
Non-coding DNA: A Methodology for Detection and Analysis of
Pseudogenes
Gabriella Trucco and Vittorio Cerioli
Department of Computer Science, University of Milan, via Celoria, Milan, Italy
Keywords:
Pseudogenes, CpG Island, Alignment, Viterbi Algorithm, Gibbs Sampling.
Abstract:
It is well known that elements lying outside the coding regions of the human genome are involved in many
human diseases. Therefore, the efforts to detect and characterize functional elements in the non-coding regions
are rapidly increasing. Among many types of non-coding DNA, pseudogenes are sequences that share some
similarities with their parental genes but have lost their ability to code for proteins. In this paper, we propose
a methodology for detection and analysis of pseudogenes, based on transition probabilities of the nucleotides
and their occurrences. The 1000 base pairs length downstream region of each detected pseudogene is analyzed
in order to find a polyA tail and a polyadenylation signal. We implemented a Hidden Markov Model with the
Viterbi algorithm to decode the upstream regions of the previously detected pseudogenes in order to search
for CpG islands. In order to identify motif signals in the selected pseudogenes, we implemented the Gibbs
sampling algorithm and we executed it on the flanking regions of some pseudogenes. Results demonstrate
that the proposed methodology is an efficacious solution to detect new potential loci, especially when the
query coverage of the alignment is shorter than the coding strand. These loci can be classed as pseudogene
fragments.
1 INTRODUCTION
Completing the human genome reference sequence
was a milestone in modern biology. It was quickly
recognized that nearly 99% of the ∼ 3.3 billion nu-
cleotides that constitute the human genome does not
code for proteins (Lander et al., 2001). More recently,
studies have discovered many loci that contribute to
human diseases and susceptibility to disorders lying
outside the protein coding regions (Maurano et al.,
2012; Schaub et al., 2012; Martinez et al., 2016;
Amiel et al., 2010; Braconi et al., 2011; Bao et al.,
2016; Zhang and Zhangm 2015). These findings sug-
gest that the non-coding regions of the human genome
contain a plentiful and variegated set of functionally
significant elements. There are several segments of
non-coding regions including: non-coding RNA, cis-
and trans-regulatory elements, introns, pseudogenes,
telomeres, transposons and repeat sequences. These
regions seem to be responsible for a varied number
of diseases in humans and, therefore, understanding
their roles in the genome is of utmost necessity (Mau-
rano et al., 2012; Schaub et al., 2012; Martinez et al.,
2016; Amiel et al., 2010; Braconi et al., 2011; Bao et
al., 2016; Zhang and Zhangm 2015).
A pseudogene is a genomic DNA sequence that is
closely related to a gene but has lost the capacity to
produce a functional protein. The estimated number
of pseudogenes in the human genome is comparable
to that of protein coding genes (∼ 20.000) (Koonin,
2005; Zheng et al., 2007). Some pseudogenes are
clearly non-functional gene relics (Niimura and Nei,
2007). Other pseudogenes, on the contrary, although
not translated into proteins, are capable of influenc-
ing the activity of other genes by means of long non-
coding RNA (lncRNA) transcripts.
Characterizing the pseudogenes and understand-
ing their regulatory role is essential to discover the
genetic background of many diseases and to elabo-
rate new pharmacological treatments. Moreover, the
correct identification of pseudogenes is important also
for gene annotation (Zheng et al., 2007; Zheng and
Gerstein, 2006). Despite protein sequence similar-
ity to parent genes is the main feature used to detect
pseudogenes, because it is deemed the most sensitive
indicator (Harrow et al., 2006; Zhang et al., 2006),
we developed an algorithm capable to detect pseudo-
genes (in particular, processed pseudogenes). Our al-
gorithm is based on raw nucleotide identity (DNA se-
quence similarity) with the coding sequence (CDS) of
the corresponding gene and on its transition probabil-
ities. The coding sequence is the portion of the gene
Trucco, G. and Cerioli, V.
Non-coding DNA: A Methodology for Detection and Analysis of Pseudogenes.
DOI: 10.5220/0010190400930100
In Proceedings of the 14th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2021) - Volume 3: BIOINFORMATICS, pages 93-100
ISBN: 978-989-758-490-9
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
93

that remains in the mature messenger RNA after the
splicing and, therefore, it is the portion that is actually
translated into protein. It is composed by the exons.
Once identified a putative pseudogene, we analyzed
both the upstream (before the pseudogene 5’ extreme)
and the downstream (beyond the pseudogene 3’ ex-
treme) regions in order to find out biologically inter-
esting features. In particular, we searched for a CpG
island and promoter signals in the upstream region.
The downstream region, instead, was analyzed in or-
der to detect the presence of a polyA tail and, when
a polyA tail was found, a polyadenylation signal was
searched for. The polyadenylation signal (typically
AAUAAA) is a binding site on the messenger RNA
where polyadenylation starts. Moreover, we imple-
mented the Gibbs sampling algorithm with the aim of
finding a common motif in the upstream regions con-
taining a CpG island (Das and Dai, 2007; Thompson
et al., 2003).
