
Tragus based Vagus Nerve Stimulation for Stress Reduction
Surej Mouli
1 a
, Ramaswamy Palaniappan
1 b
, Jane Ollis
2
, Ian McLoughlin
3 c
,
Rahul Kanegaonkar
4,5
and Sunil Arora
6
1
Data Science Research Group, School of Computing, University of Kent, U.K.
2
MindSpire Ltd., U.K.
3
ICT Cluster, Singapore Institute of Technology, Singapore
4
Canterbury Christ Church University, U.K.
5
Kent Surrey Clinical Research Network, U.K.
6
Frimley Park Hospital, Camberley, U.K.
Keywords:
Electrocardiogram, Stress Reduction, Tragus, Vagus Nerve Stimulation.
Abstract:
Non-invasive vagus nerve stimulation is fast becoming a popular alternative treatment method for various
health disorders. The authors investigated the effects of auricular vagus nerve stimulation at tragus for acti-
vating the parasympathetic nervous system to reduce stress, in light of mixed results from other studies. Stim-
ulation frequency of 25 Hz with a pulse-width of 200 µs was administered at tragus with ECG data recorded
during pre- and post-stimulation trials to investigate changes in the low-frequency (LF) and high-frequency
(HF) parameters of heart rate variability (HRV). The results from five subjects demonstrate an increase in
the HF component and a decrease in LF when comparing pre- and post- stimulation values, denoting that
VNS stimulated more of the parasympathetic activity. The LF/HF ratio was reduced for all participants after
stimulation, with an average reduction of 64.5% observed. Overall, this study has indicated the feasibility of
using tragus as a stimulation site to stimulate the vagus nerve; tragus being easier to administrate than many
alternative sites while still being effective for stress reduction.
1 INTRODUCTION
Constant demands in routine daily life are a catalyst
for increased stress and anxiety issues leading to var-
ious mental disorders (Hidaka, 2012; Bandelow and
Michaelis, 2015; Kessler et al., 2009). Stress is the
leading contributory factor and major cause to the de-
velopment of diseases such as cardiovascular, chronic
skin conditions, chronic cluster headaches and vari-
ous other psychiatric illnesses (Blixen et al., 2016).
For clinicians, treating patients with stress-related ill-
ness through drug administered approaches is not a
good solution as the average efficacy rate of most
drugs does not exceed 50%, and moreover can cause
intolerable adverse side effects (Ambrosini and Cop-
pola, 2020). To address these issues and to minimise
the adverse side effects of drugs, alternate treatments
a
https://orcid.org/0000-0002-2876-3961
b
https://orcid.org/0000-0001-5296-8396
c
https://orcid.org/0000-0001-7111-2008
have been sought in recent years, notably methods
based on nerve stimulation are being explored widely.
Such methods are sometimes referred as neuromodu-
lation, due to their ability to modulate the nervous sys-
tem. Amongst various neuromodulation techniques,
vagus nerve stimulation (VNS) has been widely in-
vestigated since 1990 using implanted or invasive
VNS devices (Akerman and Romero-Reyes, 2020).
The vagus nerve is the tenth cranial nerve that
consists of approximately 80% afferent fibres project-
ing into the brain and 20% efferent fibres that project
to the rest of the body. It is considered to be the
major parasympathetic innervation of the autonomic
nervous system (Akerman and Romero-Reyes, 2020;
Johnson and Wilson, 2018; Kaniusas et al., 2019b;
McClintock et al., 2009). Since the first human im-
plant of VNS devices in 1989, over 50, 000 patients
have been treated with VNS worldwide and the vagus
nerve is often considered protective, defensive and re-
laxing (Vonck et al., 2009). VNS has been recently
approved by the FDA in the US for therapeutic use in
164
Mouli, S., Palaniappan, R., Ollis, J., McLoughlin, I., Kanegaonkar, R. and Arora, S.
Tragus based Vagus Nerve Stimulation for Stress Reduction.
