
Hydrothermally Fluorinated Graphene Oxide Chemiresistive Sensor
for Detecting NH
3
and Acetone under Atmospheric Conditions
Ivan Amor, Bruno Gamero, Siziwe Bebe and Ravi Prakash*
Department of Electronics Engineering, Carleton University, Ottawa, Canada
Keywords: Biosensor, Health Monitoring, Electronic Sensor, Chemiresistor, Graphene Oxide, Exhaled Breath Detection,
Multi-analyte Sensing.
Abstract: Emergence of graphene-derived highly functional materials has transformed chemical and bio sensing, with
several novel approaches utilizing chemical modification of graphene oxide (GO). These materials have been
implemented in device fabrication for the detection of biomolecules, volatile organic compounds (VOC), and
other chemical analytes. The detection methods rely on using specificity of the modified graphene material to
target selective and quantifiable electrical responses. In this work, we report ultra-low-level detection of NH
3
(1-10 ppm) and extend the same chemiresistor sensor to additionally detect acetone and distinguish between
their individual transient responses. The low-level detection of both these gaseous analytes is highly relevant,
and the comparable physiological detection range of these analytes makes the device suitable for continuous
health monitoring and detecting specific gas molecules in exhaled breath. The sensor system is compactly
designed to make it low cost and ideal for wearable health monitoring and environmental monitoring, using
an in-house hydrothermal fluorination technique to synthesize fluorinated-GO (FGO) suspension, and its
solution-phase deposition onto interdigitated chrome electrodes to create the chemiresistive gas sensor. The
sensor device reported a highly linear detection range for NH
3
, ranging over 1 – 10 ppm and was additionally
able to detect acetone in a similar low concentration range, whilst distinguishing the two gases based on its
rapid transient response.
1 INTRODUCTION
Chemiresistors, along with electrochemical sensors,
are widely used as gas sensing devices, and for a
variety of chemical sensing applications (Yadava et
al., 2012; Sanchez et al., 2006; Lin et al., 2015).
Electrochemical sensors adequately target
metabolites and electrolytes through enzymatic, ion-
selective, electroactive reactions (Zhao et al., 2020).
However, there are several disadvantages to their
implementation for non-invasive and continuous
health applications (Gargiulo et al., 2020). Their
dependence on elaborate patterning steps and
temperature sensitivity results in higher complexity.
Chemiresistors on the other hand, offer the benefit of
sensing various analytes due to the configurable layer
properties of the sensor. In addition, chemiresistive
sensors are simpler to fabricate, require less operating
power, and possess a longer operational life span than
electrochemical detectors (Sanchez et al., 2006; Lin
et al., 2015; Zampolli et al., 2007). They prove to be
excellent candidates for ultra-low molecular
concentration detection in a non-invasive, continuous
health care monitoring approach, to potentially
observe and indicate important physiological
conditions by identifying target gaseous analytes
(Majumder et al., 2017). Chemiresistors are
commonly implemented using metal oxide (MOx)
sensitive films such as tin or titanium oxide and
require high temperature for gas detection (Wang et
al., 2010; Ponzoni et al., 2017). This is achieved using
a thermistor integrated into the device, or a sensing
layer is deposited on a micro-hot plate for optimal
operating temperature (Lin et al., 2015; Manginell et
al., 1997).
Graphene has attracted great attention among
available 2-D materials for both chemical sensing
applications. Its morphological characteristics,
especially its high surface to volume ratio has been
attractive for gas sensing applications. Graphene
contains high electronic charge mobility, Young’s
modulus, and thermal conductivity, which makes it an
ideal candidate material for integrated circuitry,
energy storage, and chemical and bio-electronic
Amor, I., Gamero, B., Bebe, S. and Prakash, R.
Hydrothermally Fluorinated Graphene Oxide Chemiresistive Sensor for Detecting NH3 and Acetone under Atmospheric Conditions.
DOI: 10.5220/0010241200990106
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 1: BIODEVICES, pages 99-106
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
99

sensors. During the oxidation process of graphene,
sp
3
bonding is heavily interrupted and makes
electrical conductivity of graphene oxide (GO)
decrease drastically compared to pristine graphene
(Zaaba et al., 2017). GO can be modified to produce
functionalized GO (FGO), which has lower or higher
conductivity properties than pristine graphene and
GO (Huang et al., 2011; Kavinkumar et al., 2015).
This makes FGO a suitable candidate for replacing
MOx in chemiresistors. Another benefit is that FGO
can be produced using cost-effective chemical
methods, and in high yields from the inexpensive
graphite raw material. In addition, due to its
hydrophilic nature, stable aqueous colloids can be
formed to facilitate the assembly of macroscopic
structures by simple, low-cost solution deposition
processes (Park et al., 2016; Chronopoulos et al.,
2017). Reducing graphene oxide by fluorination
offers several advantages, owing to the low polarity
of C–F bonds and the high polarity of oxygenic
groups, characteristics of FGO could be tuned by
controlling the C–F and C–O ratios. Oxygen or
fluorine atom concentration can be tuned for different
end-product usage (Karlický et al., 2013). The main
advantage of FGO for sensing is its high
electrocatalytic activity (augmented electron
transfer), and specificity toward certain analytes due
to the presence of C–F bonds (Urbanova et al., 2016;
Lu et al., 2009). It has been observed that low
fluorinated graphene exhibits excellent sensing
properties for a range of studied biomolecules -
including ionized (NH4+), unionized ammonia
(NH
3
), and gaseous NH
3
, nicotinamide adenine
dinucleotide (NADH), ascorbic acid (AA), uric acid
(UA), and dopamine (DA). The low fluoride (F)
content that augments the electron transfer from
electrode to biomolecule (or vice versa) will neither
significantly affect the electrical conductivity nor the
wetting properties of graphene (Zhu et al., 2019;
Huang et al., 2012). Exploration of ammonia sensing
using graphene-based platforms is rapidly evolving
(Huang et al., 2012; Schedin et al., 2007). Recent
Discrete Fourier Transform (DFT) studies by Yeon
Hoo Kim et al. predicted that the binding of GO with
NH
3
molecules can be enhanced by decreasing
electron density of GO (Kim et al., 2017).
