
Deep Learning Solution for Pathological Voice Detection using
LSTM-based Autoencoder Hybrid with Multi-Task Learning
Dávid Sztahó, Kiss Gábor and Tulics Miklós Gábriel
Department of Telecommunication and Media Informatics, Budapest University of Technology and Economics,
Magyar tudósok körútja 2., Budapest, Hungary
Keywords: Deep Neural Networks, Long-Short Term Memory, Autoencoder, Multi-Task Learning, Speech, Bio-signal,
LSTM, Voice Pathologies, Parkinson, Depression.
Abstract: In this paper, a deep learning approach is introduced to detect pathological voice disorders from continuous
speech. Speech as bio-signal is getting more and more attention as a discriminant for different diseases. To
exploit information in speech, a long-short term memory (LSTM) autoencoder hybrid with multi-task learning
solution is proposed with spectrogram as input feature. Different speech databases (voice disorders,
depression, Parkinson’s disease) are applied as evaluation datasets. Applicability of the method is
demonstrated by obtaining accuracies 85% for Parkinson’s disease, 86% for dysphonia, and 90% for
depression on test datasets. The advantage of this method is that it is fully data-driven, in the sense that it does
not require special acoustic-phonetic preprocessing separately for the types of disease to be recognized. We
believe that the applied method in this article can be used to other diseases as well and can be used for other
languages also.
1 INTRODUCTION
Speech as bio-signal getting more and more attention
as a discriminant for different diseases. There can be
many alterations in speech production due to
neurological and/or organic disorders caused by
illnesses. In general, any alteration from ‘normal’
speech might be an indication of pathological speech.
Alterations of speech may be caused by various
things, for example psychological conditions such as
depression. Voice disorders are also main causes of
voice alternations. Voice disorder happens once
somebody’s voice quality, pitch, and loudness are
inappropriate for an individual’s age, gender, cultural
background, or geographic location. The American
Speech-Language-Hearing Association divides voice
disorders into two groups: organic voice disorders
and functional voice disorders. Organic disorders can
be structural and neurogenic in nature. Structural
disorders involve physical changes in the voice
mechanism, such as alterations in vocal fold tissues
such as oedema or vocal nodules, polyps,
gastroesophageal reflux disease (GERD), cyst and
vocal cord paralysis. Neurogenic voice disorders on
the other hand are caused by a problem in the nervous
system, that include voice problems caused by
abnormal control, coordination, or strength of voice
box muscles due to an underlying neurological
disease such as stroke, Parkinson’s disease, multiple
sclerosis.
Classifying speech into normal and disordered is
more problematic than it first seems. There are a large
number of works (Dastjerd et al., 2019; Filiou et al.,
2020; Jeancolas et al., 2020; Kiss & Vicsi, 2017a;
Klempíř & Krupička, 2018; Low et al., 2020; Tóth et
al., 2018; Zhang et al., 2019) subjected to
classification of these diseases using various machine
learning techniques.
Deep learning (DL) is one of the most frequently
used machine learning solutions nowadays. There are
many DNN algorithms developed, each is a proper
solution to a given data type processing. In this paper,
we utilize a long-short term memory (LSTM)
autoencoder (AE) hybrid with multi-task learning
(MTL) solution to propose a DL structure for
detecting multiple diseases, using a voice disorder, a
depression and a Parkinson’s disease dataset.
An important disadvantage of these classification
methods is that they may need complex phonetic
preprocessing in order to detect different parts of the
speech and, therefore, they may be language
dependent. The proposed method in this paper is an
Sztahó, D., Gábor, K. and Gábriel, T.
Deep Learning Solution for Pathological Voice Detection using LSTM-based Autoencoder Hybrid with Multi-Task Learning.
DOI: 10.5220/0010193101350141
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 4: BIOSIGNALS, pages 135-141
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
135

automatic way without the need of segmenting the
speech by an automatic speech recognizer (ASR).
