
Optimization of Graphene Oxide Layer-by-Layer Films to Be Used as
an Enhancer Coating of Optical Fibers Sensors
Carlota Xavier
a
, Paulo Zagalo, Paulo A. Ribeiro
b
and Maria Raposo
c
CEFITEC, Departamento de Física, Faculdade de Ciências e Tecnologia, UNL, Campus de Caparica,
2829-516, Caparica, Portugal
Keywords: Optical Fiber Sensors, Graphene Oxide, Layer-by-Layer Films, Adsorption, Desorption.
Abstract: The stability of Graphene oxide (GO) layers obtained by the layer-by-layer (LbL) films was investigated
having in view the development of tunnable surfaces for optical fibre sensors in aqueous environments. For
this purpose layer-by-layer (LbL) films based polyethylenimine (PEI) and GO, were prepared and
characterized. The kinetics of adsorption of PEI/GO LBL films revealed that the adsorbed amount per bilayer
increases linearly with the number of bilayers as expected. Furthermore adsorbed amount per bilayer for short
adsorption times tends to a constant value revealing that GO layer growth also follows the adsorption
behaviour expected polyelectrolytes in which electrostatic interaction is ruling the process. Finally desorption
studies carried out to infer about GO layer stability revealed that GO layers are more stable at higher solutions
pHs and if the adsorption time for each layer is short. These results/conclusions allowed to infer on the
possible range of applications of PEI/GO LbL films.
1 INTRODUCTION
Graphene oxide thin films, prepared by the layer-by-
layer (LbL) technique (Oliveira, 2001), have been
used as sensing layers for different kind of sensors
namely for detection of triclosan in water (Marques,
2017) and wastewater (Magro, 2019) using the
electronic tongue concept (Magro et al, 2019a),
(Magro et al, 2019b) and by measuring the impedance
spectra. However, studies on the contribution of pH
of wastewater matrices for the stability of the GO thin
films revealed to be strongly influenced by
pH((Magro et al, 2019a; Magro et al, 2019b).
Recently, it was demonstrated the possibility of using
GO as a coating material for enhancing sensing
properties of fiber sensors (Monteiro, 2019).
Moreover, GO LbL deposited on optical fibers
revealed to work as temperature sensors of aqueous
solutions (Costa, 2018). It should be also referred that
the optical fiber devices are widely explored in
literature for hydrostatic pressure (Xu, 1993), lateral
load (Novais, 2017), and strain (Liu, 2015; Monteiro,
a
https://orcid.org/0000-0003-3970-4155
b
https://orcid.org/0000-0001-9665-7610
c
https://orcid.org/0000-0003-4710-0693
2017) sensing, having therefore general application as
optical sensing devices.
In this work, LbL films prepared from
polyethylenimine (PEI) and GO, were prepared and
characterized in order to optimize its stability on
different pHs.
2 MATERIALS AND METHODS
Thin films were prepared by the LbL technique
(Oliveira et al., 2001) using the polyelectrolyte
polyethyleneimine (PEI) and graphene oxide (GO).
These compounds were acquired from Sigma-Aldrich
(St Louis, MO, USA). These films were adsorbed
onto quartz supports by adsorbing alternate layers of
PEI and GO at solid/liquid interface. PEI aqueous
solutions with a monomeric concentration of 2x10
-2
M and GO solution with a concentration of 2mg/mL
were prepared by diluting these compounds in ultra-
pure water, produced with a Millipore system
(Bedford, MA, USA). The adsorption times for each
layer were of 5, 15 and 30 s, and after adsorption of
192
Xavier, C., Zagalo, P., Ribeiro, P. and Raposo, M.
Optimization of Graphene Oxide Layer-by-Layer Films to Be Used as an Enhancer Coating of Optical Fibers Sensors.
DOI: 10.5220/0009380701920195
In Proceedings of the 8th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2020), pages 192-195
ISBN: 978-989-758-401-5; ISSN: 2184-4364
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
each layer the solid support was washed with
ultrapure water to remove the molecules which are
not completely adsorbed on the adsorbed layer. After
the adsorption of each layer, the thin-film was dried
using a flux of nitrogen gas. Films of PEI/GO were
prepared with different number of bilayers. To
analyse the effect of pH of PEI/GO LbL films
prepared with 12 bilayers, (PEI/GO)
12
were
immersed in aqueous solutions with different pHs to
characterize the desorption as a function of time. The
adsorbed/desorbed amounts were characterized by
ultra-violet spectroscopy using a double beam
spectrophotometer UV-2101PC (Shimadzu).
