
Low Energy ECG Features Extraction for Atrial Fibrillation
Detection in Wearable Sensors
Manan AlMusallam
1
and Adel Soudani
2
1
Department of Computer Science, Imam Mohammad Bin Saud Islamic University, Riyadh, Saudi Arabia
2
Department of Computer Science, King Saud University, Riyadh, Saudi Arabia
Keywords: ECG Signal Processing, Atrial Fibrillation, Wavelet Analysis, Features Extraction, WBSN.
Abstract: The Internet of Health Things plays a key role in the transformation of health care systems as it enables
wearable health monitoring systems to ensure continuous and non-invasive tracking of vital body parameters.
To successfully detect the cardiac problem of Atrial Fibrillation (AF) wearable sensors are required to
continuously sense and transmit ECG signals. The traditional approach of ECG streaming over energy-
consuming wireless links can overwhelm the limited energy resources of wearable sensors. This paper
proposes a low-energy features’ extraction method that combines the RR interval and P wave features for
higher AF detection accuracy. In the proposed scheme, instead of streaming raw ECG signals , local AF
features extraction is executed on the sensors. Results have shown that combining time-domain features with
wavelet extracted features, achieved a sensitivity of 98.59% and a specificity of 97.61%. In addition,
compared to ECG streaming, on-sensor AF detection achieved a 92% gain in energy savings.
1 INTRODUCTION
Atrial fibrillation (AF) is a prevalent arrhythmia that
is associated with an increased mortality, increased
hospitalization rate, and a higher risk of strokes.
Moreover, its prevalence is expected to increase
significantly in the next years due to an ageing
population (Mairesse et al., 2018). A major challenge
in AF diagnosis is that its early stages episodes are
short self-terminating with little or no symptoms
experienced by the patient. The electrocardiogram
(ECG) (Petty, 2016), a graphical representation of the
heart’s electrical activity, is an essential tool in AF
diagnosis. However, standard ECG recordings that
are done in hospitals provide only a snapshot of the
heart’s activity. Therefore, AF can go undiagnosed
until a patient has a routine checkup or suffer from a
serious complication such as a stroke. Ambulatory
ECG monitoring is an alternative tool for AF
diagnosis where ECG recordings are acquired,
outside of hospitals, over a pro-longed period of time.
Thus, it can capture short-lived and silent episodes of
AF.
However, traditional ECG recorders cannot
provide real time ECG monitoring because patients
are required to bring the recorder back to the doctor
office for analysis. Recent technology advances
resulted in the development of wearable ECG
monitors (Lin et al., 2010) that provide unprecedented
mobility for patients and provide doctors with real-
time data that increases AF diagnosis accuracy and
allows instant response to alarming events.
In a typical set-up, a wearable ECG monitor can
be programmed to capture and wirelessly transmit
raw ECG signals. However, transmitting raw data
over energy-consuming wireless links severely
reduces the sensor’s battery life time. Currently
available Wireless Body Sensor Networks (WBSN)
platforms depend on limited batteries and it is
essential to reduce as much as possible the
inconvenience associated with battery replacements
and recharges.
A key strategy is to implement ‘energy-ware’
signal processing algorithms on the sensor node. This
way, the sensor node will only be required to transmit
a minimal number of features instead of a full stream
of raw data. However, the main challenge is to
implement on-sensor signal processing within the
constrained resources of a sensor node.
This paper, studies the feasibility of on-sensor AF
features extraction. Instead of streaming ECG signals,
the sensor locally extracts AF relevant features. When
an AF episode is detected by the sensor it sends a
AlMusallam, M. and Soudani, A.
Low Energy ECG Features Extraction for Atrial Fibrillation Detection in Wearable Sensors.
DOI: 10.5220/0010245200690077
In Proceedings of the 10th International Conference on Sensor Networks (SENSORNETS 2021), pages 69-77
ISBN: 978-989-758-489-3
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
69
minimum number of bytes to alert the server. The
underlying hypothesis evaluated in this paper is that
low complexity on-sensor ECG signal processing can
decrease the energy consumption of wireless
transmission and therefore extend the lifetime of the
sensor.
2 RELATED WORK
The electrical patterns, captured by the ECG (Petty,
2016), are manifested as a sequence of waveforms
representing the sequence of contraction and
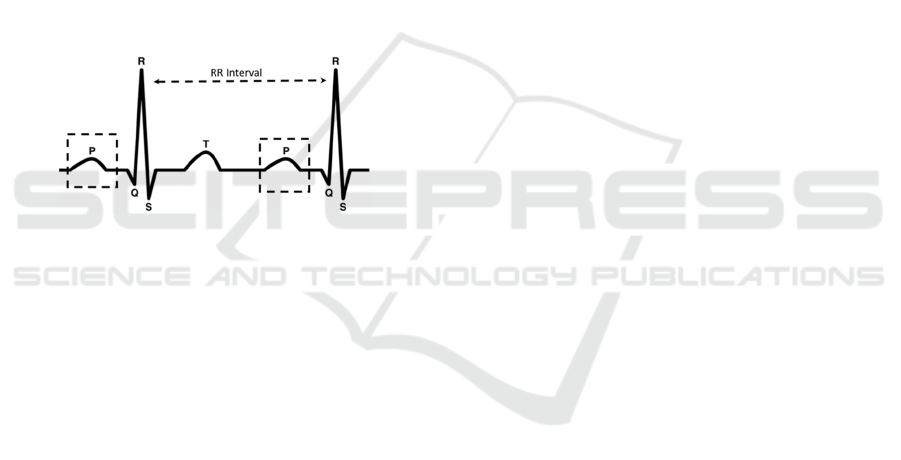
relaxation of the heart. A normal cardiac cycle has
distinct waveforms called the P wave, QRS complex
and T wave as shown in Figure 1. The QRS complex
is the most dominant feature of the ECG cycle with a
sharp peak in the middle, called the R wave. A
significant ECG feature is the interval between two
consecutive R peaks, referred to as the RR interval.
Figure 1: ECG Waveforms.
An ECG of a healthy heart shows a Normal Sinus
Rhythm (NSR) where RR intervals are regular and the
P waves are present. On the other hand, Atrial
Fibrillation (AF) (January et al., 2014) is an irregular
heart rhythm that is characterized on ECG signals by
irregular RR intervals and absent P waves that are
replaced by low-amplitude fibrillatory f-waves.
