
Detection of Error Correlates in the Motor Cortex in a Long Term
Clinical Trial of ECoG based Brain Computer Interface
Vincent Rouanne
1a
, Maciej Śliwowski
1,2 b
, Thomas Costecalde
1c
, Alim Louis Benabid
1,3 d
and Tetiana Aksenova
1e
1
Univ. Grenoble Alpes, CEA, LETI, Clinatec, F-38000 Grenoble, France
2
CEA, LIST, Gif-sur-Yvette, France
3
CHU Grenoble Alpes, Grenoble, France
Keywords: Brain Computer Interface, Error Correlates, Sensory-motor Cortex, Machine Learning, Clinical Trial,
Tetraplegic Subject.
Abstract: Error correlates are thought to be promising for BCIs as a way to perform error correction or prevention, or
to label data in order to perform online adaptation of BCIs’ control models. Current state-of-the-art BCIs are
motor-imagery-based invasive BCIs and thus have no access to neural data apart from sensory-motor cortices.
We investigated at the single trial level the presence and detectability of error correlates in the primary motor
cortex during observation or motor imagery (MI) control of a BCI with two discrete classes by a tetraplegic
user. We show that error correlates can be detected using a broad range of classifiers, namely Support Vector
Machine (SVM), logistic regression, N-way Partial Least Squares (NPLS), Multilayer Perceptron (MLP) and
Convolutional Neural Network (CNN) with respective mean AUC of the ROC curve of 0.645, 0.662, 0.642,
0.680 and 0.630 in the observation condition, and 0.623, 0.605, 0.603, 0.626 and 0.580 in the MI-control
condition. We also suggest that these error correlates are stable in time. These findings suggest that error
correlates could be used in clinical trials using invasive motor-imagery-based BCIs for error correction or
prevention.
1 INTRODUCTION
Brain computer interfaces (BCI) are promising tools
that use neural signal recordings to directly control
effectors. However, BCIs are currently mostly used in
research laboratories due to several limitations,
including their often too low performances and their
requirement to be calibrated in specific conditions
with the assistance of a researcher. Both of these
issues can be alleviated using a biomimetic strategy
of learning for the training of the decoder of the BCI.
In humans, brain signals that generate correct actions
can be reinforced, while action recognized as
erroneous can be corrected and may have also
reduced probability of being performed in the future.
This learning requires feedback in order to know if a
given action was correct or erroneous. In the case of
a
https://orcid.org/0000-0001-7708-8176
b
https://orcid.org/0000-0001-6744-1714
c
https://orcid.org/0000-0003-2216-4447
d
https://orcid.org/0000-0002-4479-1807
e
https://orcid.org/0000-0003-4007-2343
an action performed by a human controlled BCI, the
human receives feedback (e.g. visual) regarding the
success of the action, whereas the machine does not.
Having the user consciously (e.g. orally or physically)
transferring this feedback to the BCI may be tiring,
impractical or even impossible depending on the
condition of the user. However, the feedback received
by the user may produce specific brain activity. A
BCI able to detect such brain activity would thus have
access to learning-enabling feedback. Brain activity
correlated to errors was recorded as early as 1991 in
the experiments of Falkenstein et al. (1991).
Detection of error correlates during BCI operation
can provide a way to either correct mistakes after they
have been performed or train or update the models
used to control the BCI (Chavarriaga, Sobolewski, &
Millan, 2014). The ability to reliably detect error
26
Rouanne, V.,
´
Sliwowski, M., Costecalde, T., Benabid, A. and Aksenova, T.
Detection of Error Correlates in the Motor Cortex in a Long Term Clinical Trial of ECoG based Brain Computer Interface.
DOI: 10.5220/0010227800260034
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 4: BIOSIGNALS, pages 26-34
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

correlates in brain signals is thus valuable for the
development of BCIs.
Although error correlates can be used directly as
control signals to operate a BCI (Chavarriaga,
Iturrate, & Millan, 2016), we are interested here in
their use as a secondary signal acquired to improve
the performance of BCIs. Notably, error correlates
have been used in simulations and online experiments
to automatically correct errors during BCI operation
(Even-Chen et al., 2018; Parra, Spence, Gerson, &
Sajda, 2003) or to update control models without the
use of new externally labeled data (Blumberg et al.,
2007; Spüler, Rosenstiel, & Bogdan, 2012).
The error correlate discovered by Falkenstein et
al. (1991) is the error-related signal mostly used in
BCI applications. This waveform called the error-
related potential (ErrP) is composed of a negative
potential deflection over the fronto-central scalp area
roughly 50 to 100ms after the event that induced it,
followed by a centro-parietal positive deflection
(Chavarriaga, Sobolewski, & Millan, 2014).