In this work, we have demonstrated a small
footprint, low-cost, FGO based chemiresistive gas
sensor, implemented on a variety of substrate
materials, for rapid and ultra-low concentration
detection of NH
3
(~ 1 – 10 ppm). The operating
principle of our chemiresistive sensor relies on
adsorption of gas molecules in a sensitive 2-D film
and the resultant change in electrical resistance
(Manginell et al., 1997; Bârsan et al., 1995; Zhao et
al., 2019; Briand et al., 2000). In our approach, a layer
of chemically functionalized graphene oxide thin film
material was deposited onto the interdigitated metal
electrodes. To render the graphene oxide selective to
target gas molecules, functional groups were added
through chemical modification. We furthermore
investigated detection of Acetone to establish the
multi-analyte sensing capability of our system. We
chose ammonia as the target analyte due to its
importance in diverse biological processes.
Commercial sensors exist for detecting ammonia in
ranges above 10 ppm, but they are not adequately
sensitive for healthcare centric applications. Samples
of ammonia that are discharged from the body are
used to monitor systemic ammonia levels (Gafare et
al., 2014). Multi analyte detection of acetone is also
crucial for monitoring conditions such as diabetes.
Studies have shown that persons with diabetes would
exhibit an ultra-low concentration of acetone (>
1.8ppm) in exhaled breath (Xiao et al., 2014).
2 MATERIALS AND METHODS
XtalFluor-E (XtalFluor), hydrofluoric acid (HF),
sodium hydrogen carbonate, poly-(3,4-
ethylenedioxythiophene polystyrene sulfonate
(PEDOT:PSS), polyvinyl alcohol (PVA) and poly-
methyl methacrylate (PMMA) were acquired from
Sigma Aldrich. De-ionized water was gathered from
the local micro fabrication lab on-site regeneration
system.
A specialized Teflon beaker with a specially
formulated stabilized poly-tetrafluoro ethylene
(PTFE) carbon base to aid in heating was obtained
from Canada wide Scientific (Dynalon Labware) for
the synthesis process.
Surface oxidized (passivated) silicon (pSi) and
silicon (Si) substrates with chromium Interdigitated
Electrodes (IDEs) were fabricated at the Carleton
University micro-fabrication lab. Nitrogen (N
2
) and
NH
3
gas were obtained from Praxair Canada Inc.
2.1 In-house FGO Synthesis
Two different synthesis methods were used to make
FGO. A commercial chemical agent and an in-house
hydrothermal process were synthesized using the
FGO preparation method. The commercial
fluorination agent, XtalFluor-E 2 g of and 2 mL of 2
mg ml
-1
GO solution were chemically modified by
dissolving them in 48% HF solution. The
consolidated solution is then boiled at 180
0
C for 2 h.
BIODEVICES 2021 - 14th International Conference on Biomedical Electronics and Devices
100
The reaction was quenched with sodium hydrogen
carbonate (5%), washed thoroughly with de-ionized
water and sample dried.
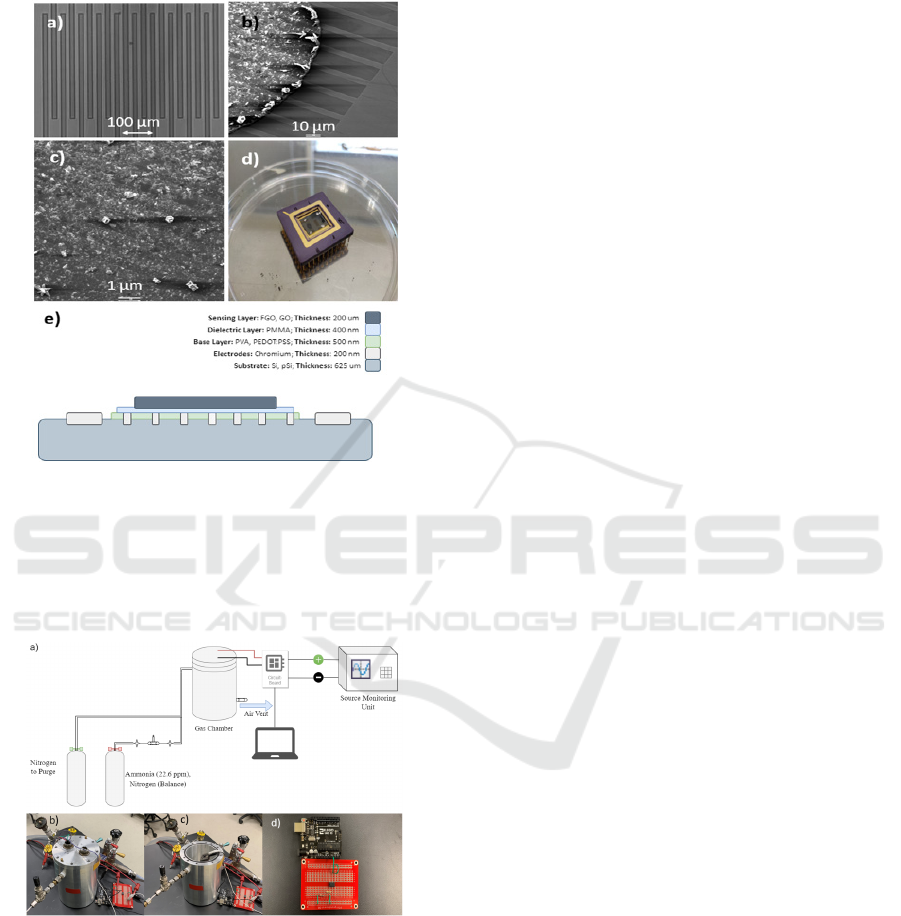
Figure 1: Micrographs showing a) Optical microscope
image of IDE chrome structure, b) Scanning Electron
Microscope (SEM) image of drop casted FGO film on the
IDE structure, c) SEM micrograph showing distribution of
FGO micro-particles on sensor surface and, d) a fully
packaged FGO NH
3
gas sensor device, e) Device Cross-
Sectional diagram.