2 METHODS
In this section, we provide detailed descriptions of the
algorithms and the strategies used in this project to
pursue the following goals:
• identification of the processed pseudogenes of
some selected genes;
• detection of a polyA tail in the downstream region
of each identified pseudogene;
• detection of CpG islands in the upstream region
of each pseudogene;
• motif discovery (search for potential promoters
sequences) in the upstream regions of the pseu-
dogenes.
2.1 Identification of Pseudogene
Sequences
The first step is design of a strategy for identifying
the pseudogenes of a gene originated from its CDS.
We developed a program that scans the entire genome
and stores all the sequences that have similarity with
a selected CDS in terms of transition probabilities
(the probabilities of transition between the different
nucleotides in the CDS) and occurrences of the nu-
cleotides. Each stored sequence is then aligned with
the CDS and, if the alignment is statistically signifi-
cant, the sequence is marked as a pseudogene.
As a first step, the program builds a matrix of the
transition probabilities of the CDS and computes the
probability of the CDS itself according to this model
(CDS
P
). The probability is computed as a sum of log-
arithms of probabilities in order to avoid floating point
underflow errors (that is numbers of smaller absolute
values than the computer can represent in its CPU)
or, worse, the production of arbitrary wrong numbers.
The nucleotides occurrences of the CDS (CDS
C
n
) are
also calculated. A sliding window that has the same
length of the CDS scans the entire genome. When
it finds a sequence with a transition probability that
is included in the interval ±CDS
P
· 0.05 and a nu-
cleotide occurrence in the interval ±CDS
C
n
· 0.2, for
each nucleotide, the extremities of the sequence are
stored in a list. The window can enlarge itself until the
above-mentioned conditions are satisfied. Sequences
longer than four times the CDS length will be dis-
carded from the list at the end of the scanning. A dis-
tinct program builds 100.000 random sequences with
the same transition probabilities of the CDS. Each se-
quence is aligned with the CDS and the program re-
turns the mean and the standard deviation of the align-
ment scores. Then we align the CDS with all the
sequences in the list. For each alignment, the main
program computes the z-score given by Z =
X−µ
σ
, us-
ing the mean µ and the standard deviation σ previ-
ously computed as explained above. A threshold of 8
is chosen for the z-score so that only sequences with
a z-score greater than the threshold are recorded as
pseudogenes. We chose a threshold of 8 because the
alignment scores between the CDSs and the random
sequences are not normally distributed (Mitrophanov
and Borodovsky, 2005). The parameters of the align-
ment algorithm are: match = 1, mismatch = 0 and gap
= -1.
2.2 PolyA Tails
A polyA tail is a stretch of RNA that has only adenine
bases. In eukaryotes, the addition of a polyA tail to a
messenger RNA 3’ end is part of a process that pro-
duces mature messenger RNA (mRNA) and is called
polyadenylation (Zhang et al., 2002). Processed pseu-
dogenes are typically characterized by the lack of in-
trons and the presence of residue of the polyA tail
(Zhang et al., 2002). We searched for a polyade-
nine tail by means of a 50 bp sliding window in the
1000 bp (base pairs) length region beyond the pseu-
dogene 3’ extremity. The 50 bp windows containing
more than 30 adenines are memorized (if they exist)
and the most promising one is considered as a PolyA
tail. When a polyadenine tail is found, the algorithm
searches for a polyadenylation signal (AATAAA or
ATTAAA) in the 100 bp length upstream region of
the tail.
BIOINFORMATICS 2021 - 12th International Conference on Bioinformatics Models, Methods and Algorithms
94

2.3 CpG Islands
CpG islands are regions of DNA in which a cytosine is
followed by a guanine in the sequence of nucleotides
along the 5
0
→ 3
0
direction with a high frequency. The
notation CpG is used to distinguish the single strand
sequence from the CG pairing on the double strand.
In vertebrate genomes, CpG nucleotides occur with
a much lower frequency than would be expected by
random chance. The frequency of CpG dinucleotides
in the human genome is 0.98% while the expected
frequency is 4.41% (Gardiner-Garden and Frommer,
1987). CpG islands play an important role in gene
expression regulation and the ability to identify them
can help us to predict the location of genes within the
DNA.
A na
¨
ıve approach to locate CpG islands in a se-
quence X of length L is to extract a sliding window of
length len L and to compute a score for each sub-
sequence of length len in X. The main disadvantage
of this strategy is that we have no information about
the lengths of the islands. If we use a value of len that
is too large, the score we get from this window may
not be high enough. The best approach for this prob-
lem is the use of a Hidden Markov Model (HMM). A
general HMM (Durbin et al., 1998) is a triplet
M = (Q, S, Θ),
where:
• Q is an alphabet of symbols;
• S is a finite set of states capable of emitting sym-
bols from the alphabet Q;
• Θ is a set of probabilities, comprised of:
- state transition probabilities, denoted as p
i j
for
each i, j ∈ S;
- emission probabilities denoted as q
k
(b) for
each k ∈ S and b ∈ Q.
The HMM for CpG islands has (Gr
¨
opl, 2012):
• 9 states: begin/end, A+, C+, G+, T+, A-, C-, G-
and T-
• 4 symbols: A, C, G and T
The letters A+, C+, G+ and T+ represent states that
belong to a CpG island. The other letters, instead,
represent states not belonging to a CpG island. The
state 0 corresponds to the state begin/end of the chain.
