Questionnaire (UEQ), n.d.). The cleaning
of data was completed by removing all the
questionnaire data related to users that presented at
least 2 inconsistencies among the answers, as it may
be associated with low attention during the
questionnaire filing.
Table 2: UEQ scales results.
UEQ Scales (Mean and Variance)
Attractiveness 2.146 0.90
Perspicuity 1.750 0.98
Efficiency 1.917 1.40
Dependability 1.984 0.75
Stimulation 2.391 0.68
Novelty 2.484 0.57
4.2.5 UEQ Questionnaire Stereoscopy
Twelve out of 16 participants filed the UEQ
questionnaire for the stereoscopy evaluation. The
questionnaire results are evaluated as consistently
with the other UEQ questionnaire as per the
methodology proposed with the questionnaire (User
Experience Questionnaire (UEQ), n.d.). The results
are cleaned with the same criteria used for the other
UEQ questionnaire. The results of the cleaned UEQ
questionnaire are presented in Table 3.
HEALTHINF 2021 - 14th International Conference on Health Informatics
488

Table 3: UEQ scales results for the stereoscopic
visualization.
UEQ Scales (Mean and Variance)
Attractiveness 2.403 1.02
Perspicuity 2.000 1.45
Efficiency 2.023 2.12
Dependability 1.977 0.72
Stimulation 2.477 1.43
Novelty 2.614 0.63
5 DISCUSSION
The study allowed the team to collect many data
regarding the user interaction with the device,
enabling the definition of improvements of the user
interface and to define the device safety. During the
formative stage, the usability issues identified during
the focus group were analyzed and mitigated with
different techniques.
The first methodology was the provision of
adequate training to the user before the device use. In
particular, the training focused on the position of the
hands and a clear explanation of the gestures. The
users had difficulties when completing the
movements associated with the modification of the
zoom of the model and the rotation of the model. In
both cases, the clear explanation of the gestures with
the provision of examples completed by the
moderators allowed the users to improve their user
experience and complete the tasks correctly.
Therefore, the designers decided to introduce the
tutorial section, intended to make the user practice
with the gestures and have an easier interaction with
the device. Other issues associated with the menu
were resolved with a modification of the user
interface, adding a back panel to the menu tiles
presentation, reducing the visibility problems, and
allowing the users to move the menu once opened,
and to place it in a more comfortable position.
Figure 7: Menu visualization of the device version
presented during the formative evaluation.
Figure 8: Menu visualization of the device version used
during the summative evaluation.
The new functionalities are included in the
version tested for the summative evaluation, and the
tutorial section became an integral part of the
simulated use testing.
The results of simulated use showed that the
device cannot lead to risks for the patients, as the
moderators did not classify any action as a critical
error. Also, the technical errors were very few and led
to a complete stop in the device use only within the
tutorial section, which is not a medical module of the
software and not intended to be used during the
intraoperative planning. The results also showed that
the percentage of use errors decreased rapidly during
the device use, suggesting that the users can learn the
correct use of the device very quickly during the
device use, producing a steep learning curve when
compared to the curves associated with surgical
procedures (Hopper et al., 2007). Further
observations can be completed by removing from the
analysis user #13, which completed only the first part
of the simulated use, completing only 13 out of 40
tasks, and removing the not completed tasks. The
number of not completed tasks is affected by a set of
6 tasks that were misleading to the users. These tasks
are the ones associated with the possibility to hide or
make transparent all the components of the model
belonging to a specific category. Many users
completed the task hide or made visible the elements
of the entire category (e.g. veins, arteries, etc.)
without using direct command buttons, but by
performing more commands than required.
Therefore, these tasks were recorded as not
completed, but not as use error, because the goal of
the tasks was correctly reached.
