
3D Printing and 3D Virtual Models for Surgical and Percutaneous
Planning of Congenital Heart Diseases
Katia Capellini
1,2
, Paolo Tripicchio
5 a
, Emanuele Vignali
1,2
, Emanuele Gasparotti
1,2
,
Lamia Ait Ali
3 b
, Massimiliano Cantinotti
4 c
, Duccio Federici
4
, Giuseppe Santoro
4
,
Francesca Alfonzetti
5
, Chiara Evangelista
5
, Camilla Tanca
5
and Simona Celi
1 d
1
BioCardioLab, Bioengineering Unit, Fondazione Toscana ”G. Monasterio”, Massa, Italy
2
Department of Information Engineering, University of Pisa, Pisa, italy
3
Institute of Clinical Physiology, CNR-Regione Toscana, Massa, Italy
4
Paediatric Cardiology Unit, Fondazione Toscana ”G. Monasterio”, Massa, Italy
5
Perceptual Robotics Lab, TeCIP Institute, Scuola Superiore Sant’Anna, Pisa, Italy
Keywords:
Virtual Model, Virtual Reality, 3D Printing, Complex Congenital Heart Diseases.
Abstract:
Despite increasing evidence of their utility, 3D models have never been extensively tested so far in pediatric
cardiac surgery planning. 3D models may offer advantages over traditional imaging examinations: 1) a deeper
understanding of 3D anatomy in complex defects allowing visual and tactile inspection from any point of view,
2) the possibility to interact with a tangible replica of the real organs, 3) the surgical planning and simulation
maneuvers on the printed and virtual model, and 4) interaction with anatomical structures thank to Virtual
Reality technologies. The work aims to test and compare the accuracy and the incremental diagnostic value of
3D printed and virtual models in patients undergoing cardiac surgery for CHDs.
1 INTRODUCTION
In the last years, the interest in 3D printed models
is increased in numerous medical fields (Vukicevic
et al., 2017) both for the operative planning of dif-
ferent surgical approaches and the development of
custom devices based on the patient-specific cases
(Kurenov et al., 2015)(Sun et al., ). Congenital heart
diseases (CHDs) are an ideal field to test the poten-
tialities, accuracy, reproducibility and clinical effec-
tiveness of 3D technologies due to the complexity
and diversity of cases, the need for a complete repre-
sentation of intra/extra-cardiac anatomy, and of per-
sonalized interventional approaches and size materi-
als (Cantinotti et al., 2017). In complex CHDs the
understanding of the 3D spatial relationship in an un-
usual anatomical arrangement is certainly a major dif-
ficulty. Currently medical imaging is able to provide
a
https://orcid.org/0000-0003-3225-2782
b
https://orcid.org/0000-0003-1672-5308
c
https://orcid.org/0000-0002-4671-9606
d
https://orcid.org/0000-0002-7832-0122
functional and anatomical detailes with hight resolu-
tion and accuracy (Burchill et al., 2017) (Greil et al.,
2017) (Celi et al., 2017) and are used as starting point
for several advanced studies based on integration with
numerical models (Celi and Berti, 2013) (Celi et al.,
2013) (Capellini et al., 2018). Despite all these ad-
vances in the research field, currently, for the rou-
tinely diagnosis of CHDs there is a strong need to in-
troduce interactive tools in clinical practise. In fact,
all current 3D imaging modalities are not interactive
and don’t allow to manipulate the 3D image or the
projected images. The standard volume rendering ap-
plications included in the image processing worksta-
tions are not able to provide tangible surfaces and
edges; it refers to a technique for generating a visual
representation of data that is contained in a three di-
mensional space. Even if volume rendering is an im-
portant graphics and visualization technique and sev-
eral techniques and algorithms have been developed
to provide high quality visualization (El Seoud and
Mady, 2019), this approach is not able to produce
a mathematical model useful for additional clinical
evaluation and planning. Reconstructed 3D models
Capellini, K., Tripicchio, P., Vignali, E., Gasparotti, E., Ali, L., Cantinotti, M., Federici, D., Santoro, G., Alfonzetti, F., Evangelista, C., Tanca, C. and Celi, S.
3D Printing and 3D Virtual Models for Surgical and Percutaneous Planning of Congenital Heart Diseases.
DOI: 10.5220/0009160702810287
In Proceedings of the 15th International Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications (VISIGRAPP 2020) - Volume 3: IVAPP, pages
281-287
ISBN: 978-989-758-402-2; ISSN: 2184-4321
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
281
can reproduce anatomical details with high accuracy
and permit both virtual and physical manipulation of
the model offering the advantage to simulate surgical
interventions in a real physical environment in terms
of spatial relationship with an adjunct tactile sense.