AF detection algorithms involve (Sörnmo,
Petrenas, & Marozas, 2018): ECG pre-processing, AF
features extraction, and finally classification. AF
features are expected to quantify RR interval
irregularity and/or provide information on the
absence/presence of P and f waves. However,
extracting reliable features that detect the
presence/absence of P waves is challenging at low
signal-to-noise ratios. Therefore, the majority of AF
detection algorithms are RR-based and are designed
to extract features that reflect the degree of
randomness, variability, and complexity of RR
interval series.
Commonly RR-based methods include comparing
the density histogram of RR series to a standard
density histogram (Tateno & Glass, 2002) and
evaluating statistical attributes that reflect the
randomness and complexity of RR series (Dash,
Chon, Lu, & Raeder, 2009). On the other hand, few
contributions proposed P-wave based AF detectors.
Ladavich et al. (Ladavich & Ghoraani, 2015)
developed a rate-independent AF classifier that
utilizes statistical and morphological features from a
model of normal sinus rhythm P-wave ; whereas
Ródenas et al. (Ródenas, García, Alcaraz, & Rieta,
2015) used wavelet entropy to quantify the
presence/absence of P waves. AF detectors have also
been designed to combine RR and P-wave features.
The AF detector proposed by Petrenas et. al
(Petrėnas, Sörnmo, Lukoševičius, & Marozas, 2015)
is based on four parameters that characterize RR
interval irregularity, P wave absence, f wave
presence, and the noise level in the signal. The
algorithm presented in (de Carvalho et al., 2012)
quantifies P wave absence by measuring the
correlation of the detected P waves to a P wave
model, assesses heart rate variability using a
statistical similarity measure, and analyzes atrial
activity using a wavelet approach. AF detection
proposed by Babaeizadeh et. al (Babaeizadeh, Gregg,
Helfenbein, Lindauer, & Zhou, 2009) involves a
statistical classifier that uses as input a combination
of P-R interval variability, a P wave morphology
similarity measure, and an R-R Markov score.
Regardless of the accuracy in AF detection, the
previously mentioned contributions may not be
technically feasible for real-time on-sensor
processing of ECG signals due to the high
computation requirements that can overwhelm the
constrained sensor resources.
Therefore, we turned our attention to AF detection
algorithms that have been designed to operate on
wearable ECG monitors. The study in (Marsili et al.,
2016) implements and tests an AF detection
framework on a wearable prototype device. The study
results demonstrate the framework capability to
provide onboard AF detection with affordable
computational burden. However, the detection
approach is based solely on the RR feature and the
prototype device used in the study is more resourceful
than a constrained wearable sensor.
Rincon et al. (Rincon, Grassi, Khaled, Atienza, &
Sciuto, 2012) implement AF detection on a WBSN
platform by using fuzzy logic to combine the output
of RR interval analysis and P-wave detection. The
proposed approach demonstrated satisfactory
accuracy but in terms of reducing energy
consumption and extending the node lifetime, it
offered a marginal 4% increase in the node’s life time
that does not play in favour of adopting it as an
efficient energy solution.
SENSORNETS 2021 - 10th International Conference on Sensor Networks
70

3 EMBEDDED AF DETECTION
This section presents the specification of the proposed
on-sensor AF detection algorithm. It describes the
QRS detection algorithm in addition to the features
extraction methods used to detect RR irregularity and
absence of the P wave.
3.1 General Approach
In the proposed approach, the sensor processes a
periodically acquired ECG segment to detect AF
episodes. If an AF episode is detected, the sensor
sends a notification to the server including relevant
features (Figure 2). Recent medical studies
(Rabinstein et al., 2013) highlighted the significance
of detecting AF episodes that are shorter than 30
seconds. Therefore, the proposed scheme is based on
the processing of a 10-seconds ECG signal. This
length is an adequate recording length of the ECG
signal that can contain a number of QRS complexes
sufficient for extracting relevant AF features. From
another side, the reduced set of samples in the
processed ECG signal saves the memory in the
wireless sensor. In addition, our approach is aligned
with typical clinical settings, where a cardiologist
examines a 10 seconds ECG strip (Meek & Morris,
2002).
Figure 2:Proposed approach for embedded AF detection.
In the proposed approach, the QRS detection
module detects the location of R peaks that act as
reference point for further features extraction. On-
sensor features extraction involves estimating the
irregularity of RR intervals and detecting the
presence/absence of the P wave. The embedded AF
decision rules are applied to determine if the 10-
seconds ECG signal is a possible AF episode. Once
an AF episode is detected the sensor will send, to the
base station, an alert notification in addition to the
extracted relevant AF features. The base station will,
in turn, forward the alert and AF features to a remote
server for advanced ECG classification.
The vast majority of AF detection algorithms
proposed in the literature are designed to classify
individual heartbeats. However, the proposed scheme
classifies a 10-seconds ECG segment that is
composed of a number of heartbeats. This design
choice is driven by the fact that an AF heartbeat does
not occur in isolation but only as part of an AF
episode.
3.2 QRS Detection
On-sensor ECG features extraction starts by detecting
the QRS complex. For that purpose, we have adopted
the Dual Slope algorithm (Wang, Deepu, & Lian,
2011) that analyses the signal in the time-domain and
detects the signal segment that represents the QRS
Complex. Once detected, we can extract R peaks from
the QRS complex segment. The RR interval, as a
relevant temporal feature, is extracted by measuring
the time between two consecutive R peaks. In
addition, we can use the R peak location to define a
search window for the detection of the P wave
presence/absence.
The Dual Slope algorithm does not require any
QRS enhancement and directly starts detecting the
QRS complex to localize the R peak. It focuses on
calculating the slope of straight lines connecting two
samples that are separated by a distance equal to the
QRS width. The rationale behind slope calculation is
that the largest value of slopes is expected to be found
in the QRS complex.
3.3 AF Detection
AF episodes are reflected in ECG signals by
irregularity of RR intervals and absence of valid P
waves. The irregularity of RR intervals is measured
using a simple statistic that gives an estimate of the
standard deviation of RR intervals (eStd). When the
eStd feature of the processed ECG segment crosses a
pre-set threshold, the segment is labeled as having
irregular RR intervals. Otherwise, the segment is
labeled as having regular RR intervals.