Figure 2: a) Schematic of the experimental setup. b) Closed-
lid chamber and c) Open-lid chamber setup. d) Amplifier
circuit for voltage monitoring and capture.
A relatively low-cost in-house hydrothermal FGO
preparation method was developed and tested. Here,
GO (50 mg) was dispersed in 20 ml of deionized
water ultrasonically, and 5 ml of HF (49%) was added
with moderate stirring. The mixture was stirred on a
hot plate at 150 °C for 20 h. The resulting solution
was quenched using sodium hydrogen carbonate
(5%), centrifuged and washed several times with de-
ionized water. The final product was then freeze-
dried.
2.2 IDE Chemiresistive Sensor
Fabrication
The IDEs used in this experiment were
micropatterned using standard photolithography of
200 nm chrome layer, deposited on Si and pSi
substrates in Fig. 1a).
The IDE structures on the pSi substrate were
layered with PEDOT: PSS (conductive) and PMMA
(di-electric). A homogenous suspension of GO and
the different samples of FGO (0.2mg/ml) were drop
coated using a micro-pipette on each of the different
devices (Fig. 1b, c).
The sensor microchip was wire bonded onto a
package shown in Fig. 1d), that was then used to
connect the device to the circuit for measurements.
The packaged sensors were exposed to NH
3
and other
target gases for an established period under both
controlled and normal environmental conditions at
ATM as seen in Figure 2b) and c). The experiments
were repeated to verify reproducibility, reversibility,
and sensor behaviour.
2.3 Experimental Setup
The initial experimental setup is shown in Figure 2a).
NH
3
and N
2
gases were released into the stainless-
steel test chamber and used to verify the concept of
targeted gas sensing in an ideal, controlled
environment. The packaged sensor was placed inside
the gas chamber. A vacuum pump was used to
achieve a chamber pressure of ≤ 0.00533 kPa (40
mTorr). The ammonia gas cylinder with a rated
concentration of 22.6 ppm, was then attached to the
chamber with a needle valve to control the flow of gas
released into the chamber with added precision. A
flow of ammonia gas was released for 3 minutes by
opening the needle valve at around 15%. The nitrogen
gas was used to dilute the ammonia and return to
atmospheric baseline readings. In certain control
experiments, a commercial Honeywell Ammonia Gas
flow sensor (MIDAS-E-NH3) was introduced in the
setup to measure and correlate the ammonia
concentration to the response of the chemiresistor gas
sensor.
The sensor device was connected in series as the
second resistor in a voltage divider circuit to feed the
input voltage, V
in
from the SMU for the
instrumentation amplifier AD 620. The voltage
measurements were then recorded over time.
Hydrothermally Fluorinated Graphene Oxide Chemiresistive Sensor for Detecting NH3 and Acetone under Atmospheric Conditions
101
•
2.4 Experimental Procedure: Data
Capture and Analysis
The data was captured with respect to the voltage of
the amplifier V
O
over time and was used to
characterize the normalized change in resistance
defined as |ΔR/R| (%): where ΔR is the resistance
change with respect to the baseline resistance R
before exposure to ammonia.
The sensing mechanism was exposed to a steady
flow of ammonia in a closed environment then
extensively verified in an open chamber in an ambient
setting to mimic practical health monitoring
applications. Responsiveness and selectivity of the
devices in these conditions were validated, in
significantly lower gas concentrations. Experiments
were repeated in an open chamber with ammonia gas
flow at concentrations below 2.26 ppm on top of the
sensor surface, to mimic exhaled breath sensor
response under atmospheric pressure conditions.
2.5 Material Characterization for FGO
Fourier transform infrared spectroscopy (FTIR) is
performed on several GO and FGO (XtalFluor) and
FGO (In-house) samples using a KBr pellet method,
scanning from 400 to 4000 cm
-1
. Absorption and
Transmittance spectra are analyzed to compare
efficacies of GO functionalization methods.
The X-ray photoelectron spectroscopy (XPS) data
was collected using AlK
α
radiation at 1486.69 eV
(150 W, 10 mA), charge neutralizer and a delay-line
detector (DLD) with three multi-channel plates.
Survey spectra are recorded from -5 to 1200 eV at a
pass energy of 160 eV (number of sweeps: 2) using
an energy step size of 1 eV and a dwell time of 100
ms. High resolution spectra for F1s, O1s, and C1s are
recorded in the appropriate regions at a pass energy
of 20 eV (number of sweeps: F1s, 15; O1s, 5; C1s, 5)
using dwell time of 300 ms and 0.1eVstep size.