The key problems in complex CHDs are the small
and often the non conventional dimensions and spa-
tial relationships (Triedman and Newburger, 2016)
(Stout et al., 2019). With this in mind, in addition
to the physician’s knowledge, and his/her experience,
an important role is played by the spatial intelligence,
defined as “a capacity for mentally generating, ro-
tating, and transforming visual image” (Park et al.,
2010). This kind of intelligence is crucial to the ef-
fectiveness of CHD physicians (Gardner, 2008) and
is considered a fundamental element in medical ed-
ucation (Hegarty, 2014) and more in particular for
anatomy education of the cardiovascular system (Ku-
malasari et al., 2017). In this context, research results
(Sajid et al., 1990) have shown the benefits of three-
dimensional approaches for learning. Indeed, physi-
cal interaction with a 3D model allows to understand
the physical structures of the organs and obtain fa-
miliarity with them (Cooper and Taqueti, 2008). In
the study presented by (Maresky et al., 2018) stu-
dents who were exposed to VR demonstrated sig-
nificant improvement in their understanding of car-
diac anatomy. The learning advantage of VR tech-
nology has been proven useful also in the training of
minimally invasive surgical procedures (Konietschke
et al., 2010). Given its complex three-dimensional
structures, cardiac anatomy may be challenging to
grasp. In this context, VR technologies offer immer-
sive and intuitive experiences that allow appreciating
the size differences of such structures and at the same
time to contextualize their relationships. Several VR
applications in cardiovascular medicine education are
currently being explored, for instance, the one de-
veloped in the Stanford Virtual Heart Project (2017)
where such technology is used in the context of pedi-
atric patients’ parents’ education to allow them visu-
alizing their child’s congenital heart disease. These
kinds of applications contributed to the research in
cardiovascular intervention by assisting the physi-
cians in learning and interpretation of cardiovascular
anatomy and pathology, increasing the precision and
reducing the invasiveness of the interventions (Silva
et al., 2018). 3D models may provide to pediatric sur-
geon/interventionalist a direct visualization of com-
plex intra-extra-cardiac anatomy. Moreover, the com-
bined use of 3D virtual and printed models may help
to plan more accurate surgical/interventional strate-
gies and to choose materials of proper size (i.e. con-
duit, ballon, prosthesis, etc) (Moore et al., 2018). Im-
mersive bimanual exploration of virtual models will
complement the understanding and planning capabil-
ities of the printed model, without requiring complex
haptic devices. In a recent study, some advantages
of VR technique respect to the 3D printing approach
have been depicted (Ong et al., 2018), however, ac-
cording to our knowledge, there are no studies in the
literature that investigate the effectiveness of these
two approaches on the same populations of cases.
Furthermore, virtual models, combining immersive
visual and vibrotactile feedback, have been scarcely
tested for pediatric cardiac surgery (Izard et al., 2018),
and no specific comparison between the 3D virtual
model and 3D printed anatomies have been proposed.
This work aims to perform a comparison of the adop-
tion of 3D printed and 3D virtual models for complex
CHDs defects in terms of the satisfaction of physi-
cians. To extend our investigation, both surgical and
endovascular planning have been investigated.
2 MATERIALS AND METHODS
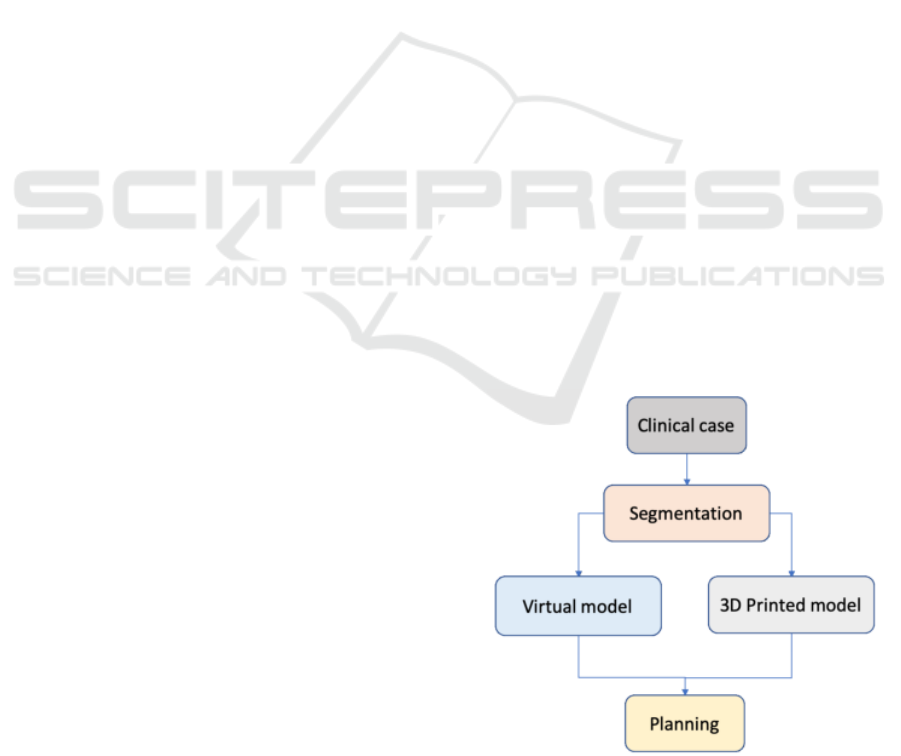
The workflow of this study is reported in Figure 1.