3 RESULTS AND DISCUSSION
3.1 Buildup of PEI/GO LbL Films
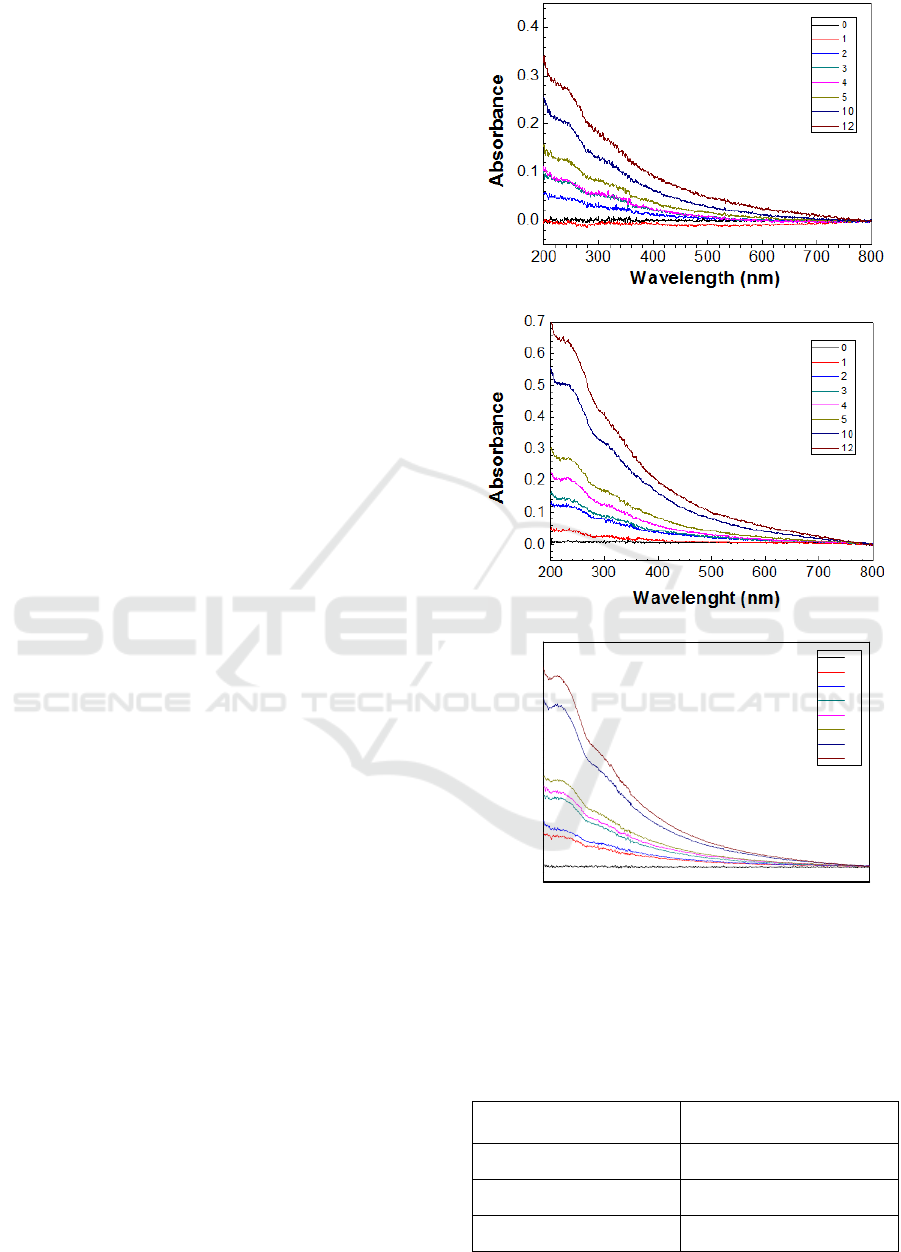
Figures 1 a), b), and c) show the obtained UV-Vis
spectra of PEI/GO LbL films, prepared with 5, 15 and
30s adsorption times.
The obtained spectra bands shown in figure 1 are
essentially associated to the electronic transitions of
GO, namely, the peak located at 230 nm is associated
with π-π* transitions of the aromatic ring (phenol)
and to n-π* transition of the group carboxylic acid.