A Markov chain is a system (S, A) consisting of a fi-
nite set of states S and a transition matrix A = a
kl
with
∑
l∈S
a
kl
= 1 for all k ∈ S that determines the probabil-
ity of the transition k → l by P(s
i+1
= l | s
i
= k) = a
kl
.
At any step i, the Markov chain is in a specific state
s
i
and the chain changes to state s
i+1
according to
the given transition probability (Gr
¨
opl, 2012). In this
model, each state emits only the corresponding sym-
bol/nucleotide (with probability 1).
The state transition probabilities matrix is reported
in Table 1. Model ”+” describes the transition proba-
bilities inside the CpG, model ”-” describes the tran-
sition probabilities outside the CpG island (Gr
¨
opl,
2012).
2.4 Motif Discovery
In order to find potential sequence signals (DNA bind-
ing sites or promoters) in the upstream regions in
which a CpG island is present, we developed a Gibbs
sampling algorithm capable of locating a pattern of
subsequences with the highest likelihood. Gibbs sam-
pling is a probabilistic inference algorithm used to
generate a sequence of samples from a joint proba-
bility distribution of two or more random variables
(Haggstr
¨
om, 2002). In bioinformatics, Gibbs sam-
pling is used to detect motif signals in multiple DNA
or protein sequences assuming no prior information
about the motifs (Das and Dai, 2007; Thompson et
al., 2003; Lawrence et al., 1993). Thus, given a
set of sequences S = S
(1)
, . . . , S
(n)
and an integer w,
the algorithm finds, for each sequence S
(i)
, a subse-
quence of length w, so that the similarity between
the n sequences is maximized (Lawrence et al., 1993;
Rouchka, 2008). Let c
i j
be the number of occurrences
of the symbol j ∈ Σ among the i
th
position of the n
subsequences. Let q
i j
denote the probability of the
symbol j to occur at the i
th
positions of pattern and
let p
j
denote the frequency of the symbol j in all se-
quences of S. The algorithm maximizes the equation:
F =
w
∑
i=1
∑
j∈Σ
c
i j
· log
q
i j
p
j
,
where c
i j
and q
i j
are computed from the complete
alignment of the subsequences. To achieve this result,
we designed an algorithm that performs the following
iterative procedures:
1. Initialization: randomly chooses a
(1)
, . . . , s
(n)
,
the starting indices of the subsequences in
S
(1)
, . . . , S
(n)
, respectively.
2. Randomly chooses 1 ≤ z ≤ n and computes c
i j
,
qi j and p
j
values for the sequences in S\S
(z)
.
3. According to the model, computes the weights of
all possible subsequences of length w in S
(z)
. The
weights are normalized and a new value of a
(z)
is randomly selected with a probability propor-
tional to the weights of the subsequences of S
(z)
.
In order to avoid local optima, the starting posi-
tion with the highest weight is not guaranteed to
Non-coding DNA: A Methodology for Detection and Analysis of Pseudogenes
95
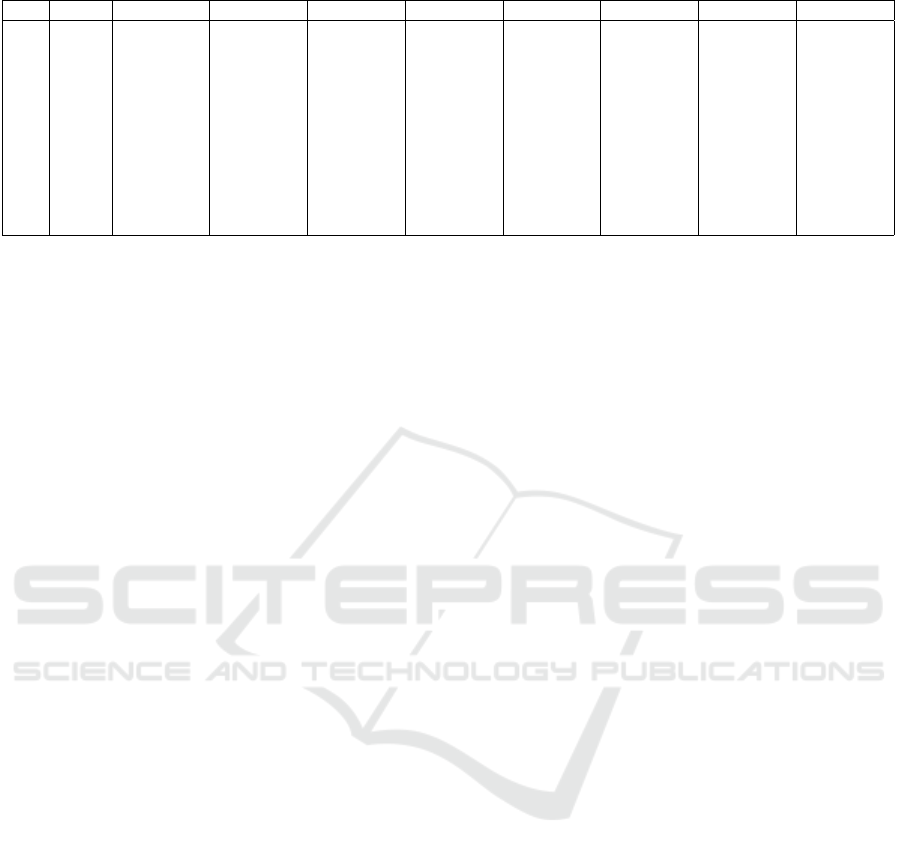
Table 1: Transition matrix.