Feature extraction is a critical step of any
classification method. DL has the ability to learn to
extract the best needed features for the best result.
There are end-to-end systems that receive raw sound
signals (amplitudes) as input and derive the proper
features themselves. For this, huge available data is
needed. Generally, dealing with speech and diseases,
this is not the case. In our work we use spectrograms
as inputs to the DL method and apply autoencoder
based feature learning (Yu et al., 2019).
Speech production process is time-varying. The
same linguistic content can be said in many durations.
Importantly, this variation may not correlate with the
given task (disease detection) at all. LSTMs, as a
special DL building element, has the property to learn
information across time due to its ability to have
memory. Therefore, it can learn information that
concerns different diseases (Gupta, 2018; Kim et al.,
2018; Mallela et al., 2020; Yang et al., 2016; Zhao et
al., 2019).
Overfitting is always an important concern in
classification trials. There are multiple ways of
overcoming this error, mostly by applying proper
dataset splitting (train-validation-test). Here, beside
the correct dataset splitting, multi-task learning
(Ruder, 2004) is also utilized. MTL is not only used
as regularization, but also for the parallel
classification and autoencoder (feature learning)
implementation.
Recent works dealing with deep learning and
disease classification include various convolutional
network assemblies, recurrent neural networks,
LSTMs and even solutions on mobile devices. Since
the majority of these works use different datasets for
evaluation (even for the same disease), it is hard to
compare their results. Most of them report about 90%
classification accuracy (Gunduz, 2019; Kaur et al.,
2019, 2020; Lam et al., 2019; Mdhaffar et al., 2019;
Mohammed et al., 2020; Rejaibi et al., 2019, p.). The
proposed AE-LSTM hybrid can be considered as
novel architecture among the found studies.
There are several approaches for the binary
classification of a healthy subject from voices
affected by some disorder. The first question is
whether to use sustained vowels or continuous
speech. Researchers achieved high accuracies using
sustained vowels (Orozco-Arroyave, 2015; Zhang,
2008; Ali, 2017; Teixeira, 2017), however, a
significant proportion of researchers use continuous
speech in their research pointing out the benefits of
using continuous speech over sustained vowels
(Vicsi, 2011; Guedes, 2019; Cordeiro, 2015). The
research findings are expected to be more applicable
to practical work since continuous speech is used in
real-world situations. In the work of (Guedes, 2019)
the German Saarbrücken Voice Database with the
phrase “Guten Morgen, wie geht es Ihnen?” to
classify dysphonia and healthy voices. A 66% f1-
score was reached in their experiment with Long-
Short-Term-Memory and Convolutional Network for
classification. In (Tulics, 2019) researchers used
acoustic features and phone-level posterior
probabilities computed by the DNN soft-max layer of
the speech recognition system and used them as an
input for an SVM and a Fully-Connected Deep
Neural Network. Classification accuracies were
ranging from 85% to 88% in their experiments.
The accuracy of distinguishing between depressed
and healthy subjects depends largely on the database
used, such as the size of the database and the severity
of the subjects included in it (Cummins et al., 2015).
The accuracy of the classification also depends on the
methods used, such as the feature extraction, or the
use of gender dependent or independent models (Low
et al., 2020). In (Kiss & Vicsi, 2017b) researchers
used gender dependent models and used selected
acoustic features as an input for an SVM, achieving
86% accuracy with a database which can be
considered similar as ours.
The paper is structured as follows: in Section 2,
the used speech datasets are described. Section 3
discusses the methods applied. Section 4, 5 and 6
contains the results, discussions and conclusions.
2 DATABASE
The proposed DL structure was tested on three
datasets of three disease types. Each dataset contained
the recording of reading a short folk tale ‘The North
Wind and the Sun’ in Hungarian. For each dataset,
healthy speakers are included as a control population
with the same sample number as the given disease
dataset and age and gender distribution matching the
patients’ statistics. Although the classification task
described is binary, for the sake of completeness, the
severity of the disease is also noted here for each
dataset. The audio samples were recorded with
external USB sound card and clip-on microphone in
PCM format using 44 kHz sampling rate and 16-bit
quantization. Informed consents were signed by each
patient before recordings.