When removing both these data from the analysis,
the improvement of the user performance during the
device use is more evident and recognizable. The
percentage of correctly completed tasks increased at
each macro task, ranging from the minimum of the
first phase of use equal to 62,14% to the maximum
reached in the last phase of the device use equal to
80,40%, while the percentage of the use error
Usability Assessment of an Intraoperative Planning Software
489
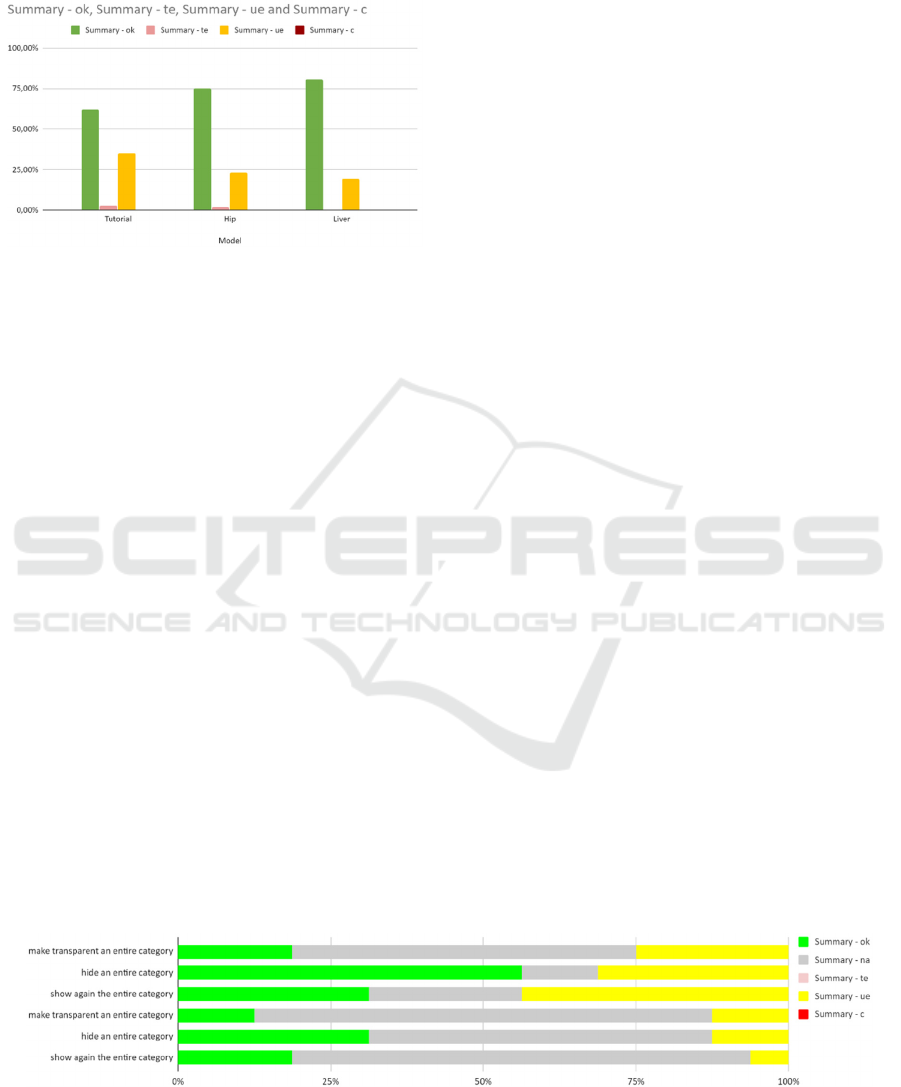
decreased ranging from the maximum of the first
microtask, equal to 34,95% to the minimum reached
in the last phase of use equal to 19,60%.
Figure 9: Analysis of task performance after removal of
user #13 and the removal of the not completed tasks.
Considering the strict protocol of the test, the
results are very promising. As a test rule, the
moderators were not allowed to help the users during
the test, neither to answer the user questions. In the
case of help provision from the moderators to the
users, the task was recorded as a use error. Therefore,
the data show that the user required less training or
help during the session and that they could remember
the information required for the correct use of the
device with a brief training session and few questions
during the first device use.
Nevertheless, during the questionnaire filling
phase, the users pointed out that they had difficulties
during the management of the model and the use of
gestures. In fact, in the heuristic questionnaire, the
highest scores are associated with the following
heuristic principles:
Match: relevant for the consistency between
the gestures of the hand and the commands
received from the software. The mismatch
could be caused by many factors, but the most
prominent one is the lack of training. All users
tried to complete actions with improper hand
gestures.
Feedback: principle applied to feedback
provided by the menu interactions. The menu
is the most difficult part of the software to use,
as it requires the user to have confidence with
the correspondence between actual hand
position and virtual hand position. Also, the
color code of the menu is intended to ease the
comprehension of the menu parts status, but at
the moment of the study was graphically
presented to the user with a use example and as
part of the brief training received by the users.
No legends were presented to the user in any
part of the user interface.
Flexibility: the main issue that users identified
is that the device requires attention and can be
tiring to use during surgery. The users
completed an intensive test that lasted from one
to two hours, while during surgery the device
will be used for a few minutes.
Undo: The main difficulties are tightly related
to the navigation of the menu, as the
visualization status is controlled by the menu.
Control: this heuristic principle is tightly
related to the menu navigation, as the control of
each model component is completed through
the menu. Therefore, the difficulties identified
by the users could be related to difficulties in
the menu navigation.
The difficulties associated with the menu are
directly related to the interaction that the user has to
complete to modify the visualization status of the
model. The user opens the menu rotating up the palm,
then selects the tiles of the menu as if they were
physical buttons, so the user had to press the tiles and
then retract the finger from the selection. While the
interaction is intuitive and does not require training to
understand the movement, it requires that the user is
aware of the position of the hand in the real and
virtual representation of the space. The brief duration
of the training phase and the short duration of the test
could lead to the difficulties of the user to have fine
control of the movements of the hand in the virtual
space, and then, as the menu is the part of the interface
Figure 10: Representation of the performance of the users in the misleading tasks: the percentage of not completed tasks is
high due to the possibility of completing the same task with different methodologies.