Conveniently for BCI, ErrPs are relatively stable
across time and tasks (Chavarriaga & Millan, 2010;
Ferrez & del R. Millan, 2008), are elicited when an
error is performed by a BCI controlled or observed by
a user (Ferrez & del R. Millan, 2008; Schalk,
Wolpaw, McFarland, & Pfurtscheller, 2000) and are
detectable at the single-trial level (Parra, Spence,
Gerson, & Sajda, 2003). However, the localization of
these ErrPs is a drawback for current state-of-the-art
BCIs. The BCIs best in terms of performance are
invasive and thus often have access to limited
recording areas over or in the brain (Benabid et al.,
2019; Wodlinger et al., 2014). The primary sensory-
motor cortex is the best candidate for the recording
area of an invasive BCI due to its ability to generate
motor imagery signals. In such circumstances, ErrPs
cannot be recorded when using these BCIs. We focus
hereafter on the specific case of BCIs that acquire
brain signals from the sensory-motor cortex only.
ErrPs are not the only error correlates that can be
recorded from brain signals. Error correlates have
been reported in the primary motor and
somatosensory cortex. In a MEG study, Koelewijn et
al. (2008) reported a stronger beta rebound after an
outcome error than after a correct task outcome, both
when observing or performing a motor task. Previous
work by van Schie et al. (2004) demonstrated the
existence of error correlates in the motor cortex by
showcasing the variability of the lateralized readiness
potential between correct and erroneous response in
an Eriksen flanker task. Although their experiment
was performed on non-human primates and using
intracortical electrodes, Inoue et al. (2016)
successfully showed that end-point errors during
reaching tasks are encoded in the primary motor
cortex. Maybe more importantly, they provided
evidence that these error signals are necessary for
adaptation in reaching movements. In an EEG-ECoG
combined study, Völker et al. (2018) showed that
error processing in the human brain involved
modulation of brain activity in the high gamma
frequency band (60-90Hz), including modulations in
the precentral gyrus and post central gyrus. These
findings are consistent with the more recent study by
Wilson et al. (2019), in which they also found an
increase in the high gamma frequency band (70-
100Hz) after erroneous BCI task outcomes with
respect to correct ones. Finally, Milekovic et al.
(2012, 2013) reported detection of errors at the single
trial-level using ECoG in the motor region
(accuracy76%) during motor execution by able-
bodied subjects. Apart from Milekovic et al., no
single-trial detection of error correlates in the motor
cortex have been reported. However, Milekovic et
al.’s studies have the drawbacks of being performed
with overt movement tasks instead of BCI operation
by tetraplegic user with motor imagery. Additionally,
these studies were performed with subjects implanted
with large ECoG grids due to intractable epilepsy.
Although they report detectability using electrodes
located over the motor cortex, this does not insure the
detectability using electrodes positioned with motor
imagery for BCI in mind.
In this study, we perform an experiment where a
tetraplegic user receives erroneous feedback from a
BCI while observing or controlling its actions. Neural
data are acquired using chronic ECoG implants
located over the left and right primary sensory-motor
cortex. The BCI is controlled using motor imagery
and errors should be detected on a single trial basis
using the brain data recorded from the motor cortex.
Several decoding models are trained for the purpose
of detecting error correlates.
2 METHODS
2.1 Data Recording
The subject in this experiment was a 28-year-old male
who had tetraplegia following a C4-C5 spinal cord
injury (ASIA scale levels of the subject are presented
in Benabid et al. (2019)). The subject was implanted
with two WIMAGINE (Mestais et al., 2015) ECoG
implants 24 months prior to the experiments in this
study, as a participant in the clinical trial “BCI and
Tetraplegia”. The “BCI and Tetraplegia” clinical trial
Detection of Error Correlates in the Motor Cortex in a Long Term Clinical Trial of ECoG based Brain Computer Interface
27
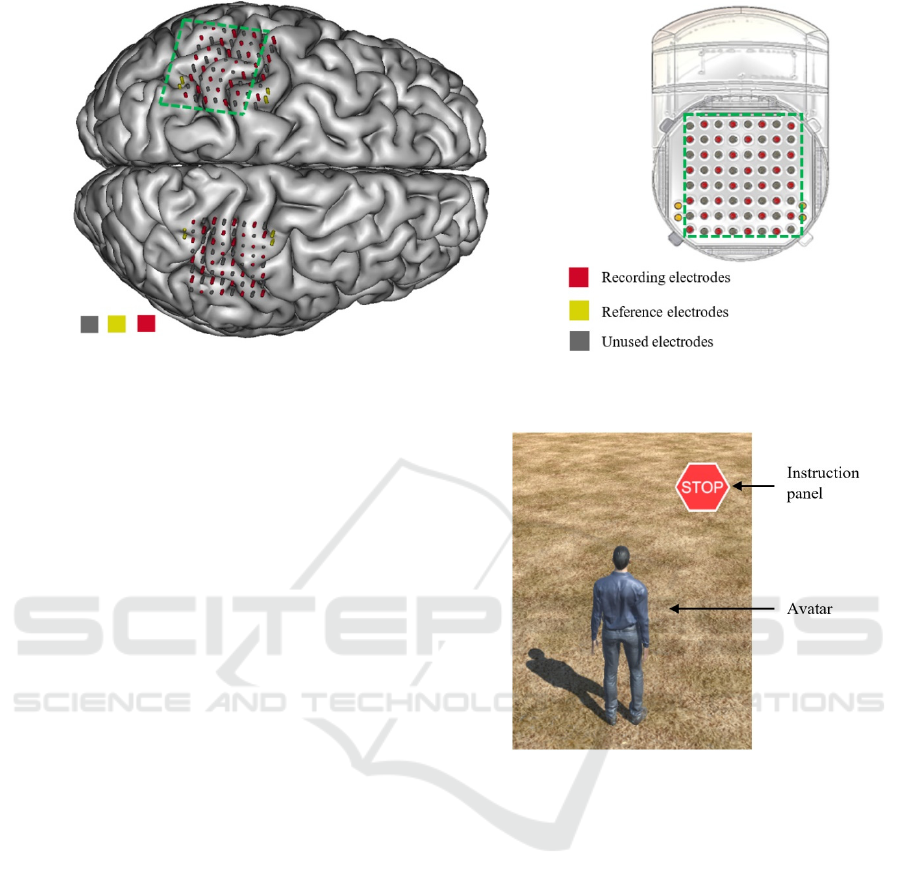
Figure 1: A. Position of the electrodes of each WIMAGINE implant over the right and left sensory-motor areas on a
reconstruction of the subject’s brain from MRI. B. Schematic view of a WIMAGINE implant.