DOI: 10.5220/0010222201640168
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 4: BIOSIGNALS, pages 164-168
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
patients aged over 12, as well as those presenting with
drug resistant epilepsy and depression (Johnson and
Wilson, 2018). However, there have been confound-
ing results in the literature where one study (Borges
et al., 2019) has indicated no difference between sham
stimulation and VNS. Hence, in this study, we have
investigated the effects of VNS at the tragus site,
and investigated its stress reduction effect. For this
analysis, we used Heart Rate Variability (HRV), as a
promising marker (Malik et al., 1996) of stress. HRV
is the fluctuation in the time intervals between adja-
cent heartbeats (Shaffer and Ginsberg, 2017). It is
sensitive to changes in sympathetic and parasympa-
thetic nervous systems from which stress levels can
be inferred. Various studies report that when stress is
induced, the HRV variable HF decreases, and LF in-
creases, to lower the parasympathetic activity (Thayer
et al., 2012; Kim et al., 2018).
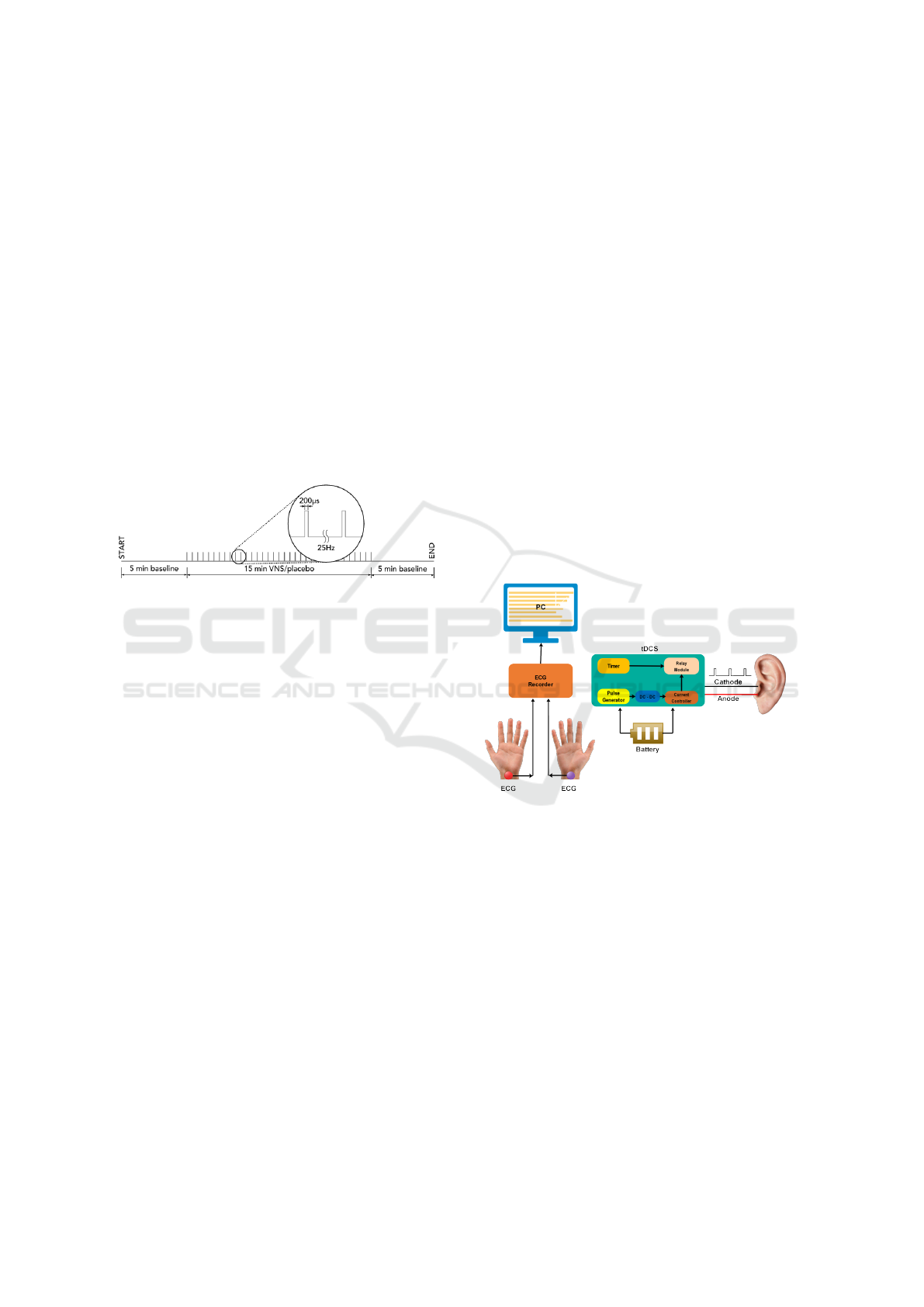
Figure 1: Stimulation protocol.