Characterization results from the FTIR and XPS
are detailed in the results and discussion section.
3 RESULTS AND DISCUSSIONS
3.1 Characterization Results for FGO
Analysis of the obtained spectra for the GO and the
FGO showed that the C–O bond at Wavenumber
~1052 cm
-1
diminishes and is subsequently replaced
by another peak at ~1166 cm
-1
. Based on literature
and results obtained previously, the resulting peak
indicated the formation of a C-F bond, hence
fluorination of the GO. A vibration characterizing a
carbonyl group C=O at wavenumber ~1720 cm
-1
,
which is originally present in the GO sample,
diminishes upon the fluorination reaction using both
methods (HF thermally, and the HF/Xtal Fluor). The
shift of wave numbers was also noted for the bond
vibration of –C=C-, ~1618 cm
-1
with a marked
decrease in the intensity of transmittance. Intensity of
the peaks associated with the carbon oxygen bonds
decrease and a strong peak at ~1166 cm
-1
,
corresponding to covalent C–F bond, appears in the
FTIR spectrum of FGO (Gong et al., 2012), indicating
that oxygen-containing functional groups are
replaced by fluorine. The bond C–F (sp
3
) vibrates at
the wave number ~ 1082 cm
-1
and is observed in the
FGO spectrum. The peak at wavenumber ~2928 Is
X=C=Y, where X and Y can be either C or O. C–F
formation was obtained from the results of the XPS
data analysis. Differences between the GO and FGO
decomposition XPS spectra in Fig. 3 a) C1s, b) O1s
and c) F1s confirmed fluorination.
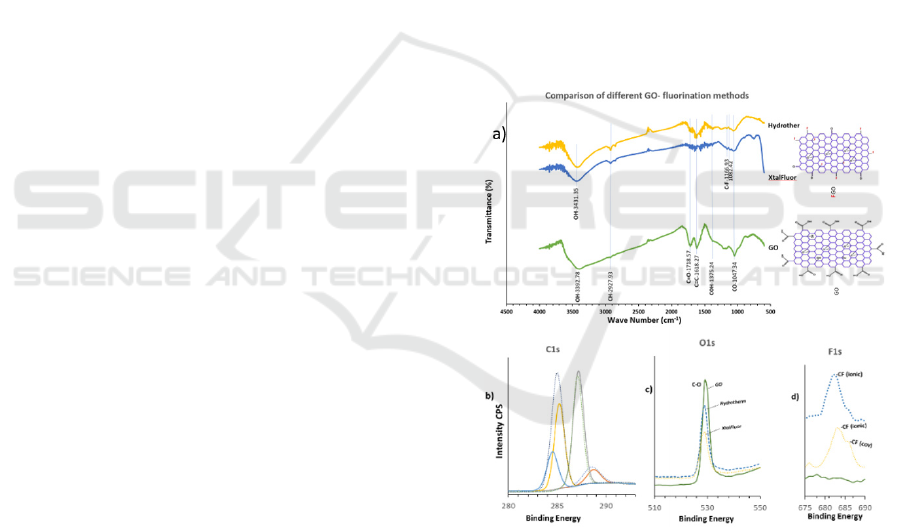
Figure 3: a) FTIR spectra of GO fluorination with XtalFluor
and with hydrothermal (Hydrother) fluorination in HF. The
GO spectrum is a reference showing the different peaks that
are formed due to the fluorination. The two molecular
structures of GO and FGO are representative structures,
showing likely fluorination positions, b) The XPS figures
show the comparison between GO before Fluorination and
after Fluorination for C1s, c) O1s and d) F1s. In addition,
c) and d) show comparison of the different synthesis
products.
BIODEVICES 2021 - 14th International Conference on Biomedical Electronics and Devices
102
Comparison of the XPS C1s spectra
decomposition showed characteristic peaks at: C=C,
284 eV, Csp3, Csp2, –CH 285 eV, C–O 287 eV and
O–C=O 288 eV. The peak intensities of C–O and
C=C/C–C display a significant increase due to the
formation of various C-F bonds. This confirmed the
reduction of the C=O in the FTIR spectrum and the
appearance of the C–F peak. Observed in XPS C1s of
the C=O is an increase and shift in the FGO spectrum.
F1s spectra Fig. 3d) shows the presence of semi-ionic
C–F bonds and the covalent C–F bonds corroborated
by the significant increase of C1s, Csp3, Csp2, –CH
peak in the FGO spectrum compared to the GO, with
no notable increases in C=O and –C–O.
From the experimental results spectra, C–F bond
formation was observed in both fluorination
processes and that using the in-house method of
synthesis would equally facilitate the formation of the
FGO–NH
3
complex molecule. A comparison of the
inhouse thermal synthesis of FGO and use of the
commercial chemical XtalFluor is shown in figure 3c.
The depletion and shift of the HC–O at 532 eV was
observed as it is consumed in the production of C–F
bonds. The graphs in figure 3c), d) also show a
comparison of the two FGO synthesis methods
products with that of GO, where the appearance of the
C–F (ionic) bond is observed at 682 eV and C–F
(covalent) bond at 687 eV for both synthesis methods
while there is no peak at all for GO. The C–F covalent
bonding is noted in the XtalFluor synthesis,
suggesting superior fluoride doping, however, the
simplicity and high yield of the inhouse thermal
process makes it more desirable.