(ClinicalTrials.gov identifier: NCT02550522) was
approved by French authorities: Agence nationale de
sécurité du médicament et des produits de santé
(ANSM) with the registration Number: 2015-
A00650-49 and the ethical committee (Comité de
Protection des Personnes - CPP) with the Registration
number: 15-CHUG-19. The implants were
positioned over the left and right sensory-motor
cortex (Figure 1). Experimental data was recorded at
a sampling rate of 586Hz from 32 out of the 64
electrodes of each implant because of limited data
rates.
2.2 Experimental Setup
The subject was sited in front of a computer screen
where a human avatar was represented from a third
person perspective. An instruction panel that either
displayed a GO or STOP label was also displayed
(Figure 2). The avatar could either stand still or walk
forward at a fixed speed. Two conditions of control
were designed. In the first condition (condition 1), the
subject had no control over the avatar, which was
controlled by the computer. In the second condition
(condition 2), the avatar was controlled by the subject
using leg motor imagery. The subject was already
trained to control a similar avatar using leg motor
imagery prior to this experiment (Benabid et al.,
2019).
In condition 1, the subject was instructed to focus
on the avatar and to expect the avatar to follow the
instructions displayed on the instruction panel as if he
was controlling the avatar’s actions through motor
imagery. In this condition, the instruction panel
switched its instruction every 5 to 15 seconds. The
Figure 2: The environment is similar to the one in Benabid
et al. (2019). The subject either watched the avatar move
automatically (condition 1), or controlled it using leg motor
imagery (condition 2). When the instruction panel showed
“STOP” the avatar was supposed to stay idle, whereas when
it showed “GO” the avatar was supposed to walk.
avatar followed the change in instruction with a
random reaction speed between 200ms and 500ms.
Additionally, error periods were automatically
introduced in this condition. During error periods, the
avatar switched its state to the opposite of the one
required from the instruction panel. Error and correct
periods always lasted at least two seconds, and error
periods never lasted more than three seconds. Error
periods were introduced at random following the
previous restrictions with an error rate of
approximately two to three errors per minute.
Nineteen sessions of eleven minutes of recording
were acquired over 268 days in condition 1.
In condition 2, the subject controlled the avatar
using leg motor imagery. Walking was triggered by
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
28

performing both legs motor imagery, while standing
still was performed by not performing motor imagery.
In this condition, the duration of error and correct
periods as well as the error rate were entirely
determined by the control of the subject over the BCI.
Since the subject was already trained for such a task
and had achieved high control of the BCI, a new
control model was built specifically for the
experiment using a purposely-reduced dataset as
training set. This was performed in order to ensure
that errors would still occur in this simple control
paradigm. Thirteen sessions of on average eleven
minutes of recording were acquired over 141 days in
condition 2.
Condition 1 was designed to ensure that error
correlates could be recorded in the motor cortex with
the present electrode setup, without any interference
from motor imagery signals. It also ensured that error
correlates detected in condition 2 were not due to
motor imagery confounds. Condition 2 was designed
to assess if error correlates could be detected while
the BCI was used.
2.3 Data Labelling
In both conditions, the goal of the experiment was to
distinguish correct from erroneous events. Events
were defined as moments when the avatar changes its
state. Specifically, correct events were defined as the
avatar changing its state to the one required by the
instruction panel, and error events were defined as the
avatar changing its state to the opposite of the one
required by the instruction panel. We expected error
correlates to appear after such erroneous events, as
was the case in Milekovic et al. (2013).
Epochs of one second and spaced by 100ms (90%
overlap) were considered for the classification of
correct or erroneous events. The first six full epochs
after an event were labeled according to the event
type. The first such epoch contained temporal data
from the event onset to one second after the event.
The last epoch contained temporal data from 0.5s
after the event to 1.5s after the event (Figure 3).