More recent studies have focused on non-invasive
methods of VNS, which can circumvent complex
implantation procedures and reduce associated risks
such as infection. To address this, electrical stimula-
tion of the auricular vagus nerve has been appropri-
ately investigated using bioelectronics with the main
focus being on the therapeutic effects (Kaniusas et al.,
2019a). Stimulating the auricular branch of the vagus
nerve (ABVN) also known as Alderman’s nerve or
Arnold’s nerve has proven to be effective in the treat-
ment of depression (Hein et al., 2013; Bermejo et al.,
2017; Trevizol et al., 2015; Fang et al., 2016; Rong
et al., 2016).
To apply electrical stimulation on the auricular
branch, the location could either be the cymba con-
chae or tragus as these have most of the vagus fibres.
Even though the conchae consists of 100% vagus fi-
bres, the tragus is easier to apply electrical stimulation
to both walls of the ear with a suitable stimulation clip
(Badran et al., 2018) despite having fewer vagus fi-
bres. Electrical stimulation frequency and pulse width
are crucial parameters that need to be chosen carefully
to activate any parasympathetic response. The stim-
ulation pattern determines the activation of parasym-
pathetic and sympathetic responses. Higher stimula-
tion frequencies of 20–25 Hz are required to stimulate
the parasympathetic system, while lower frequencies
0.5–10 Hz usually stimulate the sympathetic response
(Dietrich et al., 2008). One complicating factor is the
frequency selectivity of the skin barrier between elec-
trodes and nerve cells.
A stimulation frequency of 25 Hz is commonly
used in experimental studies related to auricular VNS
(Badran et al., 2018; Sclocco et al., 2017; Badran
et al., 2019), and a pulse width of 200 µs is considered
to be effective and safe for long periods of stimulation
(Bikson et al., 2018).
2 METHODOLOGY
To administer VNS on the left tragus, a stimulation
protocol as shown in Figure 1 was followed. The
stimulation frequency was fixed at 25 Hz with a pulse
width of 200 µs. Data collection was performed in
three trials over two sessions, starting with a rest pe-
riod of five minutes for the baseline recording, stimu-
lation/placebo session for 15 minutes and a post stim-
ulation session of five minutes. Custom hardware
was developed for administering the stimulation as
explained in the Hardware Design section.
Figure 2: Functional block diagram of the equipment devel-
oped for the experiments.
2.1 Hardware Design
Functional blocks for the hardware are shown in Fig-
ure 2. To collect the ECG data, a Biosemi Active Two
system with standalone battery pack was used with
two electrodes attached using conductive gel to the
left and right wrists of each participant. CMS and
DRL electrodes were used as reference and ground.
For stimulation, the pulse was generated using an
ARM Cortex M4 microcontroller, which was pro-
grammed to generate PWM pulses for use in our pro-
tocol (Mouli and Palaniappan, 2017; Mouli and Pala-
niappan, 2020). Along with the hardware prototype,
code was developed to generate pulses at a rate of
25 Hz with a pulse-width of 200 µs. This was fed
Tragus based Vagus Nerve Stimulation for Stress Reduction
165

to a DC-DC controller to regulate the output current
(which did not exceed 2 mA as a safety precaution
(Bikson et al., 2018)), and provided pulse amplitudes
of 2.2 volts. The output timing was controlled by us-
ing another ARM Cortex M4 microcontroller to set
the stimulation time duration of 15 minutes as well
as controlling a dual pole, dual throw relay module to
switch the pulses ON/OFF when the stimulator was
activated. The output of the relay module was con-
nected to a small ear clip as shown in Figure 3 with
two circular electrodes that can comfortably attach to
both sides of the tragus wall.