HEALTHINF 2021 - 14th International Conference on Health Informatics
490
that requires the most precise interactions, the
difficulties in the menu interactions. In these regards,
during the design phase, the developers included the
possibility to interact with the menu also with the
traditional mouse/touchpad interaction, to help the
user in the first uses of the device difficulties. During
the summative tests, the users were not deliberately
instructed about the possibility to use the mouse, to
strictly evaluate the usability of the device by the
innovative LEAP MOTION controller. This aspect
enforces the positive outcome of the test considering
that the possibility to have a well-known backup
solution in case of difficulties constitutes a distress-
relief and facilitation to accelerate the learning curve.
The same observations could be done on the
results of the UEQ questionnaire. The questionnaire
is designed to evaluate the aspects of the user
experience. In all of these aspects, the device is
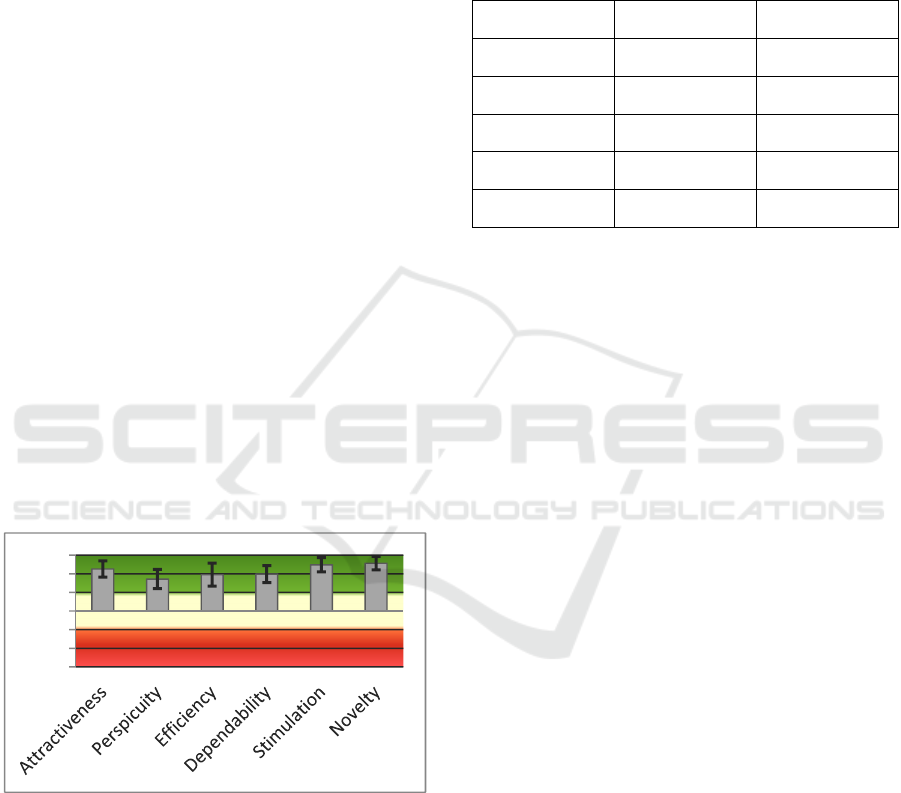
considered very good, as the mean score is always
higher than 1,6 which is twice the value considered
for a good result (0,8). Even if the sample of users is
quite small, the confidence intervals of the scores are
always higher than 0,8.
Even if the observation from the task analysis led
the moderator to the conclusion that the learning
curve of the users when using this device is very
steep, the users found perspicuity, which is the aspect
that describes how easy is for the user to learn how to
use the device, the worse usability aspect of the
device. Nevertheless, even this aspect, have a positive
score on average.
Figure 11: Scores of the UEQ Questionnaire and the
associated confidence intervals.
Similar results are obtained from the evaluation of
the UEQ questionnaire proposed to the user regarding
the stereoscopic visualization only. Between the two
questionnaires, no statistically significant differences
(p=0,05) in the scores of the usability aspects are
evidenced. Nevertheless, when comparing the results
of the two questionnaires, the greatest difference is
perceived in the perspicuity aspect. The entity of the
difference may be justified by the possibility of the
users to perceive more easily the hand position when
interacting with the menu, thanks to the capability of
presenting the third dimension provided by the
stereoscopic visualization.
Table 4: T-Test for the difference of the UEQ scores
between the general and stereoscopic visualization.