0 A+ C+ G+ T+ A- C- G- T-
0 0.000 0.0725193 0.1637630 0.1788242 0.0754545 0.1322050 0.1267006 0.1226380 0.1278950
A+ 0.001 0.1762237 0.2682517 0.4170629 0.1174825 0.0035964 0.0054745 0.0085104 0.0023976
C+ 0.001 0.1672435 0.3599201 0.2679840 0.1838722 0.0034131 0.0073453 0.0054690 0.0037524
G+ 0.001 0.1576223 0.3318881 0.3671328 0.1223776 0.0032167 0.0067732 0.0074915 0.0024975
T+ 0.001 0.0773426 0.3475514 0.3750440 0.1781818 0.0015784 0.0070929 0.0076723 0.0036363
A- 0.001 0.0002997 0.0002047 0.9992837 0.0002097 0.2994005 0.2045904 0.2844305 0.2095804
C- 0.001 0.0003216 0.0002977 0.0000769 0.0003016 0.3213566 0.2974045 0.0778441 0.3013966
G- 0.001 0.0001768 0.0002387 0.0002917 0.0002917 0.1766463 0.2385224 0.2914165 0.2914155
T- 0.001 0.0002477 0.0002457 0.0002977 0.0002077 0.2475044 0.2455084 0.2974035 0.2075844
be chosen. In order to rapidly converge to a solu-
tion, the above mentioned random sampling goes
on for a fixed amount of time (usually 15 min),
then, after the time threshold has expired, only the
position with the highest weight is chosen.
4. The algorithm repeats step 2 and 3 until it con-
verges to a fixed pattern of subsequences. The
algorithm ends when the same pattern of subse-
quences is produced for 10 consecutive iterations.
We chose this strategy with the purpose of having
many“fast”solutions rather than few“slow”ones.
3 RESULTS AND DISCUSSION
In this paper we considered 11 genes and searched
for their processed pseudogenes. Five of these genes
belong to the ribosomal protein family, which is the
family with the highest number of processed pseudo-
genes (Zhang et al., 2002). Other six genes are known
for their pseudogene-mediated expression regulation
or for their involvement in cancer disease.
The proposed algorithm was able to detect 110 of
121 pseudogenes annotated by Ensembl for these 11
genes. Moreover, it detected four loci not reported by
Ensembl, but reported by UCSC, two new potential
pseudogene loci reported neither by Ensembl nor by
UCSC and three duplicated sequences for three dis-
tinct pseudogenes. Though the algorithm didn’t cap-
ture all the annotated pseudogenes, it seems to be an
efficacious solution to detect new potential loci, es-
pecially when the query coverage of the alignment is
shorter than the coding sequence. These loci can be
classed as pseudogene fragments.
The downstream regions of the detected pseudo-
genes were analyzed in order to find polyA tails.
We found a polyA tail for 48 pseudogenes and a
polyadenylation signal for 13 of them. These num-
bers are coherent with known data. Literature reports
that a polyA tail is present in about 45-50% of the
cases (Zhang et al., 2002).
CpG islands of different lengths and at different
distances from the pseudogenes were detected in 16
upstream regions. We did not find any motif in the
upstream regions probably because a bigger set of se-
quences is needed by the Gibbs sampling algorithm.
However, we executed the algorithm on the flanking
regions of some pseudogenes and the results showed
an interesting similarity between the flanking regions
of some of them. These similarities were confirmed
also by alignments of the regions.
We implemented the algorithms in Java language
and we executed them on a Notebook Asus K72F
equipped with Intel Core i3 processor (2.5 GHz). The
entire human genome sequence was downloaded from
the repository on www.ncbi.nlm.nih.gov, the CDSs
were downloaded from the Ensembl genome browser
hosted by www.ensembl.org. In this section, we de-
scribe the results of the following experiments.
• In order to detect the pseudogenes of each gene
considered in the survey, we developed a strategy
based on raw nucleotide identity that scans the en-
tire human genome and returns the coordinates of
each detected pseudogene.
• The 1000 bp length downstream region of each
pseudogene was inquired about the presence of a
polyA tail and, when this feature was present, the
algorithm searched for a polyadenylation signal in
the 100 bp length upstream region of the tail.
• The 1000 bp length upstream region of each pseu-
dogene was decoded by the Viterbi algorithm
based on a HMM suited for CpG islands detec-
tion.
• We also performed motif discovery experiments
on the flanking regions of some pseudogenes. The
strategy used for this goal was Gibbs sampling.