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
136

2.1 Hungarian Parkinson’s Speech
Dataset (HPSD)
Speech samples were collected from patients
diagnosed with Parkinson’s disease (PD) by two
health institutes in Budapest: Virányos Clinic and
Semmelweis University. The severity of PD was
labelled according to the Hoehn & Yahr scale (H-Y)
(Hoehn & Yahr, 1967). The H-Y scale ranges from 1
to 5, where 1 indicates minimal PD while 5 is the
worst PD condition. We did not filter the patients
according to the taken medications. All patients were
in ON state.
83 speech sample were collected from patients
with PD: 43 male speakers (mean H-Y score:
2.74(±1.05); mean age: 64(±9.5)) and 40 female
speakers (mean H-Y score: 2.74(±1.10); mean age:
65.4(±9.4)).
2.2 Voice Disorder Speech Dataset
(VDSD)
Voice samples from patients were collected during
patient consultations in a consulting room at the
Department of Head and Neck Surgery of the
National Institute of Oncology. The collected speech
database contains voices from people suffering from
diseases like tumors at various places of the vocal
tract, gastroesophageal reflux disease, chronic
inflammation of larynx, bulbar paresis, amyotrophic
lateral sclerosis, leukoplakia, spasmodic dysphonia,
etc. The recorded voice samples in this experiments
were classified by a leading phoniatric according to
the RBH scale. The RBH scale gives the severity of
dysphonia, where R stands for roughness, B for
breathiness and H for overall hoarseness. The degree
of the category H cannot be less than the highest rate
of the other two categories. For example, if B = 3 and
R = 2, H is 3, and cannot be 2 or 1. A healthy voice’s
code is R0B0H0; the maximum H and respectively
RBH value is 3, so a voice’s code with severe
dysphonia is R3B3H3. Here the H score is given.
The database contains a total of 261 recordings
from patients with dysphonia: 159 females (mean H
score: 1.72(±0.77); mean age: 57.3(±14.8)) and 102
males (mean H score: 2 (±0.83); mean age:
53.7(±15.1)).
2.3 Hungarian Depressed Speech
Dataset (HDSD)
Speech samples were collected from patients
diagnosed with depression by the Psychiatric and
Psychotherapeutic Clinic of Semmelweis University,
Budapest. Patients with antipsychotic medication
which can affect the acoustical features of speech
were left out. The degree of severity of depression
was recorded using the Beck Depression Inventory II
(BDI) scale (Beck et al., 1996)). The BDI-II scale
ranges from 0 to 63, where 0 indicates a healthy state,
while 63 is the worst depression condition. The BDI-
II scale uses the following rating: 0-13 healthy, 14-19
mild depression, 20-28 moderate depression, 29-63
severe depression.
A total of 107 speech sample were recorded from
depressed patients: 64 female subjects (mean BDI
score: 28.0(±9.0); mean age: 37.5(±13.5) and 43
males subjects (mean BDI score: 26.1(±8.0); mean
age: 40.8(±13.6)).
3 METHODS
3.1 Implemented Deep Learning
Architecture and Input
The DL architecture, training and evaluation was
implemented in Tensorflow 2.1.0. The implemented
DL architecture consists of an LSTM and an
autoencoder part. Multi-task learning is applied in
order to train the network for the task-specific labels
and the autencoder-based feature extraction. Figure 1
shows the structure of the implemented network.
Spectrogram is fed to the network as input. For each
audio sample, the spectrogram was extracted by 10
ms timestep and 256 FFT size (resulting in 16 ms
window size), commonly used in speech analysis.