3.2 Substrate Selection for IDEs
A novel component of our chemiresistor sensor is the
ability to use a readily available, inert substrate. We
used pSi in our tests due to its low cost, non-
conductive nature. PEDOT:PSS has a proven
conformal contact with textured silicon, and was used
as a thick hydrophobic conductive film, with high
porosity (Jiang et al., 2018; Vosgueritchian et al.,
2012).
We observed that PEDOT:PSS was effective in
lowering the baseline resistance from bare pSi at 1.2
MΩ to 133.3 kΩ, however its response to ammonia
gas was undesirable (see Fig. 4), and necessitated
another coating layer to prevent the interaction
between the target molecules and PEDOT:PSS.
PMMA was chosen due to its fibrous composition
and very low chemical affinity. The thin film stack
aids in the retention of gas molecules, possess
excellent dielectric properties (Zhang et al., 2014),
and therefore proved helpful in trapping analyte
molecules and facilitated interaction of FGO with
NH
3
.
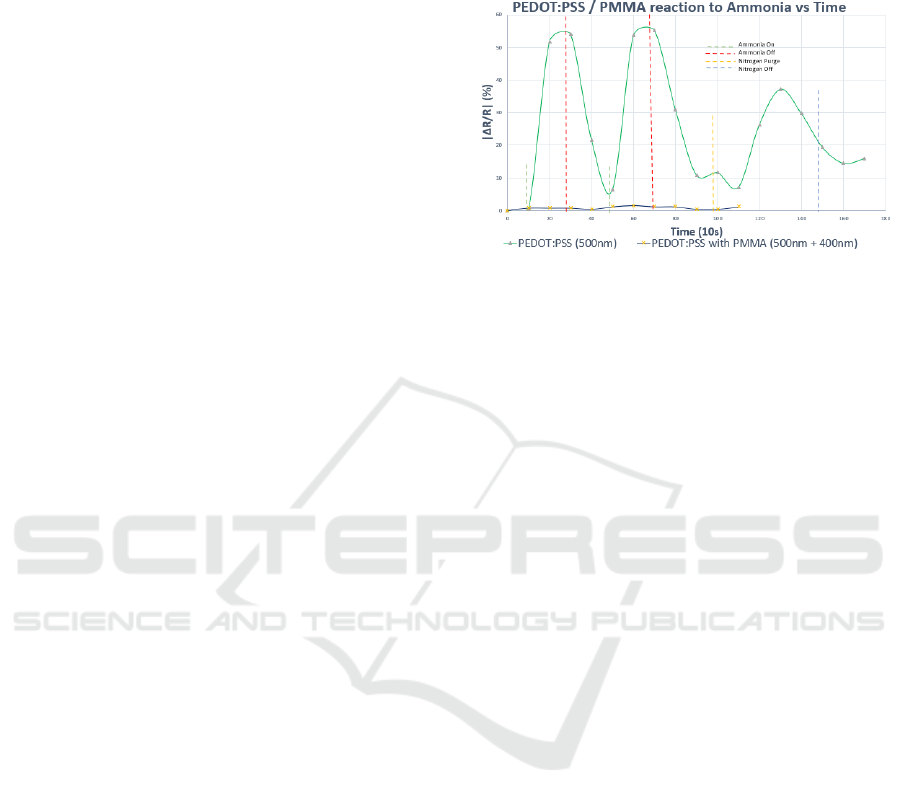
Figure 4: Substrate responsiveness coated with
PEDOT:PSS (500 nm), PEDOT:PSS & PMMA (500 nm +
400 nm) with respect to ammonia exposure over time
(sampled every 10s).
The results in Fig. 4, confirmed that adding an
adequately thick layer of PMMA on to the
PEDOT:PSS base layer renders it dormant. Having a
high surface to volume ratio also enhanced the
effectiveness of PMMA as it covers the layer of
PEDOT:PSS and limits interaction with the exposed
gas molecules.
3.3 Sensor Performance
The initial transient results of the four tested
structures were captured and delineated, as shown in
Fig. 5a). The traditional graphene oxide
demonstrated no response to the presence of ammonia
as expected. All three functionalized graphene oxide
sensors were measured in terms of absolute
percentage change and demonstrated a visible
response to the presence of ammonia. After the
nitrogen purge, the voltage output approached
nominal readings.
The graphene oxide demonstrated no discernible
change in resistance upon initial exposure, whilst the
three functionalized sensors all reacted within the first
10 s. The resistance change would continue to rise
for the first 90 s of each test, at which point gas
concentration then diminished. The two
hydrothermally processed FGO sensors had similar
maximum resistance changes, with the peaks being at
7.4% for 30% HF and 6.1% for the 49% HF sensors.
The XtalFluor processed sensor demonstrated a
larger resistance shift with a peak change of 8.9%.