In our research we identified and analyzed the
pseudogenes of the following genes: RPL14, RPL19,
RPL22, RPL36 and RPL37 that are ribosomal pro-
tein genes (RP family) (Zheng et al., 2007); PTEN
(phosphatase and tensin homolog) codes for a tumor
suppressor (Chiefari et al., 2010); KRAS (GTPase
BIOINFORMATICS 2021 - 12th International Conference on Bioinformatics Models, Methods and Algorithms
96
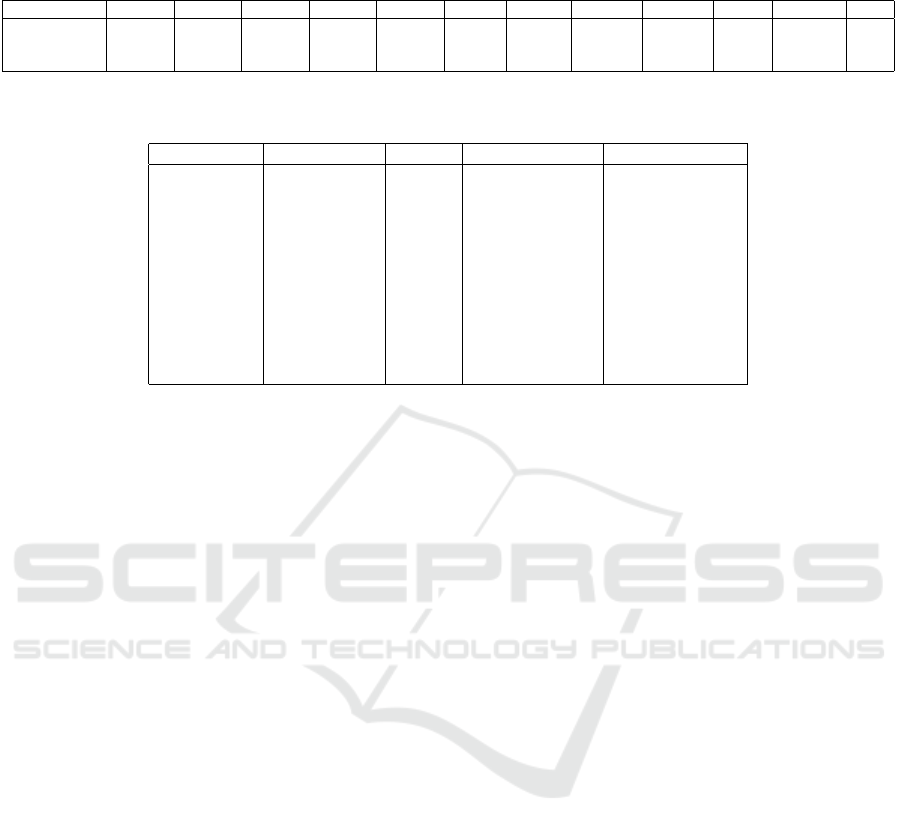
Table 2: The first row reports the number of annotated pseudogenes for each gene, the second and the third rows report the
number of attested pseudogenes and of unannotated pseudogene loci identified by our method, respectively.
RPL14 RPL19 RPL22 RPL36 RPL37 PTEN KRAS RAP1A RAP1B CX43 HDAC1 sum
annotated 9 22 23 25 28 2 1 2 5 1 3 121
attested 9 19 22 21 26 1 1 2 5 1 3 110
not reported 1 2 2 0 1 0 0 0 0 0 0 6
Table 3: The locus AL356967.1 is annotated by Ensembl as “novel pseudogene” residing on chromosome 6 (forward strand)
at 104.687.241-104.687.879. UCSC reports it as RPL14 retrogene.
chromosome z-score query coverage percent identity
RPL14 3 (+1)
RPL14P1 12 (+1) 69.86 84% 98.21%
AC017079.1 2 (+1) 24.92 42% 86.44%
AC012519.1 3 (-1) 42.78 84% 83.89%
AC126615.1 12 (+1) 56.17 82% 91.28%
RPL14P3 4 (-1) 53.20 83% 90.50%
AC108039.1 2 (-1) 17.78 37% 90.00%
AL024507.1 6 (-1) 13.32 43% 82.95%
RPL14P5 X (-1) 34.15 58% 85.58%
AC117522.3 5 (-1) 26.41 45% 86.96%
AL356967.1 6 (+1) 9.75 30% 71.53%
KRAS) is a proto-oncogene (Poliseno et al., 2010);
RAP1A and RAP1B are members of the oncogene
RAS family; CX43 (gap junction protein alpha) is an-
other cancer-related gene; GJA1P1, a pseudogene of
CX43, is expressed in breast cancer but not in normal
cells (Bier et al., 2009); and finally HDAC1 (histone
deacetylase 1) (Tam et al., 2008).
3.1 Pseudogenes Detection
The Ensembl genome browser reports 121 pseu-
dogenes for these 11 genes. We attested 110 of
them and we identified 6 pseudogenes loci not pre-
viously annotated by the Ensembl genome browser,
two of them annotated neither by the Ensembl
genome browser nor by the UCSC genome browser
(www.genome.ucsc.edu). The statistical significance
of the alignments was confirmed by the z-score and
by the BLASTN alignment online application hosted
by the National Center for Biotechnology Information
(NCBI) website (www.ncbi.nlm.nih.gov). The posi-
tion and the annotation of the sequences found were
confirmed by the Ensembl genome browser. Table 3
reports, for each gene, the number of pseudogenes an-
notated by Ensembl (first row), the number of loci at-
tested by our method (second row) and the loci not
reported by Ensembl (third row), but detected by our
method.
Table 3 shows the detection results for RPL14.