Because Tensorflow did not manage the varying
duration of the samples, this was solved by using a
Masking layer at the input. Technically, each
spectrogram was padded with 0.0 elements to reach
the duration of the longest audio sample. By using the
Masking layer, the 0.0 elements were skipped during
training and prediction processes.
The DL architecture consists of two parts. An
autoencoder part learns feature representation
(dimensionality reduction) for the audio sample
spectra. This is intended to encode information in the
spectra. Part of the bottleneck layer (fc
autoencoder
) has
shared neurons that are also trained to the task-
specific target labels. This tries to ensure that part of
the encoded spectra contains information that is
specific to the given disease. This also serves as a
regulation technique to avoid overfitting. The idea
behind this multi-task learning is that this forces the
encoded spectra to contain information partly about
the disease characteristics.
Deep Learning Solution for Pathological Voice Detection using LSTM-based Autoencoder Hybrid with Multi-Task Learning
137
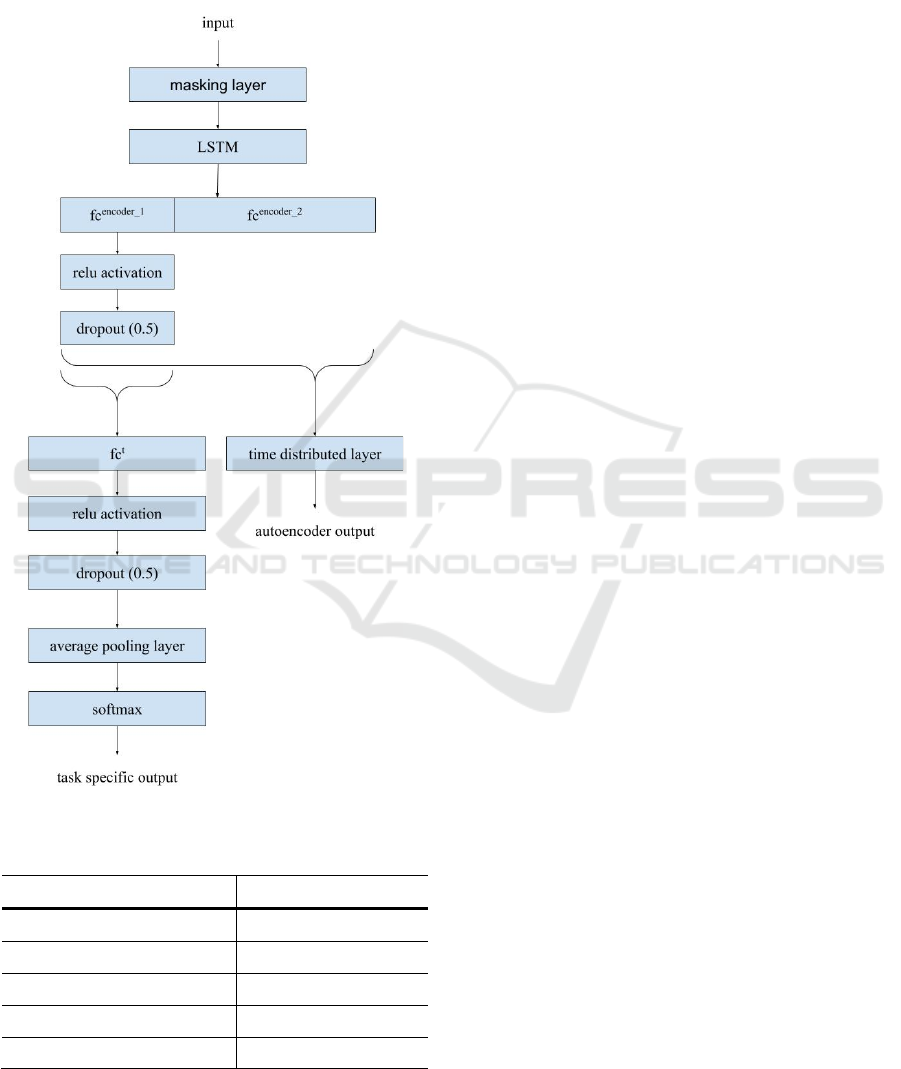
The other part of the DL architecture performs
disease-specific classification. This part contains
two fully connected layers (one is a shared layer in
the bottleneck layer) with relu activation functions,
dropout layers (dropout parameter set to 0.5) and
softmax layer at the end. Before the softmax layer an
Figure 1: Structure of the implemented network.
