
End-user Need based Creation of a Medical Device: An Experience of
Co-design to Struggle Pathological Scars
Thomas Lihoreau
1
, Brice Chatelain
2
, Gwenaël Rolin
1,3
, Chrystelle Vidal
1
, Nadia Butterlin
4
,
Emmanuelle Jacquet
5
, Aflah Elouneg
5
, Jérôme Chambert
5
, Xavier Bertrand
6
, Christophe Meyer
2,7
and Aurélien Louvrier
2,3
1
CHU Besançon, INSERM CIC 1431, Centre d'Investigation Clinique, Besançon, France
2
CHU Besançon, Department of Maxillo-Facial Surgery, Besançon, France
3
Univ. Bourgogne Franche-Comté, INSERM, EFS BFC, UMR1098, Besançon, France
4
Univ. Bourgogne Franche-Comté, Institut Supérieur d'Ingénieurs de Franche-Comté ISIFC, Besançon, France
5
Univ. Bourgogne Franche-Comté, FEMTO-ST Department of Applied Mechanics,
CNRS/UFC/ENSMM/UTBM, Besançon, France
6
CHU Besançon, Hygiène Hospitalière, UMR 6249 Chrono-Environnement, Université de Bourgogne-Franche-Comté,
Besançon, France
7
Univ. Bourgogne Franche-Comté, Nanomedicine Lab EA 4662, Besançon, France
Keywords: User-need, Keloid Scars, Medical Device, Innovation Cycle, Clinical Investigation.
Abstract: Scar is a common visible mark of human tissue healing. Sometimes pathological phenomena lead to abnormal
hypertrophic or keloid scars, with evolutions varying depending on different conditions: origin of the tissue
barrier disruption, concerned body area, or ethnic origin. Based on these statements, care procedures have
been developed to avoid aesthetical or functional impairments: drugs injection, surgery, cryotherapy or
mechanical compression. The story will relate the matching of a multi-disciplinary team that focused on
covering an unmet need for ear lobe keloid treatment, providing patients an optimal and holistic care. The
benefits researched lied in improving the understanding of the disorder, avoiding the recidivism of the scars,
diminishing the frequency and duration of care, and in end improving patients’ quality of life. The paper will
not only narrate the building of a health innovation, on technological, clinical, user points of view, but will
also try to detail the evaluations planned at the different stages of development, as well as the challenges,
conditions and prerequisites allowing to produce concrete solution.
1 INTRODUCTION: THE
MEDICAL PROBLEM
The keloid scar is defined as a pathology of tissue
healing resulting from a proliferation of fibrous
tissues that extend beyond the limits of the initial
wound (Butler et al., 2008). This pathology, described
as “pseudo-cancerous”, does not put the patient's vital
prognosis into threat, but could constitute a severe
aesthetic disturbance in addition to inducing serious
functional problems, pain and itching, seriously
impacting the quality of life of the patients, especially
for scars on visible areas of the skin. Available
epidemiological data indicate an incidence that can be
very high (16%) in subjects with ethnic skin (Bayat et
al., 2003).
The management of this pathology by the sur-
geons is difficult and seems randomly addressed.
Indeed, different treatments are proposed, ranging
from the injection of corticosteroids, to cryotherapy
and the administration of anticancer molecules (Ud-
Din et al., 2013). At present, no treatment, or
combination of treatments, have been described as
effective. The main classical management remaining
intra keloid resection, it too often leads to a more
serious recurrence of keloid in 45 to 100% of cases
(Andrews et al., 2016).
The lack of standard and effective treatment is
mainly due to the poor understanding of the cellular
and tissue mechanisms involved in the appearance
and evolution of keloids. For many years homemade
compression techniques have been described in the
literature to prevent recurrence of keloids after
surgery. Especially the handiworks were dedicated to
the ear, area of frequent occurrence of this type of
Lihoreau, T., Chatelain, B., Rolin, G., Vidal, C., Butterlin, N., Jacquet, E., Elouneg, A., Chambert, J., Bertrand, X., Meyer, C. and Louvrier, A.
End-user Need based Creation of a Medical Device: An Experience of Co-design to Struggle Pathological Scars.
DOI: 10.5220/0009369403170322
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 1: BIODEVICES, pages 317-322
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
317

pathology, often developed after piercing (Brent et
al., 1978, Vachiramon et al., 2004, Chang et al., 2005,
Yigit et al. Coll., 2009; Park and Chang, 2013;
Tanaydin et al., 2016). The effectiveness of these
means of compression relies on the reduction of the
post-surgical relapse of the earlobe keloid, observed
to a range of 10 to 30% (Vachiramon et al., 2004, Park
and Chang, 2013, Tanaydin et al. 2016).