The first column reports the position in the sequence
(the number of the chromosome, where +1 stands for
forward strand and -1 for reverse strand) of the gene
itself and of each detected pseudogene. The second
column reports the z-score of the alignments. The
third and the fourth columns report the query cov-
erage and the percent identity of the alignments re-
spectively. The latter two parameters are provided by
BLASTN.
Similarly, we calculated the results for RPL19,
RPL22, and RPL37, which are not reported here due
to lack of space.
The computation time of pseudogenes detection
depends on CDS length because the optimal align-
ment is computed in O(L
2
), where L is the length of
the sequence. However, the main factor that influ-
ences the computation time is the number of homolo-
gous sequences found, which is unknown before exe-
cution. Table 4 reports the computation time of each
experiment.
3.2 PolyA Tails
We found 48 polyA tails (41% of the cases) and 13
polyadenylation signals (AATAAA or ATTAAA). It’s
worth to notice that the sequence (1) of RPL37, re-
ported neither by Ensembl nor by UCSC, has a polyA
tail at 571 bp from its 3’ and a polyadenylation sig-
nal at 13 bp from the 5’ of the tail. Table 5 shows
the number of tails and the number of polyadenyla-
tion signals found for each group of pseudogenes.
3.3 CpG Islands
The upstream 1000 bp length regions of the detected
pseudogenes were analyzed in order to check the
presence of CpG islands. Table 6 displays the number
of CpG islands found for each group of pseudogenes
and the maximum CpG island length in each group.
It is worth to notice that the length of the CpG islands
Non-coding DNA: A Methodology for Detection and Analysis of Pseudogenes
97

Table 4: The table shows the length of each CDS and the computation time needed to scan the entire genome in search of its
pseudogenes.
gene RPL14 RPL19 RPL22 RPL36 RPL37 PTEN KRAS RAP1A RAP1B CX43 HDAC1
CDS length 552 591 387 320 294 1212 570 555 555 1146 1449
time (min) 86 70 124 46 102 422 179 254 342 192 273
Table 5: The first row reports the number of pseudogenes analyzed for each gene (attested+not reported), the second and the
third rows report the number of tails and the number of polyadenylation signals for each group respectively.
RPL14 RPL19 RPL22 RPL36 RPL37 PTEN KRAS RAP1A RAP1B CX43 HDAC1 sum
analyzed 10 21 24 21 27 1 1 2 5 1 3 116
tail 4 11 8 12 12 0 0 1 0 0 0 48
signal 1 3 4 1 4 0 0 0 0 0 0 13
varies a lot and, therefore, a sliding window cannot be
used to detect CpG islands.
3.4 Motif Discovery and Flanking
Regions
It was observed that half of mammalian CpG islands
(∼ 10.000) are “orphan”, that is, they are not asso-
ciated with annotated promoters. There are evidences
that many orphan CpG islands play a role as transcrip-
tional initiator during development and, after that,
they are subject to DNA methylation loosing their
active promoter features. Thus, orphan CpG islands
may correspond to undetected promoters that are ac-
tive during development (Illingworth et al., 2010).
With the aim of finding a possible DNA signal in the
CpG islands found, we analyzed the 500 bp length
pseudogenes upstream regions that contain CpG is-
lands. We run the Gibbs sampling algorithm in order
to find common subsequences of length 14. We did
not find any significant common motif. Nevertheless,
we identified a similarity between the upstream re-
gions of RAP1B pseudogenes. We noticed that the
subsequences of the best pattern for these regions are
located at similar distances from their respective pseu-
dogenes 5’ extremities. The same happens for the
subsequences of other high-scored patterns. A similar
feature was observed also in the downstream regions
(excepting AL161670.1). This feature was not ob-
served in the upstream (and downstream) sequences
of the pseudogenes of RAP1A, PTEN and HDAC1.
We didn’t test the ribosomal pseudogenes for this fea-
ture.
A further confirmation of the similarity between
the flanking regions of these pseudogenes is provided
by the alignment BLASTN online application hosted
by the NCBI website. In Table 7, the bottom-left tri-
angle contains the alignments scores (qc=query cov-
erage and pi=percent identity) of the upstream re-
gions. The top-right triangle contains the results of
the downstream regions alignments.
4 CONCLUSIONS
Though the genomes of higher organisms do not have
more genes than lower organisms, the greater abun-
dance of regulatory ncRNAs, found in the higher
organisms, could give reasons to a more complex
phenotype from the same building blocks (Pink and
Carter, 2013). Characterizing the pseudogenes and
understanding their regulatory role will help in dis-
covering the genetic origin of many diseases but also
in finding new pharmacological treatments. More-
over, the prevalence of pseudogenes in mammalian
genomes can introduce artifacts in automatic gene an-
notation pepelines in which pseudogenes are often
mistakenly annotated as genes. This is due to the
high sequence similarity of pseudogenes with their
parental genes (Zheng et al., 2007; Zheng and Ger-
stein, 2006). Therefore, the correct identification of
pseudogenes is important also for gene annotation.