Table 1: Number of units in DL layers.
layer name
units
lstm 100
fc
autoencoder1
30
fc
autoencoder2
30
fc
t1
200
time distributed layer 128
average pooling layer is needed in order to make one
decision for one audio sample (by averaging the
outputs of layer fc
t
).
A shared LSTM layer is also applied in order to
learn time varying information.
Layer sizes are shown in Table 1. The appropriate
numbers were selected according to preliminary
experiments.
3.2 Dataset Splitting and Network
Training
Evaluation was performed by splitting each database
into training, validation and test sets by the following
method.
Due to the limited number of audio samples
available, all samples were used for testing by a 10-
fold cross-validation process. 10 test sets were
created by 10-fold cross-validation (stratified). In
each cross-validation iteration, the remaining 90% of
the samples were split into training and validation
sets, 70% and 20% respectively, by stratified random
sampling. Training was done on the training set and
an early stopping was applied on the validation set.
Minimum cross-entropy of the disease classification
was used as a cost function for early stopping.
Maximum 1000 training epochs were done with 50
patience steps for early stopping. During training,
‘Adam’ optimizer was used.
4 RESULTS
Accuracy, sensitivity and specificity was used as
evaluation metrics. Tables 2, 3 and 4 show the results
in each set during tests (training, validation and
testing). The confusion matrices for the test sets are
also shown in Table 5. The values in the cells are the
number of samples (subjects).
The results show that the applied DL performs
well on all three datasets. The lowest accuracy on the
test sets is 0.86, which means that 86% of the samples
are correctly classified into healthy or disease
categories. The highest score is 0.90 in the case of
depression.
The DL method doesn’t seem to overfit. Balanced
results metrics are achieved in the training, validation
and test steps.
Also, sensitivity and specificity scores are well
balanced in the case of VDSD and HDSD datasets.
Parkinson samples are a little bit unbalanced
according to these metrics. Higher specificity is
reached, which means that healthy samples are
classified more accurate than the Parkinson samples.
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
138

Table 2: Results on the HPDS samples.
accuracy sensitivity specificity
training 0.86 0.77 0.95
validation 0.88 0.80 0.96
test 0.85 0.75 0.95
Table 3: Results on the VDSD samples.
accuracy sensitivity specificity
training 0.90 0.89 0.91
validation 0.87 0.86 0.89
test 0.86 0.85 0.87
Table 4: Results on the HDSD samples.
accuracy sensitivity specificity
training 0.93 0.92 0.93
validation 0.90 0.90 0.89
test 0.90 0.91 0.89
Table 5: Confusion matrices obtained on the test datasets.
PDSD
predicted positive predicted negative
true positive 62 21
true negative 4 79
PDSD
predicted positive predicted negative
true positive 226 35
true negative 28 164
HDSD
predicted positive predicted negative
true positive 95 12
true negative 9 93
5 DISCUSSION
The achieved results on the applied three datasets
show that the proposed DL method is able to extract
information from the samples in order to make
distinction between negative (healthy) and positive
cases. These actual accuracy, sensitivity and
specificity scores are, naturally, dependent on the
datasets. However, they can be considered large
enough, that some statements could be concluded
based on them. With the extension of the recordings
and by applying more datasets in different languages
may also prove the proposed DL method to be more
robustly usable.
Based on the results, the highest evaluation scores
were achieved on the depression database test set (and
also on training and validation sets). Based on
personal experience of clinical experts, intonation is
also highly affected by depression. By applying an
LSTM layer, this intonational disorder (which can be
captured through temporal analysis) can be more
accurately modelled.