Figure 3: Tragus clip.
The chosen pulse width and rate are based on
other VNS experimental studies (Kaniusas et al.,
2019b; Badran et al., 2019), although in this case we
stimulated the tragus site. The hardware prototype is a
standalone unit with pre-programmed stimulation fre-
quency and powered by 5 V DC battery pack, which
makes it safer to use and avoids any external power
surges and interferences.
2.2 Procedure
For this study, five healthy participants (three females,
two males, with age 38.6 ± 12.5 years) took part in
the data recording for two sessions, which comprised
stimulation and placebo on different days. Each par-
ticipant was seated comfortably with electrodes at-
tached using gel to both wrists for ECG collection.
The current stimulator ear clip was coated with con-
ductive paste and was connected to the left tragus with
the anode on the inner side of the tragus and the cath-
ode on the outer side of the tragus. Each session
involved taking three separate ECG recordings be-
ginning with a five-minute baseline (pre-stimulation)
followed by a 15 min session without stimulation
(placebo) and ending with a five-minute post ‘stimu-
lation’ session, as shown diagrammatically in Figure
1. The recordings were repeated for the same partici-
pant on a different day, once with placebo and once
with active current stimulation. The precise order
of stimulation/placebo was randomised between par-
ticipants who were not informed which session was
placebo and which involved active VNS. Ethics ap-
proval for the experiment was obtained from the Sci-
ences Research Ethics committee at the University of
Kent. The collected data were anonymised and anal-
ysed to study the effects of current stimulation.
2.3 Data Processing
Two sets of data were analysed separately for placebo
and VNS sessions in each participant. Data was con-
verted from the 128 Hz sample rate of the 24 − bit
Biosemi Data Format (BDF) recordings to European
Data Format (EDF) using the EEGLAB plug-in in
MATLAB. Data was filtered from 1 to 35 Hz using
an IIR Elliptic filter and the ECG was extracted from
the channel corresponding to the left wrist with the
right wrist used as a re-reference channel. R-R peaks
were detected using a peak detection method in MAT-
LAB that involved a pre-defined threshold that was
verified manually for each experiment to ensure that
artefact induced peaks were excluded. These R-R in-
tervals were fed into Kubios HRV 3.3.1 analysis soft-
ware (Tarvainen et al., 2014) to compute the values
for LF (0.04-0.15 Hz), HF (0.15-0.4 Hz) and the ratio
LF/HF.
3 RESULTS AND DISCUSSION
From HRV, the LF and HF components can be com-
puted to evaluate the influence of VNS. Computed
values from pre- and post- VNS sessions are shown in
Table 1 for all the five participants. Based on these re-
sults, it is evident that LF values were reduced and HF
values were increased with VNS, denoting a stronger
activation of the parasympathetic system compared
to the sympathetic system. The overall reduction in
the LF/HF ratio from the pre-VNS and post-VNS ses-
sions was also computed. Over all five participants,
an average reduction of 64.5% was observed between
pre- and post- VNS sessions. Meanwhile an aver-
age reduction of 6.8% was observed between pre- and
post- placebo sessions. Figure 4 also shows the reduc-
tion in electrocardiogram derived respiration (EDR).