Additionally, epochs that were too close to another
event were discarded. The inclusion of several epochs
for each event was performed with two goals in mind.
The first one is to counterbalance the issue of
synchronization. Indeed the timing of the brain
response to the erroneous or correct events may vary
depending on several conditions, such as the attention
level, the tiredness of the subject, or the workload as
is the case in classical ErrPs paradigms (Iturrate,
Chavarriaga, Montesano, Minguez, & Millán, 2012).
Additionally, there was some jitter in the reaction
Figure 3: Example of an error event where the avatar starts
walking when it is supposed to stay idle. Red dots indicate
epochs belonging to the error class. The first epoch included
contains neural data from the onset of the error to one
second after.
time of the avatar itself to a change of command (e.g.
the avatar must finish a step before stopping), which
was estimated to be up to 300ms. Adding several
epochs for each event increased the probability of
having the desired brain signal in one of them at the
cost of some label uncertainty.
2.4 Feature Extraction
Time-frequency decomposition of brain signals is
classically performed in the literature for the
detection of error correlates in the motor cortex
(Milekovic, Ball, Schulze-Bonhage, Aertsen, &
Mehring, 2012; Wilson et al., 2019). Therefore, time-
frequency information was extracted for each 1s
epoch and for the 64 electrodes. Continuous complex
wavelet transform was applied using a family of
fifteen Morlet wavelets of central frequencies from
10Hz to 150Hz. For each 1s epoch, the absolute value
of this time-frequency data was averaged over the
temporal dimension into ten non-overlapping
windows of 100ms. The resulting feature tensor for
each epoch was thus of shape 10 15 64,
respectively along the temporal, frequential and
spatial dimensions.
2.5 Data Balance
Due to the design of the experiment, there was an
imbalance in the class repartition of the data in both
condition 1 and 2 (Table 1). Additionally, error
epochs and correct epochs could each be of two
separate types. Error epochs could be due to the
avatar starting to walk when expected to stand idle, or
due to the avatar stopping when expected to walk.
Similarly, correct epochs could be due to the avatar
starting to walk when expected to walk or due to the
Detection of Error Correlates in the Motor Cortex in a Long Term Clinical Trial of ECoG based Brain Computer Interface
29

avatar stopping when expected to stop. The existence
of these sub-classes could potentially create strong
confounds for the error correlate detection if they
were not balanced (e.g. a motor imagery confound in
condition 2). The training dataset was balanced by
oversampling the three sub-classes with the least
number of epochs to the same number of epochs as
the most populated sub-class. Oversampling was
performed by repetition of the epochs present in the
sub-classes.
Table 1: Number of epoch in each class for each condition.
2.6 Decoders
Several decoder types were trained and compared on
both condition 1 and 2. We trained classical decoders
used in BCI studies, namely support vector machine
(SVM), logistic regression, multilayer perceptron
(MLP), convolutional neural networks (CNN) and N-
way partial least squares (NPLS). These decoders
share a characteristic of simplicity as the dataset in
this problem is of high dimensionality (9600 input
features), and with a relatively low amount of samples
(~9000 and ~8000 in condition 1 and 2 respectively).
For various example of use of these decoders for BCI,
the interested reader may refer to Lotte et al. (2018).
2.6.1 SVM & Logistic Regression
SVM and logistic regression are considered as state-
of-the-art methods for binary classification. These
methods are most often used in combination with
kernels, which can act as nonlinear projections of the
input data into high dimensional spaces without
having to specify the transformed input data. Regular
kernels (Gaussian and polynomial) were not used as
in preliminary studies they tended to strongly overfit
the training datasets, even with strong regularization
parameters and low Gaussian kernel scale (<10-5) or
low polynomial kernel order (order of 2 or 3).
Since we have more features in our input dataset
than sample points, regularization was used for both
SVM and logistic regression. For both methods, ridge
regularization was applied. After preliminary results,
lambda was set to one.
2.6.2 NPLS
NPLS is a less known method in the field of BCIs. It
is a linear method that is particularly suitable for
tensor-based high dimensional datasets. It also has the
advantage of being updatable using low
computational power and without requiring to save
the full original training dataset (Eliseyev et al.,
2017).
2.6.3 MLP
MLP is a fully connected feedforward artificial neural
network. It may be interpreted as a logistic regression
model preceded by a nonlinear transformation which
increases predictive power of the model. Proposed
MLP model consisted of one hidden layer with 100
neurons (with learnable weights) followed by a ReLU
activation. As all neurons are connected to each input
component and produce linear combination of input
features, it results in a huge number of parameters to
train. Considering the size of the dataset and number
of parameters we decided to regularize the model by
applying batch normalization, dropout with
probability of a neuron being zeroed 0.5, L2
regularization on model’s weights with lambda equal
0.1 and early stopping on validation set.