The suggested method not only captured
intonational features of speech, but voice
characteristics related to dysphonia, such as
hoarseness and breathiness.
In case of Parkinson’s disease lower sensitivity
scores are achieved than specificity scores. Although
a high sensitivity-specificity balance is more
desirable, the present case doesn’t mean that the
method can’t be used as pre-screening. Less positive
samples will be detected, but the overall accuracy can
be considered sufficient for the task. In fact, every
method is usable with over random performance.
The results achieved are comparable to the
previous results in the field. Since actual results are
dataset dependent, direct comparison of accuracy and
other metrics is problematic. (Kiss & Vicsi, 2017b)
reported 86% accuracy for a former version of the
depression dataset. Here, we achieved 90%. In case
of PD, (Sztahó et al., 2019) reported around 88%
accuracy using cross-validation setup, without
separate independent test set. The 85% achieved here
is comparable, especially if we add that here we
applied an appropriate test set. In the case of VDSD
dataset, a previous result is reported in (Tulics, 2019).
In that, a 95% accuracy was described with possible
overfitting effect, and between 85-88% without
overfitting. Here, we achieved 86% without probable
overfitting. All these researches used segmentation
information to obtain the highest performance. In our
case here, this computationally intensive step is not
needed.
Among many other usages, actual practical
applicability can be pre-screening in general
practitioner offices or home-care environments. A
cheap, easy to use devices (software) can be
implemented to detect various diseases that affect
speech.
Deep Learning Solution for Pathological Voice Detection using LSTM-based Autoencoder Hybrid with Multi-Task Learning
139

6 CONCLUSION
The goal of this work is to introduce a novel method
for pathological speech recognition using continuous
speech without the need of a voicing detection or
speech segmentation application. The proposed DL
architecture consists of two parts: an autoencoder part
learns feature representation and a disease-specific
classification.
We demonstrated the applicability of the method
by classifying three different diseases: Parkinson’s
disease, dysphonia related voice disorders and
depression. The method achieved 0.85 for
Parkinson’s disease, 0.86 for dysphonia, and 0.90 for
depression on the test datasets. These classification
accuracies correspond to the classification accuracies
mentioned in the literature. The advantage of this
method is that it is fully data-driven, in the sense that
it does not require special acoustic-phonetic
preprocessing separately for the types of disease to be
recognized. The speech recordings can be directly
given to the deep neural network (using
spectrographic extraction only).
We believe that the applied method in this article
can be used to other diseases as well and can be used
for other languages also.
ACKNOWLEDGEMENTS
Project no. K128568 has been implemented with the
support provided from the National Research,
Development and Innovation Fund of Hungary,
financed under the K_18 funding scheme. The
research was partly funded by the CELSA
(CELSA/18/027) project titled: “Models of
Pathological Speech for Diagnosis and Speech
Recognition”.
REFERENCES
Ali, Z., Talha, M., & Alsulaiman, M., 2017. A practical
approach: Design and implementation of a healthcare
software for screening of dysphonic patients. IEEE
Access, 5, 5844-5857.
Beck, A. T., Steer, R. A., Ball, R. & Ranieri, W. F., 1996.
Comparison of beck depression inventories -IA and -II
in psychiatric outpatients. Journal of Personality
Assessment 67, 588–597.
Cordeiro, H., Meneses, C., & Fonseca, J., 2015. Continuous
speech classification systems for voice pathologies
identification. In Doctoral Conference on Computing,
Electrical and Industrial Systems, pp. 217-224.
Cummins, N., Scherer, S., Krajewski, J., Schnieder, S.,
Epps, J., & Quatieri, T. F., 2015. A review of depression
and suicide risk assessment using speech analysis.
Speech Communication, 71, 10-49.
Dastjerd, N. K., Sert, O. C., Ozyer, T., & Alhajj, R., 2019.