A valid P wave would typically occur in the
second half of the RR interval which we refer to as
the search interval. The number of search intervals in
a 10-seconds segment varies according to the heart
rate. Therefore, we consider that there are N search
intervals where N is equivalent to the number of RR
intervals in the segment. From every search interval,
the P wave detection algorithm extracts features that
indicate if a valid P wave is absent or present. The
algorithm maintains the number of search intervals
that did not include a valid P wave (referred to as a
Miss).
The number of Misses (M) is evaluated as a
percentage of the total number of search intervals in
the 10-seconds segment (N). The percentage can
range from 0 to 100%. In our approach, the 10-
seconds segment is assigned one of three
Low Energy ECG Features Extraction for Atrial Fibrillation Detection in Wearable Sensors
71
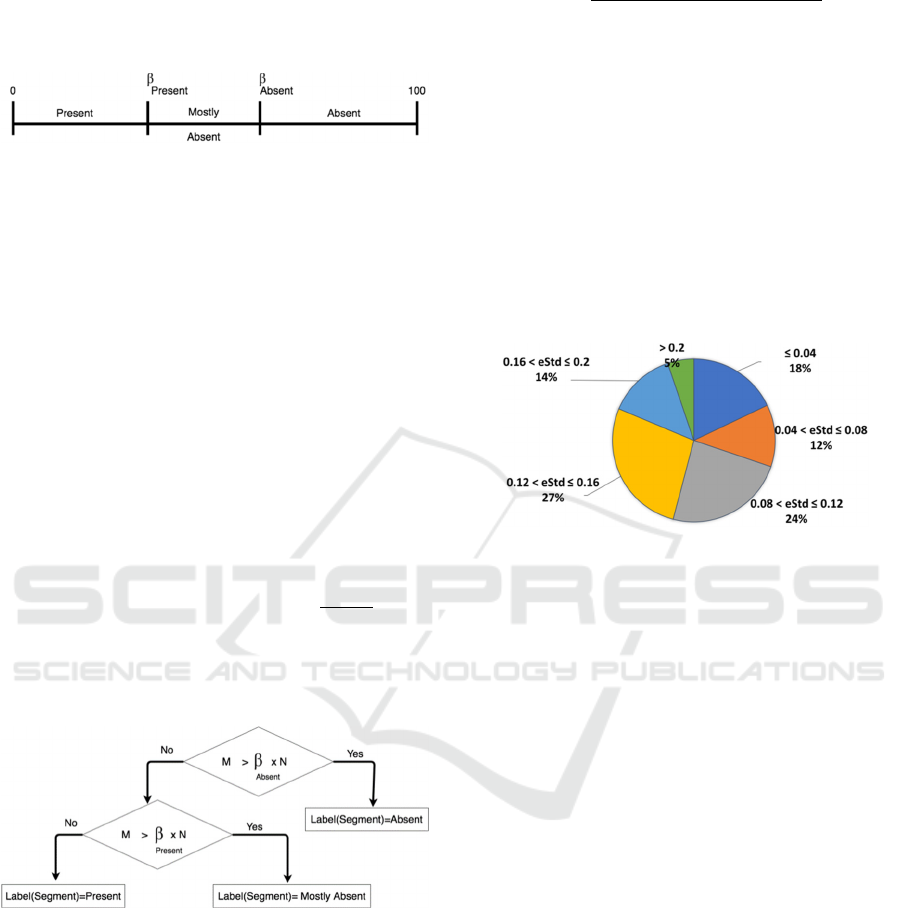
classification labels according to the percentage of
Misses in that segment. Each classification label is
associated with an interval on the real number line as
depicted in Figure 3.
Figure 3: P-wave Miss Ratios and corresponding
classification label.
To define the intervals, we need two values which
we refer to as {ß
Absent
, ß
Present
}. If the percentage of
Misses in the segment is in the interval [ß
Absent
,100]
then the segment is labeled “Absent” to reflect that
the number of search intervals that did not have a
valid P wave is high. The interval [0, ß
Absent
] covers
two classification labels “Mostly Absent” and
“Present”. The label “Present” is assigned to
segments in which the number of search intervals that
did not have a valid P wave is low. The label “Mostly
Absent” is assigned to segments in which the number
of search intervals that did not have a valid P wave is
in between the two extremes defined by the labels
“Present” and the label “Absent”. Therefore, to
discriminate between the labels “Present” and
“Mostly Absent” we define ß
Present
as the middle point
of the interval [0, ß
Absent
] given by (
). The
values {ß
Absent
, ß
Present
}are experimentally evaluated
as later show in section 4. Figure 4 illustrates the
labeling of the segment according to the values {
ß
Absent
, ß
Present
}.
Figure 4: Classification labels based on P wave Detection.
3.3.1 RR Analysis
The RR feature is evaluated as the time interval
between two consecutive R peaks. To capture RR
irregularity, we used a simple statistical measurement
(Bluman, 2009) that gives an estimate of the standard
deviation (Std) of RR intervals ( 1). We refer to this
measurement as eStd (RRs) where RRs is the set of
RR intervals extracted from the 10-seconds ECG
signal.
eStd (RRs) =
max
RR
s
- min
RR
s
4
(1)
The process of irregularity detection is based on
the comparison of eStd (RRs) value of a 10-seconds
ECG signal with a pre-set threshold ( TH
Std
). To
estimate the value of the threshold (TH
Std
) we used
268 10-seconds segments of ECG signals that were
annotated with AF episodes in the MIT/BIH
Arrhythmia Database (G. Moody & Mark, 2001).