Even if these works have been presented, there is
no current consensus or shared “gold standard”
practice for the treatment of the ear, particularly its
compression lobe. One of the causes being the lack of
solid clinical trials on the subject (Louis and Gracia,
2010). We proposed then a work assembling from the
beginning different experts around the development
of a quite unpretentious medical device, which
materialize in fact the center of complex
considerations.
2 THE ADVENTURE OF
EMERGENCE OF THE IDEA
In 2014, a surgeon from our university hospital,
contacted the clinical investigation research center for
a need related to his clinical practice, in fact the
medical problem announced in part 1 of this abstract.
His difficulty concerned then the reccurrences of
keloid scars on an important proportion of his
patients, which he yet treated consciensioulsy with
intralesional resection plus corticosteroids -
triamcinolone acetonide injection.
Meeting the research engineers, he explained his
needs in a system to add to the current care, relatively
to the litterature arguments in favor of a compression
of these specific tissues on one point, and to the
existing proposed solutions on another point.
At the beginning the deal seemed to be fairly
simple:
the possibility of adjustment of the pressure by the
patient himself (within a limit of the maximum
number of magnets imposed by the clinician) would
favorize the observance of the device and its comfort.
For the few existing studies on the subject, correlation
between keloid recurrence of the ear and discomfort
in wearing a device has been proved to be correlated
(Tanaydin et al, 2016), which may be related to poor
adherence to the application of pressure procedure.
Following works in collaborations with other
clinicians (to confort the shared property of the
expressed need), engineering and business local
schools (bibliographic, research & development,
clinical, and market analysis successive training
periods), as well as with engineering research center,
permitted to formalize a state of the art, and the first
drafts of the value analysis and specifications of the
innovation, in terms of ergonomy, adaptability, cost,
aesthetic...
The collaborations led then to the design of a
product as well as evaluations all along the
progression.
3 TECHNOLOGICAL
DEVELOPMENTS: THE “SCAR
WARS” PROJECT
Based on brainstorming and on the kind of “Santa
letter-writing” desires from the clinicians, but also
from the specific anatomical area, and from the
technical constraints, the prototypes were first
computer-aided designed (Figure 1 left) to format,
modelize the idea and project the skateholders into a
first view of the possible object.
From that, discussions led - beginning of 2015 -
to adaptations before an agreement on the general
shape and on primary dimensioning options.
The next step consisted in a 3D printing thanks to
stereolithography: few samples of different sizes
where produces, manipulated and confronted to the
ear lobes of healthy volunteers (from the
team…Figure 1 right).
It permitted to define then the size, but also to
determine the fact that our idea would need to be
constitued of a clip on which magnets could be easily
inserted. We had our proof of concept definition
prototype.
Figure 1: Digital and first physical version of the clip.
At the same time, bench lab tests on magnets
figured out their sizing - and in fact the possible
applied strengths.
A support associated with a dynamometer system
measured the forces in work with different magnets
and depending of the distance between.
According to the results, for a coherent lobe
thickness plus a pressure to be applied (from the
litterature) of 25 to 35 mmHg, we defined that we
ClinMed 2020 - Special Session on Designing Future Health Innovations as Needed
318

would need 2 to 8 neodymium-iron-bore magnets
with nickel coating magnets (1mm thickness,
diameter 12mm) to be placed on ear lobe.
We needed then to securize the product before
thinking of a first use in human. The contact with an
industry allowed to produce a mold from which the
first clips made with flexible medical grade silicone
were manufactured on February 2016 (Figure 2).
In terms of idea protection, an anteriority mark
tool was used in December 2016.
It was then the time to think about testing it on
targetted concerned patients with keloid scars.
Figure 2: The Scar Wars clip.
4 THE REAL LIFE TEST
4.1 Requirements
In order to provide a product that could be tested
during a clinical trial, the responsible manufacturer
need to follow regulatory requirements, centralized
by the european Medical Device Regulation MDR
2017/745 (repealing Council Directive 93/42/EEC).
Our ambition was to test a product which was not
yet CE marked. As a reminder, the CE mark is obtain
by a procedure in which notified bodies examine the
conformity of the product. On this particular situation
(without yet industrial part identified as a owner), our
hospital assumed the responsibility as a regulatory
manufacturer, for the clinical trial. Actually to obtain
the authority agreement to perform the clinical study,
we had to provide a file quite similar to the one that
would be presented for CE mark obtention.