Identification. No consensus computational
scheme for detecting and defining pseudogenes has
yet been developed. Distinct pseudogene annotation
strategies produced rather distinct set of pseudogenes
(Zheng et al., 2007). The algorithm based on raw nu-
cleotide identity, even if it did not “capture” all the
pseudogenes annoted by Ensembl, proved to be an
efficacious tool for detection of new potential pseu-
dogene sites not discovered by other strategies. In
particular, it seems capable to cut off statistically sig-
nificant alignments with a low query coverage, which
we can regard as pseudogene fragments (Zhang et al.,
2002). The algorithm parameters (thresholds for the
transition probability and for the nucleotides occur-
rences), which we chose empirically, have to be re-
fined in order to improve the performance of the al-
gorithm. Moreover, it should be tested also for detec-
tion of duplicated pseudogenes. These are longer than
processed pseudogenes because they include introns.
However, unlike processed pseudogenes, they reside
near their parental genes and, as a consequence, they
do not need the scanning of the entire genome to be
BIOINFORMATICS 2021 - 12th International Conference on Bioinformatics Models, Methods and Algorithms
98

Table 6: The first row reports the number of pseudogenes analyzed for each gene (attested+not reported), the second row
reports the number of CpG islands identified in each group. The last row displays the maximum CpG island length in each
group.
RPL14 RPL19 RPL22 RPL36 RPL37 PTEN KRAS RAP1A RAP1B CX43 HDAC1 sum
analyzed 10 21 24 21 27 1 1 2 5 1 3 116
CpG 0 2 2 4 1 1 1 2 3 0 0 16
max len. 132 142 268 90 805 95 100 111
Table 7: The table reports the scores of the alignments among the upstream regions (bottom-left) and among the downstream
regions (top-right) of the pseudogenes of RAP1B.
AC113404.3 RAP1BP1 RAP1BP2 RAP1BP3 AL161670.1
AC113404.3 qc=100%, pi=92.83% qc=100%, pi=87.23% qc=99%, pi=91.40% no significant similarity
RAP1BP1 qc=32%, pi=83.45% qc=100%, pi=82.47% qc=99%, pi=86.17% no significant similarity
RAP1BP2 qc=16%, pi=73.33% qc=31%, pi=70.34% qc=100%, pi=81.27% qc=2%, pi=100%
RAP1BP3 qc=35%, pi=72.15% qc=27%, pi=70.75% qc=28%, pi=65.94% no significant similarity
AL161670.1 qc=32%, pi=89.44% qc=33%, pi=82.68% qc=18%, pi=85.07% qc=2%, pi=92.26%
detected (Zheng et al., 2007).
PolyA tails. Although it was observed that a
polyA tail is present beyond a processed pseudogene
in about half of the cases (Zhang et al., 2002), the
presence of a polyA tail (with a possible polyadeny-
lation signal) could help the definition of a sequence
as a processed pseudogene.
CpG islands and motif discovery. The accepted
definition of what is a CpG island was proposed
in 1987 as being a 200 bp stretch of DNA with a
C+G content of 50% and an observed CpG/expected
CpG in excess of 0.6 (Gardiner-Garden and Frommer,
1987). However, any definition of CpG island, af-
ter all, is arbitrary (Takai and Jones, 2002). Using a
HMM designed for the purpose, we found some CpG
islands of different lengths and located at different
distances from the pseudogenes. Then we tried to find
a motif (or signal) in the upstream regions in which a
CpG island is present. The issue of searching for pos-
sible promoter sequences within these orphan CpG re-
gions is a promising future development of this work.
The experiments with the Gibbs sampling showed a
surprising similarity between the flanking regions of
some pseudogenes of the same gene. This suggests
that generation of the processed pseudogenes should
be further investigated.
REFERENCES
E. S. Lander et al., International Human Genome Sequenc-
ing Consortium Initial sequencing and analysis of the
human genome, in Nature 409 (2001), 860-921, doi:
10.1038/35057062
M. T. Maurano et al. Systematic localization of common
disease-associated variation in regulatory DNA. in
Science 337 (2012), 1190-1195. doi: 10.1126/sci-
ence.1222794
M. A. Schaub et al. Linking disease associations with
regulatory information in the human genome. in
Genome Research 22(9) (2012), 1748-2759. doi:
10.1101/gr.136127.111
A. F. Martinez et al. An ultraconserved brain-specific en-
hancer within DGRL3 (LPHN3) underpins attention-
deficit/hyperactivity disorder susceptibility. in Bi-
ological Psychiatry 80 (2016), 943-954. doi:
10.1016/j.biopsych.2016.06.026
J. Amiel, S. Benko, C.T. Gordon and S. Lyonnet. Disrup-
tion of long-distance higly conserved noncoding ele-
ments in neurocristopathies. in Annals of the New York
Academy Sciences 1214 (2010), 34-46
C. Braconi et al. Expression and functional role of a tran-
scribed noncoding RNA with an ultraconserved ele-
ment in hepatocellular carcinoma. in Proceedings of
the National Academy of Sciences 108 (2011), 786-
791. doi: 10.1073/pnas.1010198108.