Fuzzy Classification Methods Based Diagnosis of
Parkinson’s disease from Speech Test Cases. Current
Aging Science, 12(2), 100–120.
Filiou, R.-P., Bier, N., Slegers, A., Houzé, B., Belchior, P.,
& Brambati, S. M., 2020. Connected speech assessment
in the early detection of Alzheimer’s disease and mild
cognitive impairment: A scoping review. Aphasiology,
34(6), 723–755.
Guedes, V., Teixeira, F., Oliveira, A., Fernandes, J., Silva,
L., Junior, A., & Teixeira, J. P., 2019. Transfer
Learning with AudioSet to Voice Pathologies
Identification in Continuous Speech. Procedia
Computer Science, 164, 662-669.
Gunduz, H., 2019. Deep learning-based Parkinson’s disease
classification using vocal feature sets. IEEE Access, 7,
115540–115551.
Gupta, V., 2018. Voice disorder detection using long short
term memory (lstm) model. ArXiv Preprint
ArXiv:1812.01779.
Hoehn, M. & Yahr, M. D., 1967. Parkinsonism onset,
progression, and mortality. Neurology 17, pp. 427–427
Jeancolas, L., Petrovska-Delacrétaz, D., Mangone, G.,
Benkelfat, B.-E., Corvol, J.-C., Vidailhet, M., Lehéricy,
S., & Benali, H., 2020. X-vectors: New Quantitative
Biomarkers for Early Parkinson’s Disease Detection
from Speech. ArXiv:2007.03599 [Cs, Eess, q-Bio].
http://arxiv.org/abs/2007.03599
Kaur, S., Aggarwal, H., & Rani, R., 2019. Diagnosis of
Parkinson’s Disease Using Principle Component
Analysis and Deep Learning. Journal of Medical
Imaging and Health Informatics, 9(3), 602–609.
Kaur, S., Aggarwal, H., & Rani, R., 2020. Hyper-parameter
optimization of deep learning model for prediction of
Parkinson’s disease. Machine Vision and Applications,
31(5), 32.
Kim, M. J., Cao, B., An, K., & Wang, J., 2018. Dysarthric
Speech Recognition Using Convolutional LSTM
Neural Network. INTERSPEECH, 2948–2952.
Kiss, G., & Vicsi, K., 2017a. Comparison of read and
spontaneous speech in case of automatic detection of
depression. 2017 8th IEEE International Conference on
Cognitive Infocommunications (CogInfoCom), 213–
218.
Kiss, G., & Vicsi, K., 2017b. Mono-and multi-lingual
depression prediction based on speech processing.
International Journal of Speech Technology, 20(4),
919-935.
Klempíř, O., & Krupička, R., 2018. Machine learning using
speech utterances for Parkinson disease detection.
Lékař a Technika - Clinician and Technology, 48(2),
66–71.
Lam, G., Dongyan, H., & Lin, W., 2019. Context-aware
deep learning for multi-modal depression detection.
ICASSP 2019-2019 IEEE International Conference on
BIOSIGNALS 2021 - 14th International Conference on Bio-inspired Systems and Signal Processing
140

Acoustics, Speech and Signal Processing (ICASSP),
3946–3950.
Low, D. M., Bentley, K. H., & Ghosh, S. S., 2020.
Automated assessment of psychiatric disorders using
speech: A systematic review. Laryngoscope
Investigative Otolaryngology, 5(1), 96–116.
Mallela, J., Illa, A., Suhas, B. N., Udupa, S., Belur, Y.,
Atchayaram, N., Yadav, R., Reddy, P., Gope, D., &
Ghosh, P. K., 2020. Voice based classification of
patients with Amyotrophic Lateral Sclerosis,
Parkinson’s Disease and Healthy Controls with CNN-
LSTM using transfer learning. ICASSP 2020-2020
IEEE International Conference on Acoustics, Speech
and Signal Processing (ICASSP), 6784–6788.