2.6.4 CNN
CNNs take advantage of data structure. They are
capable of capturing invariant patterns that may occur
in different parts of the signal. They have less
trainable parameters than similar MLP because of
filters weight sharing which means that the same set
of small filters is applied all over the data. We decided
to use CNN as there is a possible shift in error
correlates synchronization inside epochs. By sliding
convolutional filter over the signal in the time domain
we expected network to recognize error correlates
(which we expect to be time invariant) occurring in
different epoch’s moment with the same filter. It
results in lower number of parameters and possible
higher performance in detecting time invariant
patterns. Proposed CNN used 128 filters of shape
51564 respectively in time, frequency and
channels dimension. Each filter was slid only over
time dimension with stride equal 1. We applied the
same regularization methods as for the MLP.
2.7 Decoder Application and
Performance Evaluation
We report the performance of each model regarding
the desired task, which is the detection of error or
correct events. Up to 6 epochs were used for each
event but the signal corresponding to an error may not
be found in all of these epochs. Events were classified
E
p
och t
yp
e Condition 1 Condition 2
Number of
e
p
ochs
Correct 7539 4412
Erro
r
2307 3580
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
30
as errors as soon as one of their associated epochs was
classified as an error. The number of correct and error
events in each fold are summarized in Table 2 and 3.
The performance of the event decoder was
assessed over a five-fold cross-validation performed
across sessions, which means that an equal number of
sessions was presented in each of the five data splits.
Each split was used as a testing fold once, with the
corresponding other four splits used as training fold.
The performance of each decoder was evaluated
using the area under the curve (AUC) of the receiver
operating characteristic (ROC) curve for each test
fold of the cross-validation, for the task of event
classification.
Table 2: Repartition of error and correct events in each fold
for condition 1 (observation).
Event
t
yp
e
Fold
1
Fold
2
Fold
3
Fold
4
Fold
5
Train
Correct 1006 992 985 981 1072
Erro
r
283 308 316 317 332
Test
Correct 253 267 274 278 187
Erro
r
106 81 73 72 57
Table 3: Repartition of error and correct events in each fold
for condition 2 (control with motor imagery).
Event
t
yp
e
Fold
1
Fold
2
Fold
3
Fold
4
Fold
5
Train
Correct 633 554 558 590 609
Erro
r
519 457 464 469 483
Test
Correct 103 182 178 146 127
Erro
r
79 141 134 129 115
3 RESULTS
In the first condition the avatar was not controlled by
the subject and the subject monitored only the actions
taken by the BCI. In the second condition the state of
the avatar was controlled by the user through leg
motor imagery. The results for each decoder and
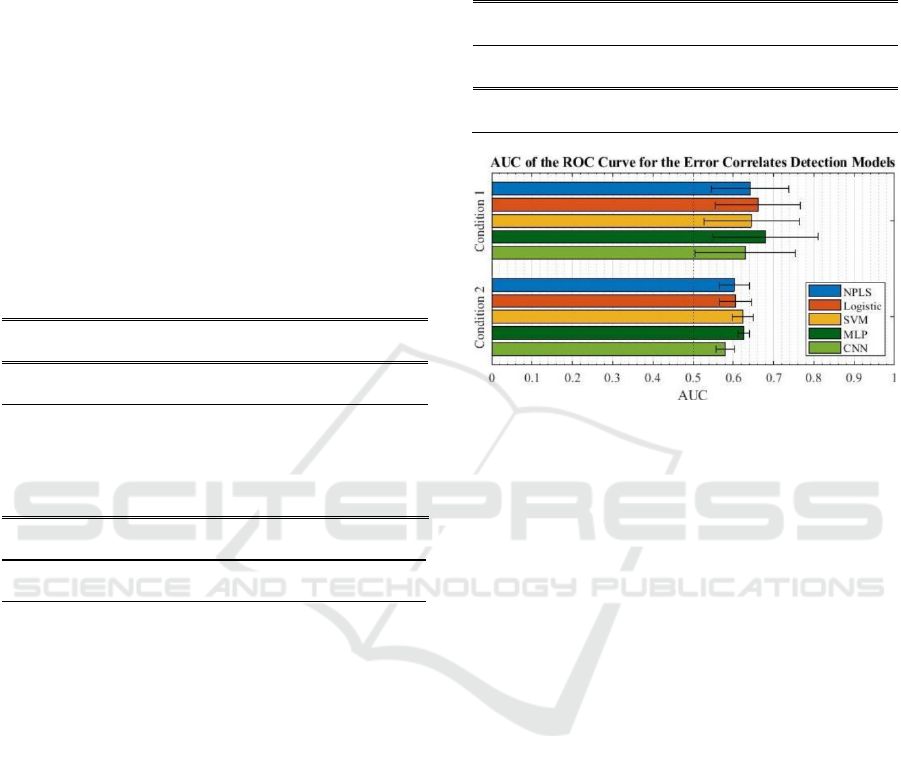
condition are summarized in Table 4 and Figure 4. A
Friedman test was performed to compare model
performances within each condition group. No
significant differences was found between models in
condition 1 (p-value = 0.13) or between models in
condition 2 (p-value = 0.08).
The results we report show that MLP achieved the
best performance in both the observation and MI-
control conditions. CNN and MLP were the two
models that allowed for the most complex
representations, such as nonlinear relationships.