Thus the team formalize a technical file, including
conception plans, laboratory tests, risk analysis,
essential requirements answers (list of all applicable
standards and the way we addressed it), user manual,
labelling and packaging.
This technical file (or “investigator brochure” on
a clinical trial language) aim to present the product
that will be tested and the security measures taken by
the manufacturer to ensure its safety use.
The medical device under study was then defined
as related to a Class I according to the requirements
of the EC Directive.
On another side, the clinical trial running (or
“design”) needed to be described in study documents,
the master ones being the study protocol, the
informed consent and case report forms. A specific
budget had also been searched and obtained to
finance the clinical project (hospital internal research
call).
4.2 Design of the Clinical Trial
The building of the study protocol was an important
phase of our project, and its writing needed to
mobilize all the partners. It helped to define the
objectives, the criteria of evaluations, the targetted
population (characteristics and number), the progress
in terms of duration… all this taking into account the
data already availables (in the litterature and thanks
to our previous advances), as well as the previous
realized tests and obtained results.
The main objective was defined as the evaluation
of the effectiveness and safety of the compressive
device; the main endpoint being then the reccurence
(yes/no) of the pathology. The study concerned 27
male and female patients (more than 18 years old)
presenting keloid lobe ear scars that needed to be
treated by reconstructive surgery; it excluded patients
with known allergy to nickel (even if the magnets are
not in direct contact, silicone clip making the
interface).
After usual management of the keloid scar of the
ear (reconstructive surgery and injection of
corticosteroids - triamcinolone acetonide), the
concerned patients had to wear the compressive
device and to adjust the compression with the
magnets provided. By consulting the literature, which
proposes that the patient wears his compression
device 8 to 24 hours a day (Louis and Garcia, 2010),
it was decided to recommend to the patient to apply a
compression allowing him to wear the device at least
12 hours a day, daily and throughout the duration of
study, ie one year. The compression must be
sufficient, without being painful. The clinician will
rely on these data, individually for each patient, to
dictate the maximum number of magnets to be used
based on the measured thickness of the patient's ear.
The clinician may reduce the frequency of use of
the device, or even stop it according to the evaluation
of the quality of healing during visits.
End-user Need based Creation of a Medical Device: An Experience of Co-design to Struggle Pathological Scars
319

The patients were planned to be seen at 3, 6 and 12
months after intervention, in the traditionnal course
of visits during the usual care (no modification due to
the trial).
The secondary objectives of the Scar Wars trial
focused on a multimodal and interdisciplinary
assessment of scar tissues by (Chambert et al., 2019):
- evaluation of patient acceptance and satisfaction,
evaluation by the surgeon (specific scars evaluation
scales, Draaijers et al., 2004, Deslauriers et al., 2009),
- biometric characterization of the area of interest,
- non-invasive imaging assessment of tissue
evolution,
- analysis of the bacterial flora present at the level of
the keloid scar,
- the creation of a keloid cell bank, basis of a
biological ancillar study allowing our biologists to
focus on pathological healing process and anti-
fibrotic drug evaluation.
4.3 Official Agreements
The “pilot study evaluating the effectiveness and
safety of a compressive device intended to prevent
recurrence of keloid scars after surgical resection” file
was submitted on February 2017 onto French
authorities, with a final positive agreement by ethical
committee and national agency for health products –
ANSM Agence nationale de sécurité du médicament
et des produits de santé – obtained in August 2017.
The trial was recorded on official web platform
ClinicalTrials.gov, and the first patient was included
in October 2017.
To date, 10 patients have been included, without
presenting a reccurence.
5 NEXT STEPS
The enrolment of the last patients and results of the
study will feed the CE mark file, which is then already
initiated.
Apart for the CE mark class I obtention, the next
important stage will be to build the business model
and development associated to an official regulatory
manufacturer that will handle the responsibilities and
assure the distribution.
Concerning the material, the perspectives could
lie on the development of different sizes of the clip
and magnets, in order to fit as much as possible to
different morphologies, or even other area on ear or
even face. 3D printing technologies offer also
prefigure tailor-made medical devices.
Functionalization with specific drugs or molecules
could be the future of such innovations.
In projection, next evaluations could focus for
sure on safety aspects once devices will be on the
market and largely diffused (material vigilance), and
on aggregation and reinforcement of clinical
evidences of the innovation. Medico-economics
studies will aim to test and possibly prove advantages
of the invention relatively to the current costs for
patients, hospital, society.