The maximum absorption band located at 247 nm can
be associated with transitions of π-π* type of the
benzene aromatic ring. The band at 299 nm
correspond to n-π* transitions of the carbonyl group
(Silverstein et al, 1991). These curves also reveal that
the PEI/GO layers grow linearly with the number of
bilayers as demonstrated in graph of Figure 2 where
the absorbance at 230 nm is plotted versus the number
of bilayers. The slopes of obtained from these curves,
displayed in table 1, reveal that the adsorbed amount
per unit of area and per bilayer increases with
adsorption time up to a constant value. This indicates
that the adsorption kinetics curve of GO onto PEI
layer at lower adsorption times presents a first short
characteristic time, in accordance with what is
normally observed in polyelectrolyte adsorption,
followed of a second adsorption process associated to
diffusion process (Raposo, 1997; Ferreira, 2013), as
observed in the adsorption of GO onto optical fibers
measured by reflectance (Monteiro, 2019).
The decrease of adsorption time also lead to
increased films the uniformity as observed by optical
microscopy, data not shown here, result which is
crucial for optical applications.
a)
b)
c)
Figure 1: Absorbance spectra of PEI/GO LbL films with
different number of bilayers prepared with: a) 5s, b) 15 and
c) 30s of adsorption of each layer.
Table 1: Calculated values of absorbance at 230 nm per
bilayer, i.e, the slope of data of figure 2.
Adsorption Time per
Layer (s)
Absorbance @ 230 nm per
Bilaye
r
5
0.0226 ±0.0009
15
0.0510 ±0.0008
30
0.052 ±0.002
200 300 400 500 600 700 800
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
Absorbance
Wavelength (nm)
0
1
2
3
4
5
10
12
Optimization of Graphene Oxide Layer-by-Layer Films to Be Used as an Enhancer Coating of Optical Fibers Sensors
193
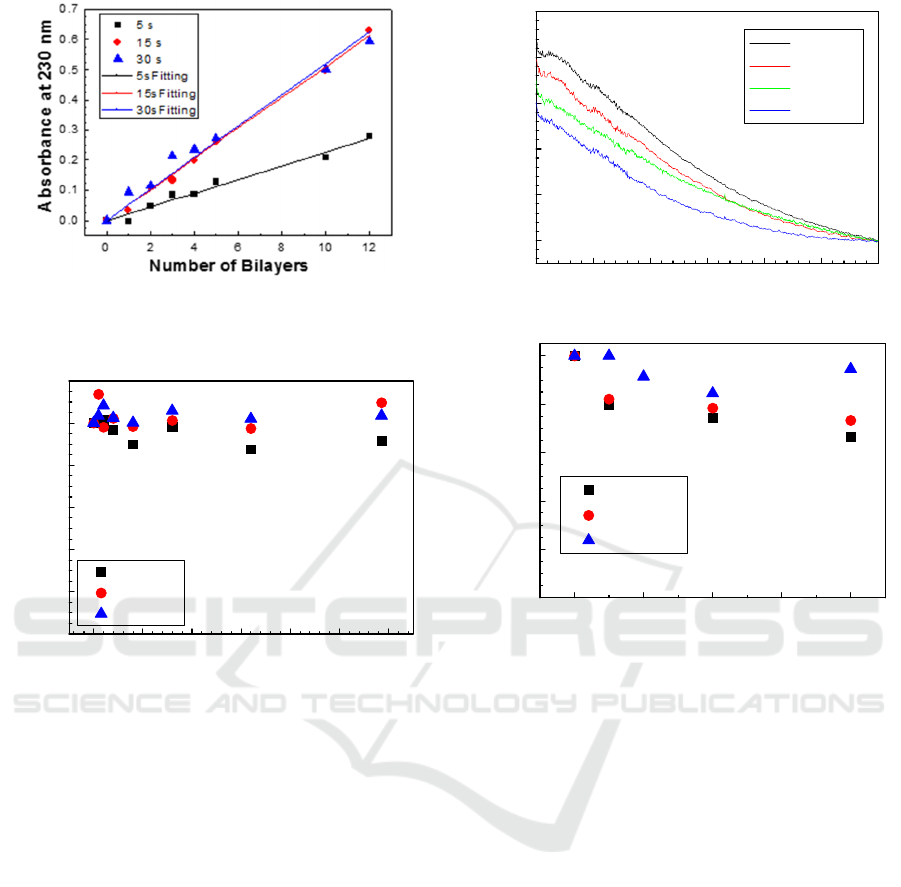
Figure 2: Absorbance at 230 nm as a function of the number
of bilayers of PEI/GO LbL films prepared at different
adsorption period of time, namely, 5, 15 and 30 s.