A small reduction is to be expected when someone
is sitting down relaxed for short periods of time, how-
ever the increased reduction observed for the VNS
sessions may indicate the effectiveness of the stimula-
tion in reducing stress. Figure 4 shows an example of
the HRV frequency analysis from one participant for
both pre- and post- VNS sessions where the higher HF
increase over LF increase in post-VNS can be seen
showing an increase in the parasympathetic activity
as compared to sympathetic. Changes in the HRV pa-
rameters as a result of VNS has also been reported
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
166
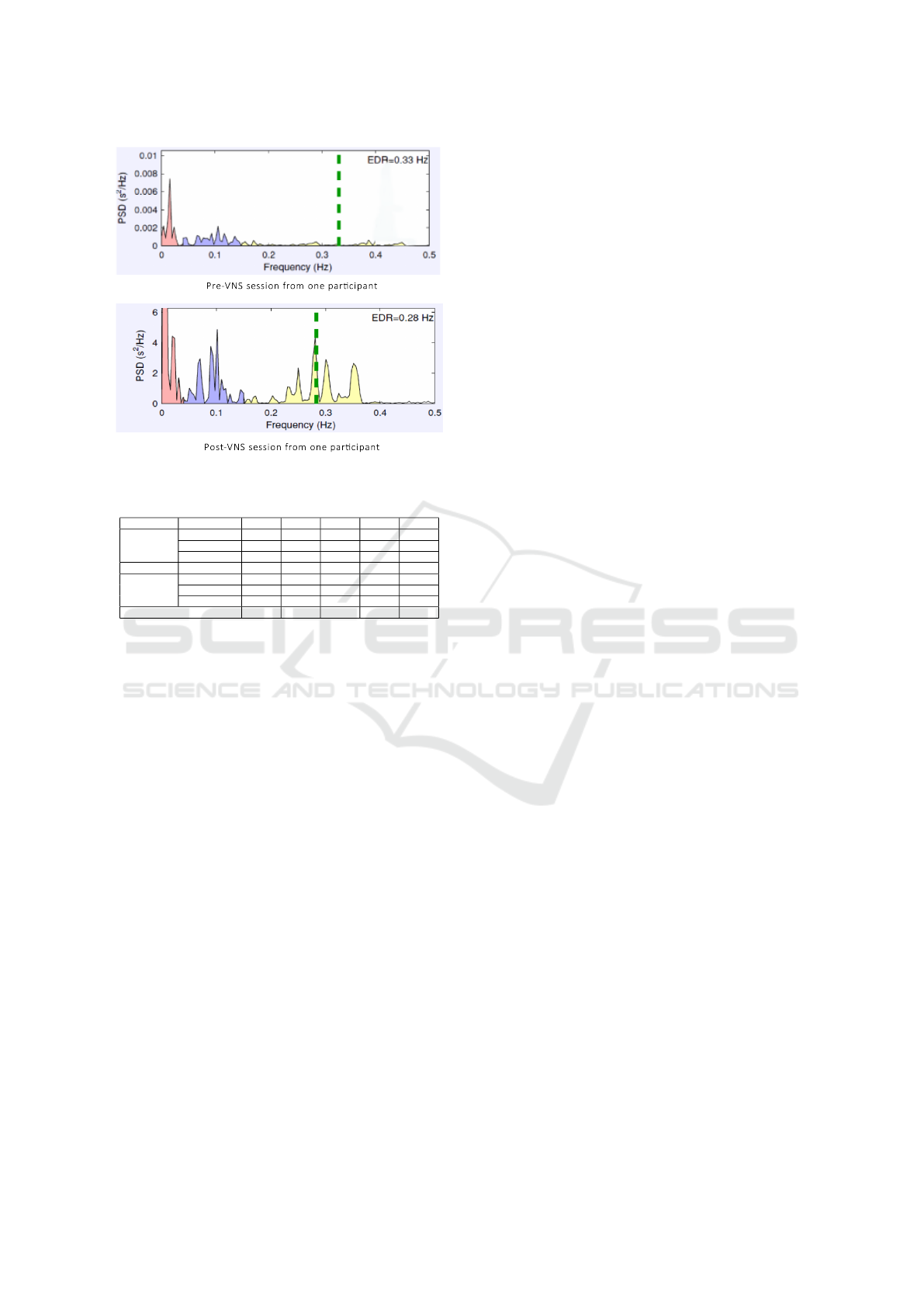
Figure 4: Pre - and Post - VNS session from one participant.
Table 1: Pre- and Post - VNS results from all participants.