Mdhaffar, A., Cherif, F., Kessentini, Y., Maalej, M.,
Thabet, J. B., Maalej, M., Jmaiel, M., & Freisleben, B.,
2019. DL4DED: Deep Learning for Depressive
Episode Detection on Mobile Devices. International
Conference on Smart Homes and Health Telematics,
109–121.
Mohammed, M. A., Abdulkareem, K. H., Mostafa, S. A.,
Khanapi Abd Ghani, M., Maashi, M. S., Garcia-
Zapirain, B., Oleagordia, I., Alhakami, H., & AL-Dhief,
F. T., 2020. Voice Pathology Detection and
Classification Using Convolutional Neural Network
Model. Applied Sciences, 10(11), 3723.
Orozco-Arroyave, J. R., Hönig, F., Arias-Londoño, J. D.,
Vargas-Bonilla, J. F., Skodda, S., Rusz, J., & Nöth, E.,
2015. Voiced/unvoiced transitions in speech as a
potential bio-marker to detect Parkinson's disease. In
Sixteenth Annual Conference of the International
Speech Communication Association.
Rejaibi, E., Komaty, A., Meriaudeau, F., Agrebi, S., &
Othmani, A., 2019. MFCC-based Recurrent Neural
Network for Automatic Clinical Depression
Recognition and Assessment from Speech. ArXiv
Preprint ArXiv:1909.07208.
Ruder, S., 2017. An overview of multi-task learning in deep
neural networks. ArXiv Preprint ArXiv:1706.05098.
Sztahó, D., Valálik, I., & Vicsi, K., 2019. Parkinson’s
Disease Severity Estimation on Hungarian Speech
Using Various Speech Tasks. In International
Conference on Speech Technology and Human-
Computer Dialogue (SpeD). pp. 1-6.
Tóth, L., Hoffmann, I., Gosztolya, G., Vincze, V.,
Szatlóczki, G., Bánréti, Z. & Kálmán, J., 2018. A
speech recognition-based solution for the automatic
detection of mild cognitive impairment from
spontaneous speech. Current Alzheimer
Research, 15(2), 130-138.
Tulics, M. G., Szaszák, G., Mészáros, K., & Vicsi, K., 2019.
Artificial Neural Network and SVM based Voice
Disorder Classification. In 10th IEEE International
Conference on Cognitive Infocommunications
(CogInfoCom). pp. 307-312.
Vicsi, K., Imre, V., & Mészáros, K., 2011. Voice disorder
detection on the basis of continuous speech. In 5th
European Conference of the International Federation
for Medical and Biological Engineering, pp. 86-89.
Yang, T.-H., Wu, C.-H., Huang, K.-Y., & Su, M.-H., 2016.
Detection of mood disorder using speech emotion
profiles and LSTM. 10th International Symposium on
Chinese Spoken Language Processing (ISCSLP), pp. 1–
5.
Yu, J., Zheng, X., & Wang, S., 2019. A deep autoencoder
feature learning method for process pattern recognition.
Journal of Process Control, 79, pp. 1–15.
Zhang, H., Song, C., Wang, A., Xu, C., Li, D., & Xu, W.,
2019. PDVocal: Towards Privacy-preserving
Parkinson’s Disease Detection using Non-speech Body
Sounds. The 25th Annual International Conference on
Mobile Computing and Networking, pp. 1–16.
Zhang, Y., & Jiang, J. J., 2008. Acoustic analyses of
sustained and running voices from patients with
laryngeal pathologies. Journal of Voice, 22(1), pp. 1-9.
Zhao, J., Mao, X., & Chen, L., 2019. Speech emotion
recognition using deep 1D & 2D CNN LSTM networks.
Biomedical Signal Processing and Control, 47, pp.
312–323.
Deep Learning Solution for Pathological Voice Detection using LSTM-based Autoencoder Hybrid with Multi-Task Learning
141