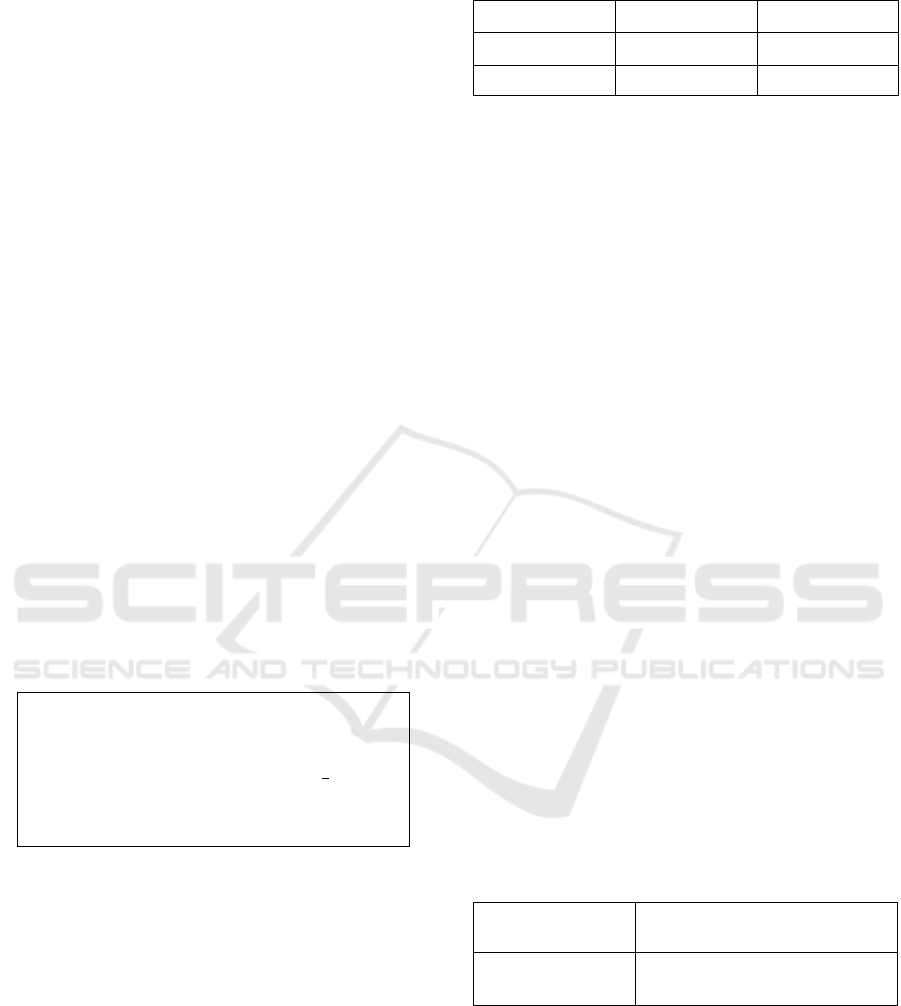
Figure 5 shows the distribution of eStd values in ECG
segments that are entirely AF episodes. No Normal
Sinus Rhythm (NSR) segments were included in the
analysis. According to the figure the majority of AF
eStd values measured (82%) were greater than 0.04.
Therefore, TH
Std
is set to the value of 0.04.
Figure 5: Distribution of eStd values in AF episodes.
To validate the eStd measurement capability in
capturing RR irregularity, we used a set of variable
length ECG segments: 10 seconds, 20 seconds, and
30 seconds. Experiments have shown that as the
segments get longer than 10 seconds, the correlation
between eStd and classical Std starts to decrease.
Thus, we can conclude that RR irregularity can be
detected in an ECG segment as short as 10 seconds.
This signal length implies lower memory
requirements, less processing time and eventually
lower energy consumption.
3.3.2 P wave Detection
In the proposed AF detection scheme, we are not
interested in detecting the temporal location of P
wave fiducial points. Instead, we are investigating:
“Is there a valid P wave in the current search
interval?”. To answer this question, a wavelet
transformation is performed to approximate the
morphology of the second half of the RR interval
(search interval). The idea is that if the approximation
extracted is similar to a pre-defined template of a
valid P wave then we can say that a P wave is present
in the search interval. Otherwise, the P wave is
considered absent.
SENSORNETS 2021 - 10th International Conference on Sensor Networks
72
The Haar wavelet (Walker, 2008) is the simplest
type of wavelet that decomposes a discrete signal into
two sub-bands where each sub-band is half the length
of the original signal. The first sub band is a running
average that approximates the shape of the original
signal. The second sub band contains the difference
that generates the detail coefficients.
Rincon et. al (Rincon et al., 2012) adopted the
quadratic spline wavelet (Martínez, Almeida, Olmos,
Rocha, & Laguna, 2004) to delineate the ECG signal.
For that purpose the sensor is expected to maintain 5
levels of wavelet decomposition including both
approximation and detail coefficients. To keep the
time-invariance and temporal resolution at different
scales, the same sampling rate has been used in all
scales.
In the proposed scheme we have selected the Haar
wavelet for its computational effeciency (Mazomenos
et al., 2013). Moreover, the sensor is designed to
maintain only the approximation coefficients at level
2. Experiments have shown that 2 levels provide
adequate noise reduction. The pseudocode of P wave
Haar based approximation is illustrated in
Figure 6.
In contrast to the wavelet approach adopted by
Rincon et. al (Rincon et al., 2012), Haar based
approach is lighter in terms of memory requirements
and computational complexity. This is due to the
simplicity of the Haar wavelet in addition to the fact
that the wavelet decomposition is only applied to a
small portion of the signal. This setup translates to
lower energy consumption.
Figure 6: A single level Haar approximation.
To create a template of the P wave, we have used
the set of NSR signals (Table 1) in the QT database
(Laguna, Mark, Goldberger, & Moody, 1997). From
each signal, we have used 1 minute of ECG recording
with an average number of 50 P waves per signal. P
waves were extracted as the second half of the RR
intervals marked by the Dual Slope algorithm. Then a
2-level Haar transform was applied to each P wave to
obtain an approximation of the P wave. The template
P wave was chosen as the average of the 400+
approximated P waves extracted from the signals.
Table 1: NSR signals used in P wave template.
sel16265 sel16272 sel16273
sel16420 sel16773 sel16539
sel16786 sel17152 sel17453
To ensure the scalability of the template, we have
normalized the sample amplitudes. This is a necessary
step since the amplitude values will vary among
signals according to the technology used in recording
the ECG signal. We have used min-max scaling to
rescale amplitudes to the unified scale [-1,1].