Session Participant P1 P2 P3 P4 P5
Pre- VNS
LF (n.u.) 88.40 67.50 46.50 57.70 93.13
HF (n.u.) 11.60 30.30 53.40 42.20 6.85
LF/HF (%) 7.62 2.23 0.87 1.37 13.60
Post- VNS
LF (n.u.) 71.20 40.00 26.20 35.70 81.8
HF (n.u.) 28.80 59.90 73.20 64.20 18.17
LF/HF (%) 2.47 0.67 0.36 0.56 4.50
% Reduction 67.56 70.02 58.90 59.33 66.89
in various studies using similar frequency (Constan-
tinescu et al., 2019; Kamath et al., 1992; De Couck
et al., 2017).
4 CONCLUSION
The study reported here explored the possibilities of
non-invasive auricle vagus nerve stimulation at the
tragus. The experiments stimulated the left tragus for
15 minutes to activate a parasympathetic response, in-
ducing a more relaxed (less stressful) state. A fre-
quency of 25 Hz using a pulse width of 200 µs was
generated using portable standalone hardware and
administered during stimulation. The vagus nerve
was easily stimulated at the tragus using a generic
ear clip for both anode and cathode, in contrast to
the cymba conchae location, which would require
custom-shaped attachments for each participant. A
reduction in LF/HF ratio after VNS was observed for
all participants even with a relatively stimulation short
period of 15−minutes, with LF components decreas-
ing and HF components increasing after the stimula-
tion to indicate a higher parasympathetic vs sympa-
thetic activation. For this study, the VNS period was
limited to 15 minutes to explore the influence of non-
invasive stimulation, but future studies could explore
longer periods of stimulation, or the effect of sessions
at regular intervals involving clinical patients. Cus-
tomised cymba conchae designs to stimulate the va-
gus nerve could also be explored, which may lead to
improved results, given that the density of vagus nerve
fibres is higher at the conchae compared to the tragus.
ACKNOWLEDGEMENTS
The project was funded by Enabling Innovation: Re-
search to Application (EIRA, Research England’s
Connecting Capability Fund, CCF) grant RD005 -
MindSpire Proof of Concept.
REFERENCES
Akerman, S. and Romero-Reyes, M. (2020). Vagus nerve
stimulation. In Lambru, G. and Lanteri-Minet, M.,
editors, Neuromodulation in Headache and Facial
Pain Management: Principles, Rationale and Clinical
Data, pages 87–98. Springer International Publishing.
Ambrosini, A. and Coppola, G. (2020). Transcranial direct
current stimulation. In Lambru, G. and Lanteri-Minet,
M., editors, Neuromodulation in Headache and Facial
Pain Management: Principles, Rationale and Clinical
Data, pages 111–118. Springer Inter. Publishing.
Badran, B. W., Alfred, B. Y., Adair, D., Mappin, G., De-
Vries, W. H., Jenkins, D. D., George, M. S., and Bik-
son, M. (2019). Laboratory administration of tran-
scutaneous auricular vagus nerve stimulation (tavns):
technique, targeting, and considerations. JoVE (Jour-
nal of Visualized Experiments), (143):e58984.
Badran, B. W., Brown, J. C., Dowdle, L. T., Mithoefer,
O. J., LaBate, N. T., Coatsworth, J., DeVries, W. H.,
Austelle, C. W., McTeague, L. M., and Yu, A. (2018).
Tragus or cymba conchae? investigating the anatomi-
cal foundation of transcutaneous auricular vagus nerve
stimulation (tavns). Brain Stimulation, 11(4):947.
Bandelow, B. and Michaelis, S. (2015). Epidemiology of
anxiety disorders in the 21st century. Dialogues in
Clinical Neuroscience, 17(3):327.
Bermejo, P., Lopez, M., Larraya, I., Chamorro, J., Cobo, J.,
Ordonez, S., and Vega, J. (2017). Innervation of the
human cavum conchae and auditory canal: anatomical
basis for transcutaneous auricular nerve stimulation.
BioMed Research International, 2017.