Figure 3: Evolution of the normalized absorbance at 230 nm
of the (PEI/GO)
12
LbL films prepared with an adsorption
time of each layer 30s as a function of immersion in
aqueous solutions with different pHs.
3.2 GO Desorption Kinetics
To analyse the stability of the PEI/PSS LbL films,
films with 12 bilayers were immersed in aqueous
solutions with different pH, namely, 5.5, 7 and 9, and
the UV –visible spectra were measured after different
immersion times. The obtained desorption kinetics
obtained for films prepared with an adsorption period
of time of 30s are shown in Figure 3. These
adsorption kinetics were obtained by plotting the
absorbance at 230 nm after normalization with
respect to the value of this absorbance before
immersing the films in the aqueous solutions a
different pHs. Similar curves are obtained for short
adsorption times. Although the obtained curves reveal
that pH does not have a strong effect in the GO
desorption, when films are prepared with short
adsorption times, a small desorption takes place at
smaller pHs.
a)
b)
Figure 4: a) Absorbance spectra of a (PEI/GO)
12
LbL film
prepared with 60s of adsorption of each layer after to be
immersed in an pH=7 aqueous solution. b) Evolution of the
normalized absorbance at 230 nm of the (PEI/GO)
12
LbL
films prepared with an adsorption time of each layer 60s as
a function of immersion in aqueous solutions with different
pHs.
However, films prepared with higher adsorption
times revealed to desorb in a large amount as
demonstrated in Figure 4 a) in which the normalized
absorbance of a (PEI/GO)
12
LbL film, prepared with
60s of adsorption of each layer after to be immersed
in an pH=7 aqueous solution during several periods
of time is seen to decrease. In fact a similar trend but
more intense to that observed in desorption kinetics
of Figure 3, is attained, when the normalized
absorbance at 230 nm of the (PEI/GO)
12
LbL films,
prepared with 60s adsorption time of each layer, are
plotted as a function of immersion time in aqueous
solutions with different pHs. This result demonstrates
that the molecules which are strongly adsorbed by
ionic interactions are less affected than the ones
adsorbed as a result of the process.
0 50 100 150 200 250 300
0.0
0.2
0.4
0.6
0.8
1.0
1.2
pH=5.5
pH=7
pH=9
Abs. Normalized
Desorption Time (min)
200 300 400 500 600 700 800
0.0
0.1
0.2
0.3
0.4
0.5
5 min
20 min
160 min
300 min
Absorbance
Wavelenght (nm)
0 10203040
0.0
0.2
0.4
0.6
0.8
1.0
pH=5.5
ph=7
ph=9
Normalized Absorbance
Desorption Time (min)
PHOTOPTICS 2020 - 8th International Conference on Photonics, Optics and Laser Technology
194

4 CONCLUSIONS
This study allowed to conclude that the adsorption of
a GO layer on PEI/GO LbL films follows the general
two stages adsorption processes found
polyelectrolytes. The first adsorption stage takes
place within the first seconds, the adsorption is
dominated by the adsorption of molecules which are
bound to the last layer of polyelectrolyte by ionic
interactions. At a given adsorption time these
adsorbed molecules somehow prevent more
molecules to be adsorbed and for larger adsorption
times, the presence of counterions and diffusion
process enable that more GO molecules to be
adsorbed. As a results these last molecules are not so
strongly bound to the last polyelectrolyte layer and
can be removed more easily by desorption. Therefore
the layers with smaller adsorption times are more
stable than for higher adsorption times. This study
also revealed that these films are more stable at higher
pHs allowing choose the adequate solutions for
sensor applications where these LbL films are stable.
ACKNOWLEDGEMENTS
The authors acknowledge the financial support from
FEDER, through Programa Operacional Factores de
Competitividade COMPETE and Fundação to
Fundação para a Ciência e Tecnologia, Portugal,
through projects “Development of Nanostrutures for
Detection of Triclosan Traces on Aquatic
Environments” (PTDC/FIS-NAN/0909/2014) and
the UID/FIS/00068/2019. PM Zagalo acknowledges
to Fundação para a Ciência e a Tecnologia for his PhD
fellowship (PD/BD/142768/2018) from RABBIT
Doctoral Programme.