6 DISCUSSION
Based on our experience of ideation from a clinical
uncovered need, formalization of an innovation,
development and testing, we would like to share
interesting points that guided us and seems conditions
of success for bringing innovation in care and in
medical devices field, which guid by definition to
complex projects.
6.1 Guiding Principles
Team effort was a key in our pathway to a concrete
solution: clinic, clinical research, technology,
regulations, ethics, usability, market / business
strategy, intellectual protection, project
management… are skills hardly or not often grouped
in the context of an hospital, or of medical devices
field which is oftenly represented by small medium
enterprise.
Contacts need to be actively researched outside,
and realized with experts motivated to answer the
questions and develop the specific project with
anticipation and relevancy. Else the innovation risks
to encounter the “death valley” located between
research and real life.
Related to that, the time and money are important
to anticipate. As we speak about little team, we can
imagine the consequences of timelines like the ones
we presented here, onto the survival of the start-up if
not planned with a strong and realistic vision.
6.2 Some Tools?
Developing innovation in health is a field on which
theory and models exist. Well known scales such as
Technology Readiness Level (TRL, Scar Wars being
today at a TRL 6-7), declined in Market RL, Financial
RL… can provide accurate marks for emergence,
development, maturity.
We can also refer to more dedicated ones to health
such as CREPS cycle (Concept, Research,
ClinMed 2020 - Special Session on Designing Future Health Innovations as Needed
320
Evaluation, Product, Care, Moreau-Gaudry A et al.
2010), Innovation RL, health tech innovation cycles
(Center for Integration of Medicine and Innovative
Technology - CIMIT, Boston) that take into
consideration the dimensions of technology,
regulatories, market, clinics…
Projects have also been provided on the subject,
let’s cite for example the European Itech “Roadmap
for Research and Innovation in Health Technology”
(FP7-HEALTH-2013-INNOVATION-1, CSA-SA –
https://cordis.europa.eu/project/id/602667/fr), that
describe 5 phases from need to industrialization,
leading to 5 outcomes from proof of concept (POC)
to reimbursment and commercialization.
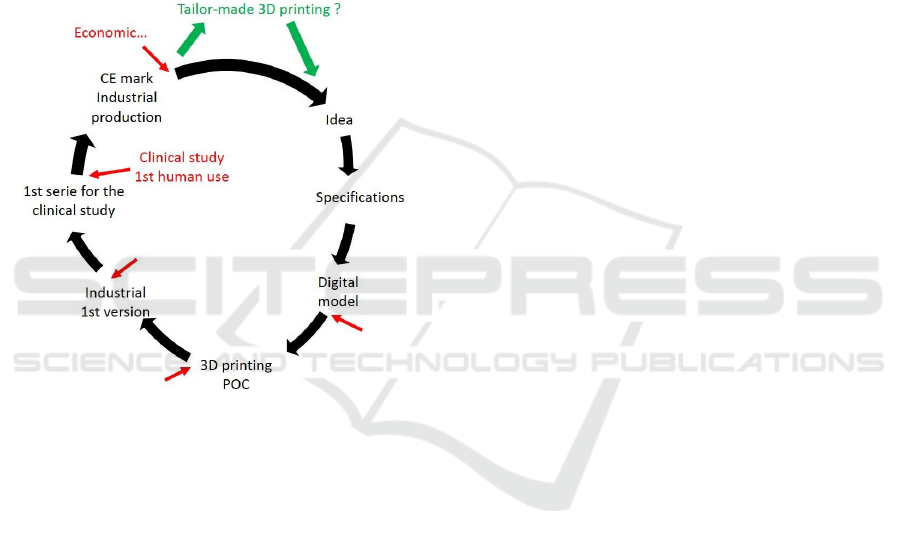
Figure 3: Scar Wars cycle of development.
The idea is to try using the scales/tools to define the
project roadmap from the beginning, to anticipate the
stages, duration, money needed, and to evaluate its
progress regularly.
We proposed on Figure 3 the cycle of
development of our innovation, with 7 steps from idea
to industrialization, evaluations indicated in red, and
perspectives in green.
7 CONCLUSION
The aim of the SCAR WARS project was to evaluate
the effectiveness and safety of an innovation in the
treatment of specific pathology on a targeted body
location.
The device is proposed in addition to the usual
care of patients seen at surgery departments, and will
provide, at the level of the lesion, a controlled
physical compression, reported in the literature as
being a determining factor for the reduction of the
volume of the scars and the rate of recurrence after
reconstructive surgery.