Bikson, M., Paneri, B., Mourdoukoutas, A., Esmaeilpour,
Z., Badran, B. W., Azzam, R., Adair, D., Datta, A.,
Fang, X. H., and Wingeier, B. (2018). Limited output
transcranial electrical stimulation (lotes-2017): En-
gineering principles, regulatory statutes, and indus-
try standards for wellness, over-the-counter, or pre-
scription devices with low risk. Brain Stimulation,
11(1):134–157.
Blixen, C. E., Kanuch, S., Perzynski, A. T., Thomas,
C., Dawson, N. V., and Sajatovic, M. (2016). Bar-
riers to self-management of serious mental illness
Tragus based Vagus Nerve Stimulation for Stress Reduction
167

and diabetes. American Journal of Health Behavior,
40(2):194–204.
Borges, U., Laborde, S., and Raab, M. (2019). Influ-
ence of transcutaneous vagus nerve stimulation on car-
diac vagal activity: Not different from sham stimula-
tion and no effect of stimulation intensity. PloS one,
14(10):e0223848.
Constantinescu, V., Matei, D., Constantinescu, I., and Cuci-
ureanu, D. I. (2019). Heart rate variability and vagus
nerve stimulation in epilepsy patients. Translational
Neuroscience, 10(1):223–232.
De Couck, M., Cserjesi, R., Caers, R., Zijlstra, W., Widjaja,
D., Wolf, N., Luminet, O., Ellrich, J., and Gidron,
Y. (2017). Effects of short and prolonged transcuta-
neous vagus nerve stimulation on heart rate variability
in healthy subjects. Autonomic Neuroscience, 203:88–
96.
Dietrich, S., Smith, J., Scherzinger, C., Hofmann-Preiß, K.,
Freitag, T., Eisenkolb, A., and Ringler, R. (2008). A
novel transcutaneous vagus nerve stimulation leads to
brainstem and cerebral activations measured by func-
tional mri. Biomedizinische Technik/Biomedical Engi-
neering, 53(3):104–111.
Fang, J., Rong, P., Hong, Y., Fan, Y., Liu, J., Wang, H.,
Zhang, G., Chen, X., Shi, S., and Wang, L. (2016).
Transcutaneous vagus nerve stimulation modulates
default mode network in major depressive disorder.
Biological Psychiatry, 79(4):266–273.
Hein, E., Nowak, M., Kiess, O., Biermann, T., Bayerlein,
K., Kornhuber, J., and Kraus, T. (2013). Auricu-
lar transcutaneous electrical nerve stimulation in de-
pressed patients: a randomized controlled pilot study.
Journal of Neural Transmission, 120(5):821–827.
Hidaka, B. H. (2012). Depression as a disease of moder-
nity: Explanations for increasing prevalence. Journal
of Affective Disorders, 140(3):205 – 214.
Johnson, R. L. and Wilson, C. G. (2018). A review of vagus
nerve stimulation as a therapeutic intervention. Jour-
nal of Inflammation Research, 11:203.
Kamath, M., Upton, A., Talalla, A., and Fallen, E. (1992).
Effect of vagal nerve electrostimulation on the power
spectrum of heart rate variability in man. Pacing and
Clinical Electrophysiology, 15(2):235–243.
Kaniusas, E., Kampusch, S., Tittgemeyer, M., Panetsos, F.,
Gines, R. F., Papa, M., Kiss, A., Podesser, B., Cas-
sara, A. M., Tanghe, E., Samoudi, A. M., Tarnaud, T.,
Joseph, W., Marozas, V., Lukosevicius, A., I
ˇ
stuk, N.,
ˇ
Saroli
´
c, A., Lechner, S., Klonowski, W., Varoneckas,
G., and Sz
´
eles, J. C. (2019a). Current directions in the
auricular vagus nerve stimulation i – a physiological
perspective. Frontiers in Neuroscience, 13(854).