The extracted P waves and the template P wave
are different in length. In addition, the length of
extracted P waves varies according to the heart rate
that defines the duration of an RR interval. Therefore,
we have selected Dynamic Time Warping (DTW) (Li,
2014) which is able to measure the distance between
time series of unequal length and that are not aligned
in time. With Dynamic Time Warping we are able to
compare any P wave to the template P wave
regardless of the heart rate and the sampling
frequency of the input signal. If the distance is within
a pre-set threshold (TH
DTW
), then approximated P
wave (𝑃
) is accepted as a valid P wave. Otherwise,
the P wave is considered absent.
To evaluate threshold (TH
DTW
), we used two sets
of signals (Table 2): AF signals obtained from the
MIT Atrial Fibrillation Database (G. B. Moody &
Mark, 1983)(Goldberger et al., 2000) and non-AF
signals obtained from MIT-BIH Normal Sinus
Rhythm database (Goldberger et al., 2000). AF
signals were extracted as entirely AF episodes that
ranged in duration from 25 seconds to 100 seconds.
The total duration of AF episodes was around 8
minutes. NSR signals were in total 10 minutes long
with 200 seconds per database record. In total there
were 690 AF distances and 830 NSR distances.
Table 2: Signals used for TH
DTW
evaluation.
AF Signals
04048, 05121, 08215,
04043, 04746, 06453
NSR Signals 19830, 16483, 16795
P: P wave , 𝑃
: approximated P wave
N = length(P)
i = 1
j = 1
while(i < N)
𝑃
(j) = (P(i) + P(i + 1) ) /
√
2
i = i + 2
j = j + 1
end
Low Energy ECG Features Extraction for Atrial Fibrillation Detection in Wearable Sensors
73
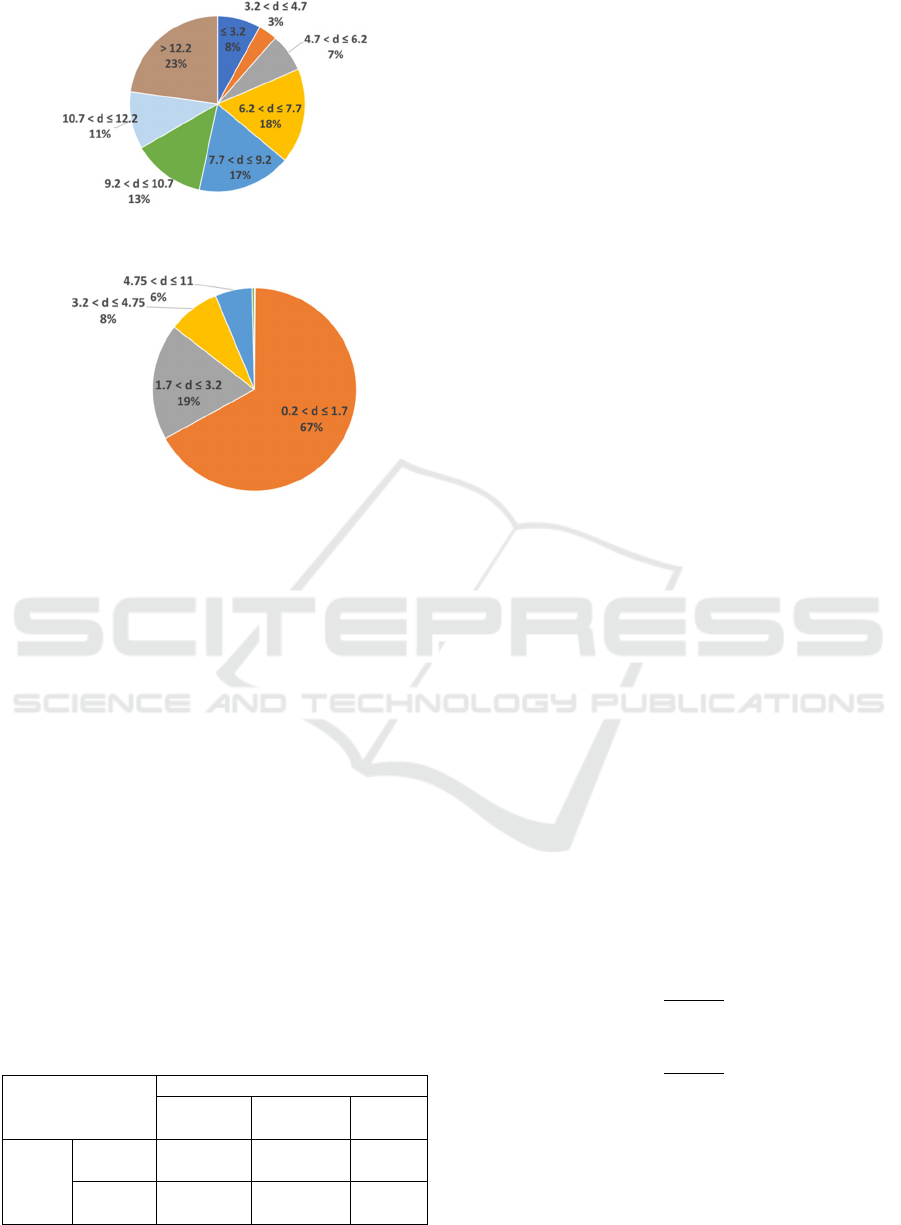
Figure 7: Distribution of DTW distances in AF signals.
Figure 8: Distribution of DTW distances in NSR signals.
Figure 7. reflects the frequency of distances
obtained from AF signals. Only (8%) of the distances
were less than or equal to 3.2. The majority of the
distances (92%) were greater than 3.2.
On the other hand, Figure 8 plots the distribution
of distances obtained from NSR signals. Only (14%)
of the distances were greater than 3.2. The majority
of the distances (86%) were less than equal to 3.2.
Therefore, we can conclude that the value 3.2 is
reasonable threshold since the majority of AF
distances were greater than 3.2 while the majority of
NSR distances were less than or equal to 3.2.
3.3.3 AF Decision Rules
The features extraction module of the proposed
scheme will produce two classification labels for each
10-seconds segment. These output labels will be used
to classify the segment as AF or non-AF (Table 3).
Table 3: AF rule- based classifier using RR and P wave
features.