Attractiveness 0.7007 No Significant
Difference
Perspicuity 0.5346 No Significant
Difference
Efficiency 0.8863 No Significant
Difference
Dependability 0.9861 No Significant
Difference
Stimulation 0.9884 No Significant
Difference
Novelty 0.8773 No Significant
Difference
6 CONCLUSIONS
The study allowed to identify the issues of the user
interface of the device at the design stage and allowed
the designers to solve the usability issues before the
summative evaluation and before of the place into
market of the device. During the summative
evaluation, the safety of the device was confirmed,
and additional information for further improvements
are collected, both in terms of improvements of the
user interface and in terms of improvement for the
training provided to users.
The study allowed the designers to observe the
learning curve of the end-users and to collect
information regarding the safety of the device as
perceived by the users and their impression regarding
the user experience. In particular, even if the
observation of the task analysis led the moderators to
think that the learning curve of the users is steep and
that there was a sensible improvement of the task
performance during the device use, the users reported
that the learnability of the device is the aspect that
needs major improvements. On the other side, we
recall that the users found the learnability of the
device still good enough.
The study presented some limits. The first is the
numerosity of the participants. While 16 participants
are considered satisfactory for the regulatory
purposes and are considered sufficient for the
determination of the usability issues of a medical
device, a greater number of participants could define
better the usability aspects evaluated with the UEQ
questionnaire. Furthermore, the setting of the test is
not representative of the device's real use. The
-3
-2
-1
0
1
2
3
Usability Assessment of an Intraoperative Planning Software
491

simulation of the operating room could not provide
the simulation complete of the device use
environment but represented only the layout of a real
use setting. The other environmental conditions like
noise, patient presence, and the timing could not be
reproduced. Also, the intensive use that is completed
during the test is not representative of the real use
condition. Even the training is not representative,
because the manufacturer intends to provide training
before the first use in a similar way to the one
completed before the simulated use, but additionally,
intend to assist in the first sessions of medical device
use.
For these reasons, this study is considered
complete in terms of identification of usability issues
and terms of confirmation for the device safety,
thanks to the worst use condition, but is not
considered complete regarding the device user
experience. Additional studies should be completed
to evaluate user perception during actual use.
REFERENCES
Center for Devices and Radiological Health. (2016).
Applying Human Factors and Usability Engineering to
Medical Devices. U.S. Food and Drug Administration.
http://www.fda.gov/regulatory-information/search-fda-
guidance-documents/applying-human-factors-and-
usability-engineering-medical-devices
Regulation (EU) 2017/745 of the European Parliament and
of the Council of 5 April 2017 on medical devices,
amending Directive 2001/83/EC, Regulation (EC) No
178/2002 and Regulation (EC) No 1223/2009 and
repealing Council Directives 90/385/EEC and
93/42/EEC (Text with EEA relevance. ), Pub. L. No.
32017R0745, 117 OJ L (2017). http://data.europa.eu/
eli/reg/2017/745/oj/eng
Hopper, A. N., Jamison, M. H., & Lewis, W. G. (2007).
Learning curves in surgical practice. Postgraduate
Medical Journal, 83(986), 777–779. https://doi.org/
10.1136/pgmj.2007.057190
International Electrotechnical Commission. (2015). IEC
62366-1:2015, Medical devices—Part 1: Application of
usability engineering to medical devices (1st ed.). IEC.
http://www.iso.org/cms/render/live/en/sites/isoorg/con
tents/data/standard/06/31/63179.html
International Electrotechnical Commission. (2016). IEC TR
62366-2:2016, Medical devices—Part 2: Guidance on
the application of usability engineering to medical
devices (1.0). IEC. https://webstore.iec.ch/publication/
24664
Laugwitz, B., Held, T., & Schrepp, M. (2008). Construction
and Evaluation of a User Experience Questionnaire. In
A. Holzinger (Ed.), HCI and Usability for Education
and Work (pp. 63–76). Springer. https://doi.org/10.
1007/978-3-540-89350-9_6
Ravizza, A., Lantada, A. D., Sánchez, L. I. B., Sternini, F.,
& Bignardi, C. (2019). Techniques for Usability Risk
Assessment during Medical Device Design.
Proceedings of the 12th International Joint Conference
on Biomedical Engineering Systems and Technologies,
207–214. https://doi.org/10.5220/0007483102070214
User Experience Questionnaire (UEQ). (n.d.). Retrieved
September 11, 2020, from https://www.ueq-online.org/
Zhang, J., Johnson, T. R., Patel, V. L., Paige, D. L., &
Kubose, T. (2003). Using usability heuristics to
evaluate patient safety of medical devices. Journal of
Biomedical Informatics, 36(1), 23–30. https://doi.org/
10.1016/S1532-0464(03)00060-1.
HEALTHINF 2021 - 14th International Conference on Health Informatics
492