The conclusions of the work carried out during the
project will make it possible to lay solid foundations
for the valorization of the device. Above all, the
original and multimodal approach of evaluation could
help identify new areas of improvement in the
pathology management, and provide to the
community new scientific data for a better
understanding of these scars, and possible successes
or failures of proposed treatments.
This first study will quickly provide the patient
with an inexpensive device, with targeted properties
of aesthetic, comfort and adjustability by the patient
himself (within the limit of the maximum number of
magnets imposed by the clinician). Those
characteristics are hoped to be source of better
compliance and therefore efficiency. The expected
decrease in recidivism rates could result in a
reduction in public health costs for resumption of
resection, which could be evaluated with specific
methodologies late. Adaptation closer to the
morphology of the patient, or to other areas of the
body by 3D printing can then constitute a potential
opening of this project.
In terms of a more global approach and for an
ambitious project, we tried to enhance the key
important guidelines; general schedule of the
pathway of innovation could be resumed by:
-writing of the project and the general objective: state
of the art, context (points to improve), positioning,
with clinicians and experts in the field;
-describing the solution (s) to be developed, broken
down into several lots (technical, regulatory, tests
(pre- and clinical, pre- and post-market…), business
marketing, etc.), and players to be brought up at each
stage.
-formalizing a consortium accordingly: do we have
all the internal or external actors identified for the
different stages ?
-establishing the budget (and means of search and
obtention) accordingly.
One of the first deliverable of any project could be
this master roadmap document, adapted and
improved all along the life of the project.
REFERENCES
Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J
(2016) Keloids: The paradigm of skin fibrosis -
Pathomechanisms and treatment. Matrix Biol J Int Soc
Matrix Biol. 51:37-46.
End-user Need based Creation of a Medical Device: An Experience of Co-design to Struggle Pathological Scars
321

Bayat A, McGrouther DA, Ferguson MWJ (2003) Skin
scarring. BMJ 326:88-92.
Brent B (1978) The role of pressure therapy in management
of earlobe keloids: preliminary report of a controlled
study. Ann Plast Surg.1:579.
Butler PD, Longaker MT, Yang GP (2008) Current
Progress in Keloid Research and Treatment. J Am Coll
Surg 206:731-741.
Chambert J, Lihoreau T, Joly S, Chatelain B, Sandoz P,
Humbert P, Jacquet E, Rolin G (2019). Multimodal
investigation of a keloid scar by combining mechanical
tests in vivo with diverse imaging techniques. Journal
of the Mechanical Behavior of Biomedical Materials,
Volume 99, Pages 206-215.
Chang CH, Song JY, Park JH, Seo SW (2005) The efficacy
of magnetic disks for the treatment of earlobe
hypertrophic scar. Ann Plast Surg. 54:566-9.
Deslauriers V., Rouleau D.-M., et al. Translation of the
Patient Scar Assessment Scale (PSAS) to French with
cross-cultural adaptation, reliability evaluation and
validation. Can. J. Surg. 2009 52: 259-263.
Draaijers LL, RHF Tempelman, Botman AMY, et al. The
Patient and Observer Scar Assessment Scale: a reliable
and feasible tool for scar evaluation. Plast Reconstr
Surg 2004; 113: 1960-5; discussion 1966-7.
Louis DD, Garcia C (2010) Pressure earring as an adjunct
to surgical removal of earlobe keloids. Dermatol Surg.
36:726.
Moreau-Gaudry A, Pazart L (2010). Development of an
innovation in health: the cycle CREPS Concept,
Research, Evaluation, Product, Care. IRBM 31 (1) pp
12-21.
Park TH, Chang CH (2013) Early postoperative magnet
application combined with hydrocolloid dressing for
the treatment of earlobekeloids. Aesthetic Plast Surg.
37:439-44.
Tanaydin V, Beugels J, Piatkowski A, Colla C, van den
Kerckhove E, Hugenholtz GC, van der Hulst RR (2016)
Efficacy of custom-made pressure clips for ear keloid
treatment after surgical excision. J Plast Reconstr
Aesthet Surg.69:115-21.
Ud-Din S, Bayat A (2013) Strategic management of keloid
disease in ethnic skin: a structured approach supported
by the emerging literature. Br J Dermatol. 169 Suppl
3:71-81.
Vachiramon A, Bamber MA (2004) A U-loop pressure clip
for earlobekeloid. J Prosthet Dent.92:389e91.
Yigit B, Yazar M, Alyanak A, Guven E (2009) A custom-
made silicon mold for pressure therapy to ear
keloids.Aesthetic Plast Surg. 33:849-51.
ClinMed 2020 - Special Session on Designing Future Health Innovations as Needed
322