AF Classifier P wave Labels
Present
Mostly
Absent
Absent
RR
Labels
Regular non-AF
(Rule1)
non-AF
(Rule3)
noisy
(Rule4)
Irregular non-AF
(Rule5)
AF
(Rule6)
AF
(Rule2)
Rule (1) will capture definite cases of Normal
Sinus rhythm that is characterized by regular RR
intervals and a valid P waves preceding each QRS
complex. Rule (2) will capture definite cases of Atrial
Fibrillation rhythm that is characterized by irregular
RR intervals and QRS complexes that are not
preceded by valid P waves.
In Rules (3) and (4), more weight is given to the
RR feature. Therefore, the segment is classified as
non-AF in Rule (3) and the absence of P waves is
attributed to noise. Rule (4) classifies the segment as
noisy since the P waves are said to be entirely absent.
Rules (5) and (6) apply for segments in which RR
intervals are irregular. In Rule (5), more weight is
given to the P wave feature. Therefore, the segment is
classified as non-AF. However, Rule (6) classifies the
segment as AF since most of the time the P wave is
absent.
4 PERFORMANCE ANALYSIS
The objective of our algorithm to efficiently
discriminate between Normal Sinus Rhythm (NSR)
and Atrial Fibrillation (AF) rhythm. Therefore, the
test signals (Table 4) do not include any other
arrhythmia such as Atrial Flutter. In addition, each
segment is either entirely NSR or AF. There is no
overlapping between segments.
Table 4: Test signals (AF Detection).
AF 04015, 07910, 04126, 04908
NSR
18177, 18184, 19088, 19090, 19093,
19140
We calculated two performance metrics of
detection accuracy: Sensitivity (Se) and Specifity
(Sp). Sensitivity defines the percentage of AF
segments that were correctly classified (2) whereas
the specificity defines the percentage of non-AF
segments that were correctly classified (3).
Se =
TP
TP+FN
(2)
Sp =
TN
TN+FP
(3)
As previously explained in section 3.2, the
detection of the P wave is based on the design
parameter that represents the percentage of Misses
in a 10-seconds segment. For the purpose of this
evaluation, we run the P wave based AF detection
algorithm at different values of . Table 5
SENSORNETS 2021 - 10th International Conference on Sensor Networks
74
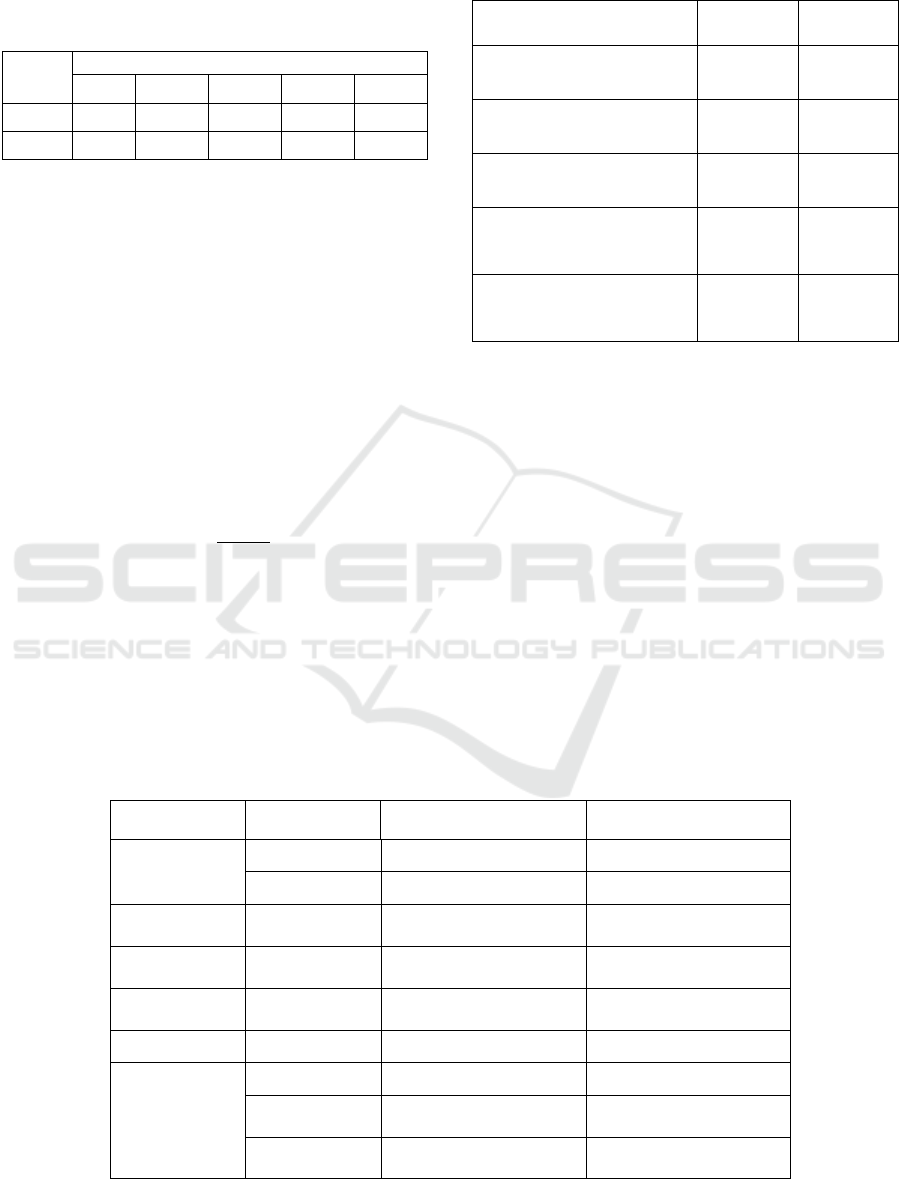
summarizes the AF detection results at = 0.3, 0.4,
0.5, 0.6, and 0.7 respectively.
Table 5: AF Detection accuracy based only on P wave.
0.3 0.4 0.5 0.6 0.7
Se % 99.8 99.2 98.5 96.4 92.9
Sp% 71.6 80.9 88.5 93.9 97.2
We can conclude from Table 5 that the best
performance was at = 0.6 and = 0.7 where both
Sensitivity and Specifity values are above 90%
However, the performance metrics at = 0.6 are
considered better since they give a higher Se 96 %,
even though it is less specific (93 %). A lower Se
might allow some AF cases to pass with no alarm.