B. Bao et al. Genetic variants in ultraconserved regions
associate with prostate cancer recurrence and sur-
vival. in Scientific Reports 6 (2016), 22124 doi:
10.1038/srep22124
Feng Zhang and James R. Zhang. Non-coding genetic vari-
ants in human disease. in Human Molecular Genetics
24 (2015), R102-R110. doi: 10.1093/hmg/ddv259
Eugene V. Koonin. Orthologs, Paralogs and Evolu-
tionary Genomics. in Annual Review of Genet-
ics 39(1) (2005), 309-338. doi: 10.1146/an-
nurev.genet.39.073003.114725
D. Zheng et al. Pseudogenes in the ENCODE regions: con-
sensus annotation, analysis of transcription, and evo-
lution. in Genome Research 17 (2007), 839-851. doi:
10.1101/gr.5586307
Yoshihito Niimura, Masatoshi Nei. Extensive gains and
losses of olfactory receptor genes in mammalian evo-
lution. in PLoS ONE 2(8) (2007), 860-921. doi:
10.1371/journal.pone.0000708
Zhaolei Zhang, Paul Harrison, Mark Gerstein. Identifica-
tion and analysis of over 200 ribosomal protein pseu-
dogenes in the human genome. in Genome Research
12(10) (2002), 1466-1482. doi:10.1101/gr.331902
L. Poliseno et al. A coding-independent function of gene
and pseudogene mRNAs regulates tumor biology. in
Nature 465 (2010), 1033-1038. doi: 10.1038/na-
ture09144
Non-coding DNA: A Methodology for Detection and Analysis of Pseudogenes
99

E. Chiefari et al. Pseudogene-mediated pstrascriptional si-
lencing of HMGA1 can result in insulin resistance and
Type 2 diabetes. in Nature Communications 12(10)
(2010), 1-7. doi: 10.1038/ncomms1040
Ryan C. Pink, David R.F Carter. Pseudogenes as regulators
of biological function. in Essays in Biochemestry 54
(2013), 103-112. doi: 10.1042/bse0540103
Jennifer Harrow, France Denoeud, Adam Frankish et al.
GENCODE: producing a reference annotation for EN-
CODE. in Genome Biology 7 (2006), S4. doi:
10.1186/gb-2006-7-s1-s4
Z. Zhang et al. PseudoPipe: an automated pseudogene iden-
tification pipeline. in Bioinformatics 22 (2006), 1437-
1439. doi: 10.1093/bioinformatics/btl116
Modan K. Das and Ho-Kwok Dai. A survey of DNA mo-
tif finding algorithms. in BMC Bioinformatics 8(7)
(2007), S21. doi: 10.1186/1471-2105-8-S7-S21.
William Thompson, Eric C. Rouchka and Charles E.
Lawrence. Gibbs Recursive Sampler: finding
transcription factor binding sites. in Nucleic
Acid Research 31(13) (2003), 3580-3585. doi:
10.1093/nar/gkg608
Alexander Yu Mitrophanov and Mark Borodovsky. Statis-
tical significance in biological sequence analysis. in
Briefings in Bioinformatics 7(1) (2005), 2-24. doi:
10.1093/bib/bbk001
Richard Durbin, Sean R. Eddy, Anders Krogh, Graeme
Mitchinson. Biological Sequence Analysis. in Cam-
bridge University Press (1998).
Clemens Gr
¨
opl. Markov Chains and Hidden Markov Mod-
els in www.mi.fu-berlin.de (2012)
Olle Haggstr
¨
om. Finite Markov Chains in Algorithmic Ap-
plications in Cambridge University Press (2002)
Charles E. Lawrence, Stephen F. Altschul, Mark S. Bo-
guski, Jun S. Liu, Andrew F. Neuwald, John C. Woot-
ton. Detecting Subtle Sequence Signals: A Gibbs
Sampling Strategy for Multiple Alignment. in Science
262 (1993), 208-214. doi: 10.1126/science.8211139
Eric C. Rouchka. A brief Overview of Gibbs Sampling.
in University of Louisville Bioinformatics Laboratory
Technical Report 02 (2008).
A. Bier et al. Connexin43 pseudogene in breast cancer
cells offers a novel therapeutic target. in Molecu-
lar Cancer Therapeutics 8(4) (2009), 786-793. doi:
10.1158/1535-7163.MCT-08-0930
Oliver H. Tam, Alaxei A. Aravin, Paula Stein et al.
Pseudogene-derived small interfering RNAs regulate
gene expression in mouse oocytes. in Nature 8(4)
(2008), 534-538. doi: 10.1038/nature06904
R. S. Illingworth et al. Orphan CpG islands identify numer-
ous conserve promoters in the mammalian genome. in
Plos Genetics 6 (2010), 786-793. doi: 10.1371/jour-
nal.pgen.1001134
Deyou Zheng and Mark B. Gerstein. A computational ap-
proach for identifying pseudogenes in the ENCODE
regions. in Genome Biology 7 (2006), S13. doi:
10.1186/gb-2006-7-s1-s13
Margaret Gardiner-Garden and Marianne Frommer. CpG
islands in vertebrate genomes. in Journal of Molecu-
lar Biology 197 (1987), 261-282. doi: 10.1016/022-
2836(87)90689-9
Daiya Takai, Peter A. Jones. Comprehensive anal-
ysis of CpG islands in human chromosomes 21
and 22. in Proceedings of the National Academy
of Sciences 99(6) (2002), 3740-3745. doi:
10.1073/pnas/05240099
BIOINFORMATICS 2021 - 12th International Conference on Bioinformatics Models, Methods and Algorithms
100