and demonstrated the fastest drop in resistance,
indicating quick molecular discharge from the
sensing film. In Figure 5a) the 49% FGO (FGO49)
had the slowest drop in percent change, indicating
Hydrothermally Fluorinated Graphene Oxide Chemiresistive Sensor for Detecting NH3 and Acetone under Atmospheric Conditions
103
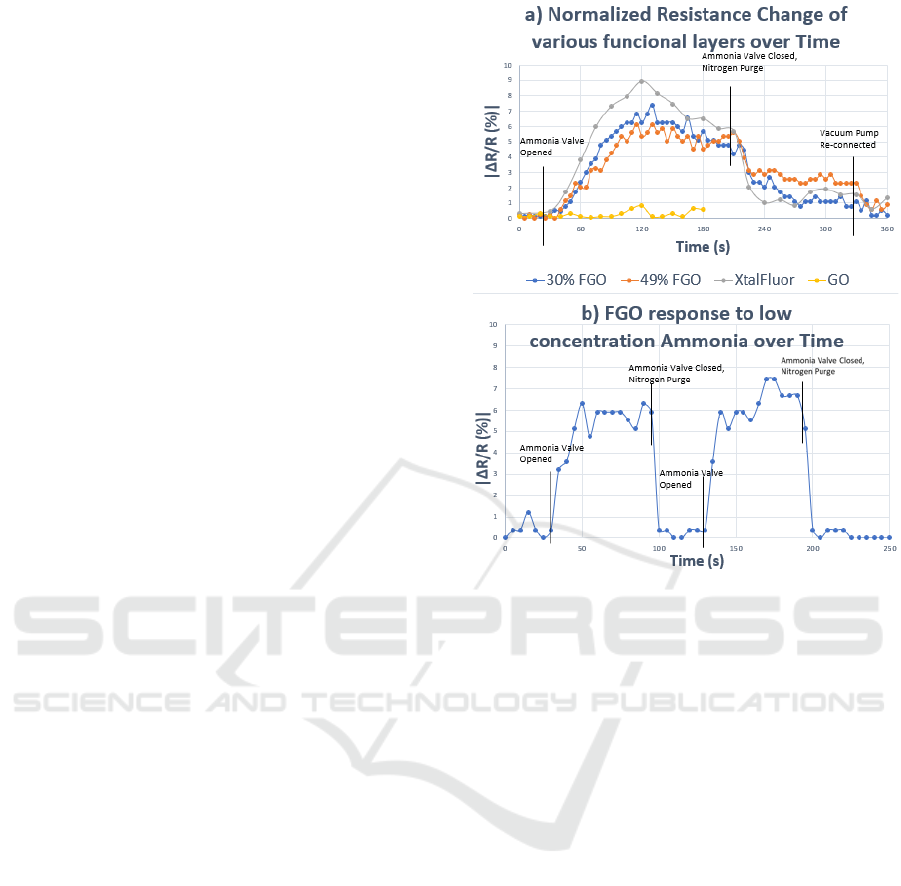
that the stronger affinity causes a retention of
ammonia particles.
Following the nitrogen purge, the FGO49 also had
the least amount of variation, further indicating its
affinity for stronger retention with ammonia particles,
while the other sensors dropped rapidly. Within the
first minute of turning the vacuum pump back on, all
sensors had returned close to the original resistance.
The results demonstrate that the functionalization
process introduced an electrical sensitivity in the
previously unmodified graphene oxide device.
3.3.1 Validation of Sensor Performance
All investigations for sensor performance validation
was done using our in-house synthesized FGO. A
commercially available ammonia sensor (Honeywell
MIDAS-E- NH3) was tested in conjunction with the
FGO sensor at atmospheric pressure to calibrate the
chemiresistor sensor response. Ammonia was
released into the chamber until it reached a
concentration of 19 ppm.
The functionalized gas sensor responds to the
ammonia within seconds of exposure while the
commercial sensor only responds after the
concentration has reached 9 ppm. The linear response
of the chemiresistive gas sensor peaks at
approximately |ΔR/R| = 8% at 19 ppm, indicating a
sensitivity of 0.42% ΔR/R per ppm.
Both sensors showed an immediate response to
the decreasing ammonia concentration, with the FGO
chemiresistive sensor having a slower response.
Within five minutes of non-exposure, the
chemiresistive sensor returned to its baseline value.
The response of the chemiresistive gas sensor is seen
to be linearly proportional to the concentration of
ammonia on the sensor coating.
Figure 5b) demonstrates the sensor exposed to a
flow of an ultra-low concentration (2.26 ppm) of
ammonia gas. The peak response was around 8%, and
the overall change was a |ΔR/R| of ~3.54% per ppm.
The adjusted scaled percentages corresponded to the
same linear response that was modelled from
previous experiments conducted in ideal conditions.
This data validates that the sensor response remains
linear over a broad concentration range and the
sensitivity is reproducible for ultra-low
concentrations (~ 1-2 ppm).
Figure 5: a) Response of FGO (30 and 49%), XtalFlour, and
GO to high concentration ammonia. b) Response of FGO to
low concentration ammonia.
3.3.2 Multi-analyte Sensing Capabilities of
Our FGO Chemiresistive Sensor
The FGO sensor was exposed to acetone to verify its
cross-sensitive performance. Acetone possesses
enhanced binding mechanisms with FGO. Its
chemical features are consistent with previously
tested analytes, as interaction with weakly bound
fluorine ions change the electronic properties of the
sensor. In this case, it provides an opposite, but
equivalent response. The experiment was conducted
in a controlled environment with both the FGO and
GO sensors exposed to acetone in closed, and open
lid situations to verify performance under ideal, and
ambient conditions.
The acetone was heated on a hot plate at 56 °C and
was placed into the sensing chamber. In Fig. 6, device
exposure to acetone vapour demonstrated the
expected, opposite response. The overall
responsiveness to ammonia |ΔR/R| (%), equal cross-
sensitivity and linear correlation of the sensor was
predictable and remained unchanged. The trend
showed that the peak exposure from the acetone
vapour occurred at 20 s.