Taking into account that regularization would limit
the drawbacks associated to their high number of
parameters, we expected these models to perform the
Table 4: Mean and standard deviation over five test folds of
the area under the curve of the receiver operating
characteristic curve for the classification of error vs correct
events.
Condition 1 NPLS Logistic SVM MLP CNN
AUC mean 0.642 0.662 0.645 0.680 0.630
AUC st
d
0.096 0.106 0.119 0.131 0.124
Condition 2 NPLS Logistic SVM MLP CNN
AUC mean 0.603 0.605 0.623 0.626 0.580
AUC st
d
0.037 0.040 0.027 0.014 0.022
Figure 4: Mean area under the curve of the receiver
operating characteristic curve for each model and each
condition. Error bars on the left and right of the mean each
represent one time the standard deviation.
best. CNN had less parameters than MLP and was
also more adapted to the task of re-synchronizing the
error correlates. However, the performances of CNN
models were the worst across all decoders. A possible
explanation for this is that both neural network
architectures (and neural networks in general) had a
high number of hyperparameters and we did not
perform an exhaustive search of these
hyperparameter spaces (e.g. learning rate, number of
filters, regularization weight).
In each condition, the three other decoders
performed similarly, with small variabilities
demonstrating better performances for logistic
regression in the first condition and for SVM in the
second condition. NPLS always performed slightly
worse than SVM and logistic regression.
Performance across different folds was
represented by the standard deviation of the AUC.
Since cross-validation was performed session-wise,
this standard deviation can be used to predict the
generalization capabilities of each model over
different datasets. In the observation condition, the
standard deviations of the AUC for each decoder
were close to one another, with NPLS having the
lowest. In the MI-control condition MLP had the
lowest standard deviation, close to twice lower than
the standard deviation of other decoders.
Detection of Error Correlates in the Motor Cortex in a Long Term Clinical Trial of ECoG based Brain Computer Interface
31

On average, the AUC of the decoders in condition
1 decreased by 6.8% for condition 2. This was
expected since the motor imagery signals used to
control the BCI in the MI-control condition can be
regarded as noise for the classification of error and
correct events. However, the standard deviation of the
AUC was up to ten times larger in the observation
condition than in the MI-control condition. We
suggest that the higher variability in the observation
condition was due to a higher variability in the
attention level of the subject than in the MI-control
condition. Indeed, in the MI-control condition the
subject was more engaged in the task since he had
active control over the avatar’s actions. In the
observation condition, the subject was more
vulnerable to distractions due to the lack of
interaction required by the condition. We hypothesize
that the attention level modulated the strength of the
error correlates in the motor cortex, similarly to how
it modulates classical ErrPs (Yeung, Holroyd, &
Cohen, 2005).
4 DISCUSSION
4.1 Impact of Event Latency
We hypothesize that the error correlate reported here
could be modulated by the length of correct or error
periods prior to an event. Although the duration of
these periods, or latency before each event, was partly
controlled in condition 1 there was no inclusion or
exclusion criterion based on it in condition 2. We
suggest that this latency may influence the brain
response to events. For example, correct events after
a long erroneous period may elicit a stronger brain
response than after a short erroneous period. Due to
the relatively small dataset acquired in this
experiment, separating the events based on latency
was not possible, but larger studies should take it into
account when possible.
4.2 Inter-session Stability of Error
Correlates
It should be noted that the different sessions of this
experiment were recorded over the course of several
months. The cross-validation was performed session-
wise, which means that the models were partly trained
on data recorded far away temporally from the data
they were tested on. This leads us to suggest that the
error correlates we report in the motor cortex may
exhibit a certain temporal stability, similarly to ErrPs.
4.3 Single Trial Detection of Error
Correlates
Although the AUCs reported in this study are not
considerably high, these are still above chance levels
for each algorithm tested here. We therefore suggest
that there effectively is an error correlate detectable at
the single trial level in the motor cortex when either
observing or controlling a BCI that performed an
erroneous action. Additionally, although the AUC
decreased between the observation and MI-control
condition, the ability to detect error correlates in the
motor cortex during operation of the BCI using motor
imagery is valuable not only from a neuroscience
perspective where it could provide some additional
insight on the motor learning mechanisms, but also
for potential applications in state-of-the-art BCIs for
which it is a requirement.
4.4 Decoders for Online BCIs
Although SVM, logistic regression and neural
networks are recognized as powerful methods, it is
not easy to update these classifier online without
retraining them on the full training dataset. This
property can be a drawback for some BCI
applications, including online training which is
considered as better than classical training with
feedback that is not generated by the control of the
BCI. More investigation would be required if these
decoders were to be trained or updated in online BCI
paradigms. In such cases, one should preferably use
NPLS over these decoders, as NPLS demonstrated
only slightly lower performances (up to 3.7% lower)
than the other decoders while being easily trainable
and updatable online.