However, lower Sp means that non-AF cases might
create some false-alarms. A false harm-less alarm is
more desirable than a harmful no-alarm.
The combination of features is performed
according to the classification rules summarized in
Table 3. The highest pair of Se and Sp achieved by
the wavelet based P wave detector was at = 0.6 ( Se
= 96.4% and Sp = 93.89%). Therefore, we can set
Absent
to 0.6 and
Present
= (
) = 0.3.
In comparison to related work in the area of
embedded AF detection, we can observe from Table
6 that the proposed approach for on-sensor AF
detection using the combination of eStd and P-wave
features is comparable to related work.
Table 6: Comparison of proposed approach to related work
in embedded AF detection.
AF Detection
Approaches
Se % Sp %
using eStd only 80.68 94.81
using P-wave only 96.4 93.89
using eStd and P-wave 98.59 97.61
AF detection on
Teleholter device
(Marsili et al., 2016)
97.33 98.67
AF detection on
Shimmer platform
(Rincon et al., 2012)
96 93
5 ENERGY EVALUATION
The underlying hypothesis evaluated in this paper is
that an efficient on-sensor processing of the ECG
signal increases the battery life time and ensures the
longevity of the application. Therefore it is important
to evaluate the energy consumption of (a) the classical
approach of full ECG transmission in contrast to (b)
the proposed approach of on-sensor AF detection.
The sensing energy is constant in both scenarios.
Therefore, we focus on evaluating the energy
consumed by local processing and wireless
transmission. For the purpose of evaluation, we
assume a 12-bit ADC with sampling frequency
(S
F
=250 Hz).
Table 7: Energy consumption of AF detection scheme.
Module Energy Label Energy units (mJ) per unit
R peak
detection
E
sample
0.03 energy per sample
E
R
75 energy per segment
RR interval
extraction
E
RR
0.01 energy per RR
P wave
detection
E
P
1.2 energy per RR
AF features
Extraction
E
FX
19 energy per segment
AF detection E
AF
0.87 energy per segment
Server
Notificaiton
E
radio
0.3 energy per byte
E
radioSample
(
2 b
y
tes
)
0.6 energy per sample
E
radioRR
(
4 b
y
tes
)
1.2 energy per RR
Low Energy ECG Features Extraction for Atrial Fibrillation Detection in Wearable Sensors
75

Table 7. lists the estimated energy cost of each
module in the proposed AF detection scheme using
the Avrora tool (Avrora, 2008) that provides a cycle-
accurate simulation of the AVR microcontroller.
Note that this evaluation considers the worst
algorithmic case of each module.
Local ECG processing is composed of R peak
detection 𝐸
, AF features extraction 𝐸
, and
AF decision 𝐸
. 𝐸
is the amount of energy
consumed to detect R peaks in a 10-seconds segment
= 75 mJ. 𝐸
is the amount of energy consumed by
the rule-based classifier = 0.87 mJ. 𝐸
is the amount
of energy consumed to extract RR and P wave
features equal to 19 mJ.
The total energy consumed for processing of a 10
seconds ECG segment given by (4):
E
AF10s
= E
R
+ E
FX
+ E
AF
=94.87 mJ
(4)
In the classical approach of full ECG
transmission, the energy is consumed by radio
transmission as there is no local ECG processing.
Therefore, the total energy consumed in this scenario
(E
ECG_transmission(a)
) is around 45 J.
On the other hand, if we consider that the sensor
performs periodic on-node AF detection every 10
seconds for 5 minutes ECG signal then the total
energy for the processing of the proposed AF
detection scheme 𝐸
= 3.4 J
The gain in energy saving measured by :
G= 1-
E
AFTotal
b
E
ECG_transmission
a
*100=92.5 %
(5)
This gain in energy saving (5) shows that our
proposed scheme for embedded Atrial Fibrillation
detection achieves a considerable gain in the energy
consumed for AF detection when compared to the
classical approach based on the full transmission of
the ECG signal to a remote server for analysis. In
fact, the energy gain achieved is higher than the
marginal 4% increase in the battery life time reported
by Rincon et. al (Rincon et al., 2012).
We note that the gain in energy consumption
increases as the ratio
decreases. Which means
as we increase the periodicity of AF detection we
increase the gain in energy. However, we have to
keep in mind the trade-off between AF detection
efficiency and energy saving to extend the network
life time. In practice, this periodicity should be based
on clinical requirements.
6 CONCLUSIONS
This paper presents a new approach of on-sensor AF
detection as a data reduction strategy. In this approach,
the body sensor node is designed to efficiently extract
and analyze relevant ECG features in order to classify
the ECG signal as a possible AF episode. This decision
will be submitted to the remote server with the
minimum representation of data to perform further
classification. Performance results have shown that the
proposed scheme achieved high sensitivity (98.59%)
and specificity (97.61%) demonstrating high accuracy
in the detection of the AF episodes. In comparison
with the transmission of full ECG signals, the
proposed approach can save around 92% of energy.
For future work, we are considering hardware
implementation of the proposed system in FPGA
platform.
ACKNOWLEDGEMENTS
This research has been generously sponsored by the
King Abdulaziz City for Science and Technology
(KACST), Riyadh, Saudi Arabia, under Grant 1-17-
02-001-0027.
REFERENCES
Avrora. (2008). Avrora - The AVR Simulation and
Analysis Framework. Retrieved July 20, 2016, from
http://compilers.cs.ucla.edu/avrora/
Babaeizadeh, S., Gregg, R. E., Helfenbein, E. D., Lindauer,
J. M., & Zhou, S. H. (2009). Improvements in atrial
fibrillation detection for real-time monitoring. Journal
of Electrocardiology, 42(6), 522–526.
https://doi.org/10.1016/j.jelectrocard.2009.06.006
Bluman, A. G. (2009). Elementry Statistics: A Step by Step
Approach (7th ed.). McGraw-Hill.