The change in |ΔR/R| (%) reached a maximum of
5% for the FGO sensor in a closed chamber. The FGO
BIODEVICES 2021 - 14th International Conference on Biomedical Electronics and Devices
104
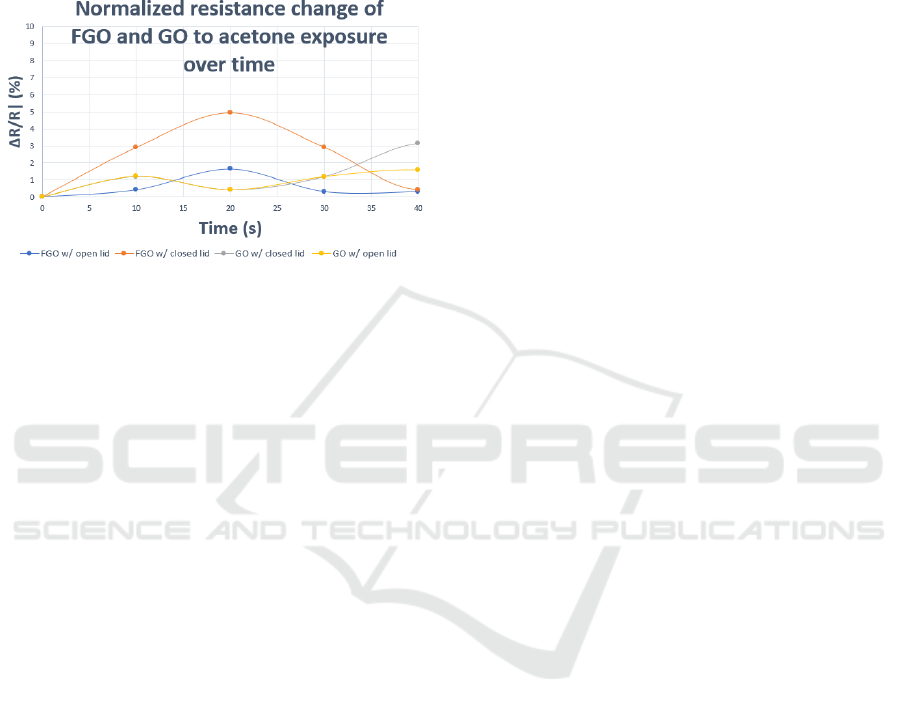
sensor in ambient conditions as well as the GO sensor
in both open and closed environments demonstrated
limited response. The device henceforth proved
capable of targeting multiple, opposite analytes, and
shows a distinct, predictable trend which can be
attributed to the selectivity of the chemiresistor
response.
Figure 6: FGO and GO response to acetone.
The change in |ΔR/R| (%) reached a maximum
value of 5% for the FGO sensor in a closed chamber.
The FGO sensor in ambient conditions as well as the
GO sensor in both open and closed environments
demonstrated limited response. The device
furthermore proved capable of targeting multiple,
opposite analytes, and shows a distinct, response
which can be attributed to the selectivity of the
chemiresistor device at an ultralow concentration
range. Since concentration ranges of acetone in
exhaled breath of people with diabetes can be as low
as ~ 1.8 ppm, the sensor response fits well within the
physiological monitoring range.
To further verify the sensing capabilities of the
coatings, the FGO and GO coated sensors were
exposed to a slow, timed-release of isopropyl alcohol
(IPA) and ethanol. Neither the GO nor FGO sensor
responded to IPA or ethanol. It furthermore proves to
be extremely promising for being highly selective
towards specific analytes in ultralow concentrations.
4 CONCLUSIONS
In this work, we achieved ultra-low-level detection
(~1-10 ppm) of NH
3
using an in-house synthesized
FGO coated chemiresistive gas sensor. The device
performance was superior to a commercial
electrochemical NH
3
sensor in detection limit,
sensitivity, and linearity. The design of these
chemiresistor devices can be configured in a way that
the sensing layer and substrate are interchangeable,
which makes them advantageous. Passivated silicon
emulated a low-cost, robust polymer substrate. In the
future, these devices will be printed on lower cost
substrates, with low production cost. The conductive
layers of, PEDOT:PSS and PMMA would provide
necessary conductivity and limit interaction with the
targeted gas. We demonstrated the miniature footprint
of the packaged device which would evolve into a
point-of-care, wearable, continuous monitoring
exhaled breath sensor. The chemiresistive sensor
device was extended to detecting another relevant
target gaseous analyte, acetone. Acetone was detected
by virtue of a different type of chemical interaction
between the FGO and the analyte molecule. Our
experiments suggested that the sensor could handle
concentrations of acetone in the ~ 1.0 -10 ppm range,
which is ideal for physiological monitoring of
diabetes. Extensive experimental validation using a
commercial gas sensor as control and ultra-low
concentration of gaseous analytes validated that our
FGO chemiresistive gas sensor has a unique, target
specific, transient response that can be easily
analysed using simplified techniques, illustrating the
versatility of these sensors over traditional electro-
chemical sensors. This work furthermore establishes
a roadmap to fabricate such sensors on a low-cost
polymer surface with configurable sensing layers, to
identify presence of multiple gaseous analytes in
ultra-low concentration range of 1 – 10 ppm. The
small footprint of the device also serves useful for
smart wearable type health monitoring applications.
ACKNOWLEDGEMENTS
The authors acknowledge Mr. Brian Kennedy at the
KennedyLabs (Ottawa, Canada) for providing
graphene and GO samples, and NSERC Canada for
the supporting the reported work through the NSERC
Engage Grant.
REFERENCES
Yadava, L., et al. (2012). Detection and sensing mechanism
of acetone with modeling using Pd/TiO2/Si structure.
Thin Solid Films, 520(7), 3039–3042.
Sanchez, J.-B., et al. (2006). A selective gas detection
micro-device for monitoring the volatile organic
compounds pollution. Sens. Act. B: Chem., 119(1),
227–233.