5 CONCLUSION
Like previous independent studies reported, we found
error correlates in the time-frequency decomposition
of brain signals recorded in the sensory-motor cortex
using ECoG. However, to our knowledge this study
is the first to report the possibility to detect at the
single-trial-level error correlates in the sensory-motor
cortex during operation of a BCI. This study is also
the first one to report error correlates in the sensory-
motor cortex of a tetraplegic subject. Additionally, in
this study the operation of the BCI is performed using
motor imagery, further highlighting the value of these
results since a BCI with access to neural data from the
motor cortex only (such as invasive state-of-the-art
motor-imagery-based BCIs) could still be able to
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
32

detect error correlates and potentially use them for
error correction or model adaptation. The fact that the
detection accuracy of the NPLS was close to other
model is also a strong point for potential online model
adaptations, as it is computationally fast to update in
real time compared to the other models presented.
The main limitation of this study is that it was
restricted to the first subject of the clinical trial.
However, this clinical trial is expected to have a total
of 5 subjects, who could later be added to this study.
Other perspective future studies include
implementing automatic error correction for this
binary BCI, as well as error correlate detection during
control of more complex BCI effectors using multiple
degrees of freedom.
AUTHOR CONTRIBUTIONS
VR and MS performed the analyses and wrote the
manuscript. VR and TA designed the task. ALB and
TA provided input and mentorship through the
analysis and writing. TC collected the data.
ACKNOWLEDGMENTS
Clinatec is a Laboratory of CEA-Grenoble and has
statutory links with the University Hospital of
Grenoble (CHUGA) and with University Grenoble
Alpes (UGA). This study was funded by CEA
(recurrent funding) and the French Ministry of Health
(Grant PHRC-15-15-0124), Institut Carnot, Fonds de
Dotation Clinatec.MS was supported by the CEA
NUMERICS program, which has received funding
from the European Union's Horizon 2020 research
and innovation program under the Marie
Sklodowska-Curie grant agreement No 800945.
Fondation Philanthropique Edmond J Safra is a major
founding institution of the Clinatec Edmond J Safra
Biomedical Research Center.
REFERENCES
Benabid, A. L., Costecalde, T., Eliseyev, A., Charvet, G.,
Verney, A., Karakas, S., … Chabardes, S. (2019). An
exoskeleton controlled by an epidural wireless brain–
machine interface in a tetraplegic patient: A proof-of-
concept demonstration. The Lancet Neurology.
https://doi.org/10.1016/S1474-4422(19)30321-7
Blumberg, J., Rickert, J., Waldert, S., Schulze-Bonhage, A.,
Aertsen, A., & Mehring, C. (2007). Adaptive
Classification for Brain Computer Interfaces. 2007 29th
Annual International Conference of the IEEE
Engineering in Medicine and Biology Society, 2536–
2539. https://doi.org/10.1109/IEMBS.2007.4352845
Chavarriaga, R., Iturrate, I., & Millan, J. del R. (2016, May
30). Robust, accurate spelling based on error-related
potentials.
Chavarriaga, R., & Millan, J. d R. (2010). Learning From
EEG Error-Related Potentials in Noninvasive Brain-
Computer Interfaces. IEEE Transactions on Neural
Systems and Rehabilitation Engineering, 18(4), 381–
388. https://doi.org/10.1109/TNSRE.2010.2053387
Chavarriaga, R., Sobolewski, A., & Millan, J. d R. (2014).
Errare machinale est: The use of error-related potentials
in brain-machine interfaces. Frontiers in Neuroscience,
8. https://doi.org/10.3389/fnins.2014.00208
Eliseyev, A., Auboiroux, V., Costecalde, T., Langar, L.,
Charvet, G., Mestais, C., … Benabid, A.-L. (2017).
Recursive Exponentially Weighted N-way Partial Least
Squares Regression with Recursive-Validation of
Hyper-Parameters in Brain-Computer Interface
Applications. Scientific Reports, 7(1), 1–15.
https://doi.org/10.1038/s41598-017-16579-9
Even-Chen, N., Stavisky, S. D., Pandarinath, C.,
Nuyujukian, P., Blabe, C. H., Hochberg, L. R., …
Shenoy, K. V. (2018). Feasibility of Automatic Error
Detect-and-Undo System in Human Intracortical
Brain–Computer Interfaces. IEEE Transactions on
Biomedical Engineering, 65(8), 1771–1784.
https://doi.org/10.1109/TBME.2017.2776204
Falkenstein, M., Hohnsbein, J., Hoormann, J., & Blanke, L.