Dash, S., Chon, K., Lu, S., & Raeder, E. (2009). Automatic
Real Time Detection of Atrial Fibrillation. Annals of
Biomedical Engineering. https://doi.org/10.1007/
s10439-009-9740-z
de Carvalho, P., Henriques, J., Couceiro, R., Harris, M.,
Antunes, M., & Habetha, J. (2012). Model-Based
Atrial Fibrillation Detection. ECG Signal Processing,
Classification and Interpretation: A Comprehensive
Framework of Computational Intelligence.
https://doi.org/10.1007/978-0-85729-868-3
Goldberger, A. L., Amaral, L. A. N., Glass, L., Hausdorff,
J. M., Ivanov, P. C., Mark, R. G., … Stanley, H. E.
(2000). PhysioBank, PhysioToolkit, and PhysioNet:
Components of a New Research Resource for Complex
Physiologic Signals. Circulation 101(23), e215–e220.
SENSORNETS 2021 - 10th International Conference on Sensor Networks
76

Retrieved from http://circ.ahajournals.org/content/
101/23/e215.full
January, C. T., Wann, L. S., Alpert, J. S., Field, M. E.,
Calkins, H., Murray, K. T., … Sellke, F. W. (2014).
2014 AHA / ACC / HRS Guideline for the Management
of Patients With Atrial Fibrillation.
https://doi.org/10.1161/CIR.0000000000000041
Ladavich, S., & Ghoraani, B. (2015). Rate-independent
detection of atrial fibrillation by statistical modeling of
atrial activity. Biomedical Signal Processing and
Control, 18, 274–281.
https://doi.org/10.1016/j.bspc.2015.01.007
Laguna, P., Mark, R. G., Goldberger, A., & Moody, G. B.
(1997). A Database for Evaluation of Algorithms for
Measurement of QT and Other Waveform Intervals in
the ECG. Computers in Cardiology, 24, 673–676.
Li, H. (2014). On-line and dynamic time warping for time
series data mining. International Journal of Machine
Learning and Cybernetics, 6(1), 145–153.
https://doi.org/10.1007/s13042-014-0254-0
Lin, C. T., Chang, K. C., Lin, C. L., Chiang, C. C., Lu, S.
W., Chang, S. S., … Ko, L. W. (2010). An intelligent
telecardiology system using a wearable and wireless
ecg to detect atrial fibrillation. IEEE Transactions on
Information Technology in Biomedicine, 14(3), 726–
733. https://doi.org/10.1109/TITB.2010.2047401
Mairesse, G. H., Ireland, P. M., Gelder, I. C. Van,
Germany, C. E., Uk, J. M., & Uk, A. B. (2018).
Screening for atrial fibrillation : a European Heart
Rhythm Association ( EHRA ) consensus document,
(March), 1589–1623. https://doi.org/10.1093/europace
/eux177
Marsili, I. A., Masè, M., Pisetta, V., Ricciardi, E.,
Andrighetti, A. O., Ravelli, F., & Nollo, G. (2016).
Optimized Algorithms for Atrial Fibrillation Detection
by Wearable Tele-Holter Devices. In 2016 IEEE
International Smart Cities Conference (ISC2).
Martínez, J. P., Almeida, R., Olmos, S., Rocha, A. P., &
Laguna, P. (2004). A Wavelet-Based ECG Delineator :
Evaluation on Standard Databases. IEEE Transactions
on Biomedical Engineering, 51(4), 570–581.
Mazomenos, E. B., Biswas, D., Acharyya, A., Chen, T.,
Maharatna, K., Rosengarten, J., … Curzen, N. (2013).
A Low-Complexity ECG Feature Extraction
Algorithm for Mobile Healthcare Applications. IEEE
Journal of Biomedical and Health Informatics, 17(2),
459–469.
Meek, S., & Morris, F. (2002). ABC of clinical
electrocardiography.Introduction. I-Leads, rate,
rhythm, and cardiac axis. BMJ (Clinical Research Ed.),
324(7334), 415–418.
https://doi.org/10.1136/bmj.324.7334.415
Moody, G. B., & Mark, R. G. (1983). A new method for
detecting Atrial Fibrillation using RR intervals.
Computers in Cardiology, 10, 227–230.
Moody, G., & Mark, R. (2001). The impact of the MIT-
BIH Arrhythmia Database. IEEE Engineering in
Medicine and Biology Magazine,
20(3), 45–50.
Petrėnas, A., Sörnmo, L., Lukoševičius, A., & Marozas, V.
(2015). Detection of occult paroxysmal atrial
fibrillation. Medical and Biological Engineering and
Computing, 53(4), 287–297.
https://doi.org/10.1007/s11517-014-1234-y
Petty, B. G. (2016). Basic Electrocardiography. New
York: Springer.
Rabinstein, A. A., Fugate, J. E., Mandrekar, J., Burns, J.
D., Seet, R. C. S., Dupont, S. A., … Friedman, P. A.
(2013). Paroxysmal Atrial Fibrillation in Cryptogenic
Stroke-A Case Control Study. Journal of Stroke &
Cerebrovascular Diseases, 22(8), 1405–1411.
Rincon, F., Grassi, P. R., Khaled, N., Atienza, D., & Sciuto,
D. (2012). Automated Real-Time Atrial Fibrillation
Detection on a Wearable Wireless Sensor Platform. In
34th Annual International Conference of the IEEE
EMBS (pp. 2472–2475).
Ródenas, J., García, M., Alcaraz, R., & Rieta, J. J. (2015).
Wavelet Entropy Automatically Detects Episodes of
Atrial Fibrillation from Single-Lead
Electrocardiograms. Entropy, 17, 6179–6199.
https://doi.org/10.3390/e17096179
Sörnmo, L., Petrenas, A., & Marozas, V. (2018). Atrial
Fibrillation from an Engineering Perspective. In L.
Sörnmo (Ed.) (1st ed., p. 316). Springer International
Publishing.
Tateno, K., & Glass, L. (2002). A method for detection of
atrial fibrillation using RR intervals.
https://doi.org/10.1109/cic.2000.898539
Walker, J. S. (2008). A Primer on Wavelets and Their
Scientific Applications (2nd ed.). CRC Press.
Wang, Y., Deepu, C. J., & Lian, Y. (2011). A
Computationally Efficient QRS Detection Algorithm
for Wearable ECG Sensors. In Engineering in
Medicine and Biology Society, EMBC, 2011 Annual
International Conference of the IEEE (pp. 5641–
5644).
Low Energy ECG Features Extraction for Atrial Fibrillation Detection in Wearable Sensors
77