Lin, C., et al. (2015). Evaluation and calibration of
Aeroqual series 500 portable gas sensors for accurate
Hydrothermally Fluorinated Graphene Oxide Chemiresistive Sensor for Detecting NH3 and Acetone under Atmospheric Conditions
105

measurement of ambient ozone and nitrogen dioxide.
Atm. Env., 100, 111–116.
Zampolli, S., et al. 2007. A supramolecular approach to
sub-ppb aromatic VOC detection in air. Chem. Com.
2790–2792.
Majumder, S., et al. (2017). Wearable Sensors for Remote
Health Monitoring. Sensors (Basel, Switzerland),
17(1), 130.
Zhao, Y. et al. (2020). A wearable freestanding
electrochemical sensing system. Science Advances,
6(12).
Gargiulo, V., et al. (2020). Graphene-like layers as
promising chemiresistive sensing material for detection
of alcohols at low concentration. Food Re. Intl.,
Volume 119., May 2019, 99-109
Wang, C., et al. (2010). Metal Oxide Gas Sensors:
Sensitivity and Influencing Factors. Sensors, 10(3),
2088–2106.
Ponzoni, A. et al. (2017). Metal Oxide Gas Sensors, a
Survey of Selectivity Issues Addressed at the SENSOR
Lab, Brescia (Italy). Sensors, 17(4), 714
Manginell, et al. (1997). Overview of micromachined
platforms for thermal sensing and gas detection. In V.
K. Varadan & P. J. McWhorter (Eds.), Smart Structures
and Materials 1997: Smart Electronics and MEMS.
SPIE.
Bârsan, N., & Tomescu, A. (1995). The temperature
dependence of the response of SnO2-based gas sensing
layers to O2, CH4 and CO. Sens. Act. B: Chem., 26(1–
3), 45–48.
Zhao, W.-J., et al. (2019). A Low-Temperature Micro
Hotplate Gas Sensor Based on AlN Ceramic for
Effective Detection of Low Concentration NO2.
Sensors, 19(17), 3719.
Briand, D., et al. (2000). Design and fabrication of high-
temperature micro-hotplates for drop-coated gas
sensors. Sens. Act. B: Chem., 68(1–3), 223–233.
Zaaba, N. I., et al. (2017). Synthesis of Graphene Oxide
using Modified Hummers Method: Solvent Influence.
Procedia Engineering, 184, 469–477.
Huang, X., et al. (2011). Graphene-Based Materials:
Synthesis, Characterization, Properties, and
Applications. Small, 7(14), 1876–1902.
Kavinkumar, T., et al. (2015). Effect of functional groups
on dielectric, optical gas sensing properties of
graphene oxide and reduced graphene oxide at room
temperature. RSC Advances, 5(14), 10816–10825.
Park, M.-S., et al. (2016). NH
3
gas sensing properties of a
gas sensor based on fluorinated graphene oxide.
Colloids and Surf. A: Phys. Eng. Asp., 490, 104–109.
Chronopoulos, D. D., et al. (2017). Chemistry, properties,
and applications of fluorographene. App. Mat. 9, 60–
70
Zhao, F.-G., et al. (2014). Fluorinated graphene: facile
solution preparation and tailorable properties by
fluorine-content tuning. J. Mater. Chem. A, 2(23),
8782–8789.
Karlický, F., et al. (2013). Halogenated Graphenes:
Rapidly Growing Family of Graphene Derivatives.
ACS Nano, 7(8), 6434–6464.
Urbanova, V., et al. (2016). Fluorinated graphenes as
advanced biosensors - Effect of fluorine coverage on
electron transfer properties and adsorption of
biomolecules. Nanoscale.
Lu, G., et al. (2009). Reduced graphene oxide for room-
temperature gas sensors. Nano., 20(44), 445502.
Zhu, W., et al. (2019). Solvent-free preparation of
hydrophilic fluorinated graphene oxide modified with
amino-groups. Materials Letters, 237, 1–4.
Huang, X., et al. (2012). Graphene-based composites.
Chem. Soc. Rev., 41(2), 666–686.
Schedin, F., et al. (2007). Detection of individual gas
molecules adsorbed on graphene. Nature Materials,
6(9), 652–655.
Kim, Y. H. et al. (2017). Chemically fluorinated graphene
oxide for room temperature ammonia detection at ppb
levels. Jou. Mat. Chem.A, 5(36), 19116–19125.
Gafare, M., et al. (2014). Detection of ammonia in exhaled
breath for clinical diagnosis- A review. 3RD INTL.
CONF. FUND. AND APP. SCI. (ICFAS 2014): Inn.
Res. App. Sci.
Xiao, T., et al. (2014). Highly sensitive and selective
acetone sensor based on C-doped WO3 for potential
diagnosis of diabetes mellitus. Sens. Act. B: Chem. 199,
210-219.
Gong, P., et al. (2012). One-pot sonochemical preparation
of fluorographene and selective tuning of its fluorine
coverage. J. Mater. Chem. 22(33), 16950.
Jiang, X., et al. (2018). High Performance of
PEDOT:PSS/n-Si Solar Cells Based on Textured
Surface with AgNWs Electrodes. Nano. Res. Let., 13(1).
Vosgueritchian, M., et al. (2012). Highly Conductive and
Transparent PEDOT:PSS Films with a
Fluorosurfactant for Stretchable and Flexible
Transparent Electrodes. Adv. Fun. Mat.
Zhang, H., et al. (2014) High-sensitivity gas sensors based
on arranged polyaniline/PMMA composite fibers. Sens.
Act. A: Phys. 219, 123-127.
BIODEVICES 2021 - 14th International Conference on Biomedical Electronics and Devices
106