REFERENCES
Costa, H.F.C. 2018. Desenvolvimento de dispositivos
sensores de fibras ópticas revestidas de filmes finos de
óxido de grafeno, Master Degree Thesis, Faculdade de
Ciências e Tecnologia, Universidade Nova de Lisboa,
Portugal.
Ferreira, Q., Ribeiro, P.A., Raposo, M. 2013.Villain's
fractal growth of poly[1-[4-(3-carboxy-4-
hydroxyphenylazo) benzenesulfonamido]-1,2-
ethanediyl, sodium salt] J-aggregates onto layer-by-
layer films and its effect on film absorbance spectrum
Journal of Applied Physics, 113, 243508.
Liu, S.; Yang, K.; Wang, Y.; Qu, J.; Liao, C.; He, J.; Li, Z.;
Yin, G.; Sun, B.; Zhou, J.; Wang, G.; Tang, J.; Zhao, J.
2015. High-sensitivity strain sensor based on in-fiber
rectangular air bubble. Sci. Rep., 5, 7624.
Magro, C., 2019. Advances in applied electrokinetics:
Treatment, by-productics reuse and sensors´system,
PhD Thesis, Faculdade de Ciências e Tecnologia,
Universidade Nova de Lisboa, Portugal.
Magro, C., Mateus, E., Raposo,M., Ribeiro, A. B, 2019
Overview of electronic tongue sensing in
environmental aqueous matrices: Potential for
monitoring emerging organic contaminants”,
Environmental Reviews, 27(2),202-214
Magro, C., Zagalo, P.M., Pereira-da-Silva, J., Mateus, E.P.,
Ribeiro, A.B., Ribeiro, P.A., Raposo, M. 2019b.
Triclosan detection in aqueous environmental matrices
by thin-films sensors: impedantiometric electronic
tongue. Proceedings 2019, 15, 24, 2019, https://doi.org/
10.3390/proceedings2019015024 .
Marques, I., Magalhães-Mota, G., Pires, F., Sério, S.,
Ribeiro, P.A., Raposo, M. 2017. Detection of Traces of
Triclosan in Water”, Applied Surface Science, 421,
142–147.
Monteiro, C. S., Raposo,M., Ribeiro, P.A., Silva, S.,
Frazão, O. 2019. Graphene oxide as a tunable platform
for microsphere-based optical fiber sensors, Proc. SPIE
11207, Fourth International Conference on
Applications of Optics and Photonics, 112070X;
https://doi.org/10.1117/12.2527267.
Monteiro, C.; Silva, S.; Frazao, O. 2017. Hollow
Microsphere Fabry–Perot Cavity for Sensing
Applications. IEEE Photonics Technol. Lett. 29, 1229–
1232
Novais, S., Ferreira, M. S., Pinto, J. L. 2017. Lateral Load
Sensing with an Optical Fiber Inline Microcavity. IEEE
Photonics Technol. Lett., 29, 1502–1505.
Oliveira, O.N., Raposo, M., Dhanabalan, A., 2001. In
Langmuir-Blodgett (LB) and Self-assembled (SA)
polymeric films, in: Nalwa, H.S.B.T.-H. of S. and I. of
M. (Ed.), Handbook of Surfaces and Interfaces of
Materials. Academic Press, Burlington, pp. 1–63.
Raposo, M., Pontes, R. S., Mattoso L. H. C., Oliveira Jr., O.
N. 1997. Kinetics of Adsorption of Poly (o-
methoxyaniline) Self-assembled films,
Macromolecules, 30, 6095-6101.
Silverstein, R. M., Bassler, G. C. & Morrill, T. C.
1991.Spectrometric Identification of Organic
Compounds.
Xu, M. G., Dakin, J. P. 1993. Novel hollow-glass
microsphere sensor for monitoring high hydrostatic
pressure. In Fiber Optic and Laser Sensors X, Vol.
1795, pp. 2–7.
Zagalo, P.M., Magro, C., Pereira-da-Silva, J., Bouchikhi,
B., el Bari, N., Ribeiro, P.A., Raposo, M. (2019).
Detection of Triclosan in tuned solutions by pH and
ionic strength using PAH/PAZO thin films.
Proceedings 2019, 15, 25, https://doi.org/10.3390/
proceedings2019015025 .
Optimization of Graphene Oxide Layer-by-Layer Films to Be Used as an Enhancer Coating of Optical Fibers Sensors
195