Kaniusas, E., Kampusch, S., Tittgemeyer, M., Panetsos, F.,
Gines, R. F., Papa, M., Kiss, A., Podesser, B., Cas-
sara, A. M., Tanghe, E., Samoudi, A. M., Tarnaud, T.,
Joseph, W., Marozas, V., Lukosevicius, A., I
ˇ
stuk, N.,
Lechner, S., Klonowski, W., Varoneckas, G., Sz
´
eles,
J. C., and
ˇ
Saroli
´
c, A. (2019b). Current directions in
the auricular vagus nerve stimulation ii – an engineer-
ing perspective. Frontiers in Neuroscience, 13(772).
Kessler, R. C., Ruscio, A. M., Shear, K., and Wittchen, H.-
U. (2009). Epidemiology of anxiety disorders. In Be-
havioral Neurobiology of Anxiety and Its Treatment,
pages 21–35. Springer.
Kim, H.-G., Cheon, E.-J., Bai, D.-S., Lee, Y. H., and Koo,
B.-H. (2018). Stress and heart rate variability: a meta-
analysis and review of the literature. Psychiatry inves-
tigation, 15(3):235.
Malik, M., Bigger, J. T., Camm, A. J., Kleiger, R. E.,
Malliani, A., Moss, A. J., and Schwartz, P. J. (1996).
Heart rate variability: Standards of measurement,
physiological interpretation, and clinical use. Euro-
pean heart journal, 17(3):354–381.
McClintock, S. M., Trevino, K., and Husain, M. M. (2009).
Vagus nerve stimulation: Indications, efficacy, and
methods. In Swartz, C. M., editor, Electroconvul-
sive and Neuromodulation Therapies, pages 543–555.
Cambridge University Press.
Mouli, S. and Palaniappan, R. (2017). Toward a reliable
pwm-based light-emitting diode visual stimulus for
improved ssvep response with minimal visual fatigue.
The Journal of Engineering, 2017(2):7–12.
Mouli, S. and Palaniappan, R. (2020). DIY hybrid SSVEP-
P300 LED stimuli for BCI platform using EMOTIV
EEG headset. HardwareX, 8:e00113.
Rong, P., Liu, J., Wang, L., Liu, R., Fang, J., Zhao, J., Zhao,
Y., Wang, H., Vangel, M., and Sun, S. (2016). Ef-
fect of transcutaneous auricular vagus nerve stimula-
tion on major depressive disorder: a nonrandomized
controlled pilot study. Journal of Affective Disorders,
195:172–179.
Sclocco, R., Garcia, R. G., Gabriel, A., Kettner, N. W., Na-
padow, V., and Barbieri, R. (2017). Respiratory-gated
auricular vagal afferent nerve stimulation (ravans) ef-
fects on autonomic outflow in hypertension. In 2017
39th Annual Inter. Conf. of the IEEE Engineering in
Medicine and Biology Society (EMBC), pages 3130–
3133.
Shaffer, F. and Ginsberg, J. (2017). An overview of heart
rate variability metrics and norms. Frontiers in public
health, 5:258.
Tarvainen, M. P., Niskanen, J.-P., Lipponen, J. A., Ranta-
Aho, P. O., and Karjalainen, P. A. (2014). Kubios
hrv–heart rate variability analysis software. Computer
methods and programs in biomedicine, 113(1):210–
220.
Thayer, J. F.,
˚
Ahs, F., Fredrikson, M., Sollers III, J. J., and
Wager, T. D. (2012). A meta-analysis of heart rate
variability and neuroimaging studies: implications for
heart rate variability as a marker of stress and health.
Neuroscience & Biobehavioral Reviews, 36(2):747–
756.
Trevizol, A. P., Taiar, I., Barros, M. D., Liquidatto, B.,
Cordeiro, Q., and Shiozawa, P. (2015). Transcuta-
neous vagus nerve stimulation (tvns) protocol for the
treatment of major depressive disorder: A case study
assessing the auricular branch of the vagus nerve.
Epilepsy & Behavior, 53:166–167.
Vonck, K., De Herdt, V., and Boon, P. (2009). Vagal nerve
stimulation — a 15-year survey of an established treat-
ment modality in epilepsy surgery. In Advances and
Technical Standards in Neurosurgery, pages 111–146.
Springer Vienna.
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
168