(1991). Effects of crossmodal divided attention on late
ERP components. II. Error processing in choice
reaction tasks. Electroencephalography and Clinical
Neurophysiology, 78(6), 447–455.
https://doi.org/10.1016/0013-4694(91)90062-9
Ferrez, P. W., & del R. Millan, J. (2008). Error-Related
EEG Potentials Generated During Simulated Brain–
Computer Interaction. IEEE Transactions on
Biomedical Engineering, 55(3), 923–929.
https://doi.org/10.1109/TBME.2007.908083
Inoue, M., Uchimura, M., & Kitazawa, S. (2016). Error
Signals in Motor Cortices Drive Adaptation in
Reaching. Neuron, 90(5), 1114–1126.
https://doi.org/10.1016/j.neuron.2016.04.029
Iturrate, I., Chavarriaga, R., Montesano, L., Minguez, J., &
Millán, J. d R. (2012). Latency correction of error
potentials between different experiments reduces
calibration time for single-trial classification. 2012
Annual International Conference of the IEEE
Engineering in Medicine and Biology Society, 3288–
3291. https://doi.org/10.1109/EMBC.2012.6346667
Koelewijn, T., van Schie, H. T., Bekkering, H., Oostenveld,
R., & Jensen, O. (2008). Motor-cortical beta
oscillations are modulated by correctness of observed
action. NeuroImage, 40(2), 767–775.
https://doi.org/10.1016/j.neuroimage.2007.12.018
Lotte, F., Bougrain, L., Cichocki, A., Clerc, M., Congedo,
M., Rakotomamonjy, A., & Yger, F. (2018). A review
of classification algorithms for EEG-based brain-
computer interfaces: A 10 year update. Journal of
Detection of Error Correlates in the Motor Cortex in a Long Term Clinical Trial of ECoG based Brain Computer Interface
33

Neural Engineering, 15(3). Scopus.
https://doi.org/10.1088/1741-2552/aab2f2
Mestais, C. S., Charvet, G., Sauter-Starace, F., Foerster, M.,
Ratel, D., & Benabid, A. L. (2015). WIMAGINE:
Wireless 64-Channel ECoG Recording Implant for
Long Term Clinical Applications. IEEE Transactions
on Neural Systems and Rehabilitation Engineering,
23(1), 10–21.
https://doi.org/10.1109/TNSRE.2014.2333541
Milekovic, T., Ball, T., Schulze-Bonhage, A., Aertsen, A.,
& Mehring, C. (2012). Error-related
electrocorticographic activity in humans during
continuous movements. Journal of Neural Engineering,
9(2), 026007. https://doi.org/10.1088/1741-
2560/9/2/026007
Milekovic, T., Ball, T., Schulze-Bonhage, A., Aertsen, A.,
& Mehring, C. (2013). Detection of Error Related
Neuronal Responses Recorded by Electrocorticography
in Humans during Continuous Movements. PLOS
ONE, 8(2), e55235.
https://doi.org/10.1371/journal.pone.0055235
Parra, L. C., Spence, C. D., Gerson, A. D., & Sajda, P.
(2003). Response error correction-a demonstration of
improved human-machine performance using real-time
EEG monitoring. IEEE Transactions on Neural
Systems and Rehabilitation Engineering, 11(2), 173–
177. https://doi.org/10.1109/TNSRE.2003.814446
Schalk, G., Wolpaw, J. R., McFarland, D. J., &
Pfurtscheller, G. (2000). EEG-based communication:
Presence of an error potential. Clinical
Neurophysiology: Official Journal of the International
Federation of Clinical Neurophysiology, 111(12),
2138–2144. https://doi.org/10.1016/s1388-
2457(00)00457-0
Schie, H. T. van, Mars, R. B., Coles, M. G. H., &
Bekkering, H. (2004). Modulation of activity in medial
frontal and motor cortices during error observation.
Nature Neuroscience, 7(5), 549–554.
https://doi.org/10.1038/nn1239
Spüler, M., Rosenstiel, W., & Bogdan, M. (2012). Online
Adaptation of a c-VEP Brain-Computer Interface(BCI)
Based on Error-Related Potentials and Unsupervised
Learning. PLoS ONE, 7(12), e51077.
https://doi.org/10.1371/journal.pone.0051077
Völker, M., Fiederer, L. D. J., Berberich, S., Hammer, J.,
Behncke, J., Kršek, P., … Ball, T. (2018). The
dynamics of error processing in the human brain as
reflected by high-gamma activity in noninvasive and
intracranial EEG. NeuroImage, 173, 564–579.
https://doi.org/10.1016/j.neuroimage.2018.01.059
Wilson, N. R., Sarma, D., Wander, J. D., Weaver, K. E.,
Ojemann, J. G., & Rao, R. P. N. (2019). Cortical
Topography of Error-Related High-Frequency
Potentials During Erroneous Control in a Continuous
Control Brain–Computer Interface. Frontiers in
Neuroscience, 13, 502.
https://doi.org/10.3389/fnins.2019.00502
Wodlinger, B., Downey, J. E., Tyler-Kabara, E. C.,
Schwartz, A. B., Boninger, M. L., & Collinger, J. L.
(2014). Ten-dimensional anthropomorphic arm control
in a human brain-machine interface: Difficulties,
solutions, and limitations. Journal of Neural
Engineering, 12(1), 016011.
https://doi.org/10.1088/1741-2560/12/1/016011
Yeung, N., Holroyd, C., & Cohen, J. (2005, May). ERP
correlates of feedback and reward processing in the
presence and absence of response choice. Cerebral
Cortex (New York, N.Y. : 1991).
https://doi.org/10.1093/cercor/bhh153.
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
34