It starts from the segmentation of clinical images to
create the 3D model of cardiac structures affected by
CHDs. The 3D models are printed with different 3D
printing techniques and materials and used as virtual
models in a virtual environment and a VR platform.
Depending on the case, the clinical team investigates
the most suitable surgical or catheter-based procedure
on both the printed and virtual models. This workflow
is illustrated for two CHDs cases: aortic coarctation
(CoA) and heart with a complex CHD.
Figure 1: Diagram of the procedural phases of the presented
approach.
IVAPP 2020 - 11th International Conference on Information Visualization Theory and Applications
282

2.1 Image Processing
Twelve Magnetic Resonance (MR) and eight Com-
puted Tomography (CT) volumetric datasets of CHDs
patients scheduled for surgical repair were analyzed.
In order to obtain a 3D model of cardiac structures of
interest, segmentation was performed by adopting dif-
ferent techniques. Semi-automatic segmentation al-
gorithms such us threshold, active contours, and re-
gion growing algorithms were adopted when possible,
together with manual segmentation slice by slice for
more complex regions of interest.
After the segmentation phase, a process of mesh
refinement was necessary to obtain the final model
without imperfections and a constant thickness was
assigned. The resulting 3D shell models were both
printed and used in the virtual environment. Figure
2 depicts, as example, the three main phases of this
process in case of a MR dataset: the medical volume
rendering (Figure 2a), the segmentation process (Fig-
ure 2b) and the final 3D result (Figure 2c).
2.2 3D Printing
In this study, two different 3D printing strategies
were tested: the fused deposition modeling technique
(FDM) and the stereolithography technique (SLA). A
thermoplastic polyurethane material (TPU Elasto85)
with shore A85 (Standard, 2005) was used in the
FDM approach. A hydrosoluble support polymer
(SSU04) was used due to the presence of cavities in
the models. The models were printed with a layer
thickness of 0.25 mm on a 3ntr A4v4 3D printer. For
the SLA technique, a Form2 3D printer was adopted
with a clear elastic resin with shore A50 (Standard,
2005) and a layer thickness of 0.1 mm. The internal
and external supports were manually removed.
2.3 Virtual Technique
Given the fact that there is no necessity for the data
to be produced in real-time, the virtual techniques in-
volve the production of 3D models and textures that
could take several hours and days. One of the objec-
tives of the VR visualization presented here is instead
that of producing a textured 3D model that is possi-
ble to visualize as soon as possible thus introducing
a trade-off between the accuracy of the representation
and immediate availability of the model. For this rea-
son, the 3D model segmented from MR and CT scans
are passed to a pre-processing step where the mod-
els are optimized for visualization and the texture co-
ordinates for each vertex are generated. A prelimi-
nary texture, not reflecting the real organic texture but
with realistic rendering, is produced and applied on
the model on the fly. This allows us to reduce the
timing for the VR simulation to be used, and this is
extremely important giving the time at disposal for
pre-operation analysis. The VR interaction with the
generated 3D model will give the surgeons some tools
to manipulate the 3D scene. In particular, the user
can rotate the view to analyze the 3D structure at its
best. A virtual cutting feature has been implemented
to enable performing some cuts on the 3D model sur-
face to explore the internal cavities and plan possi-
ble surgical exploration of the models. A second im-
plemented tool allows using simple 3D shapes (like
cubes and sphere) to perform a real-time clipping on
the 3D model thus showing internal elements in a non
disruptive way. Examples of these two types of oper-
ation are shown in figure 3 and 4. The Unity engine
was used to develop both a desktop VR application
and an immersive VR visualization for the Oculus rift
Head Mounted Display (HMD).
2.4 Pre-operative Planning
Every case under exam has been discussed by a mul-
tidisciplinary team (cardiac surgeons, pediatric cardi-
ologists, anesthetists) in two different steps: firstly,
based on conventional imaging and, secondly, with
the support of the 3D printed and virtual models.
The planning strategies were compared and the added
value of the two additional methods was evaluated.
For this study, the same team has evaluated all the 3D
printed and virtual models.
3 RESULTS
The image segmentation was feasible for all datasets
and the corresponding 3D shell models were gener-
ated for all patients. For the subjects with a CT dataset
an automatic segmentation was practicable due to the
high spatial resolution. The segmentation of the MR
datasets was more complex to be performed. It is
worth to point out that the complexity of this process
was due to the presence of breath and motion arti-
facts and, in general, due to the absence of the contrast
medium.
Case of Coarctation - In Figure 5 an example of
planning for a percutaneous procedure for an artery
affected by CoA is reported. Starting from an MR
dataset (Figure 5a), the model was 3D printed with the
SLA technique and used by the clinician to simulate
the endovascular procedure during the pre-planning
phase.
3D Printing and 3D Virtual Models for Surgical and Percutaneous Planning of Congenital Heart Diseases
283
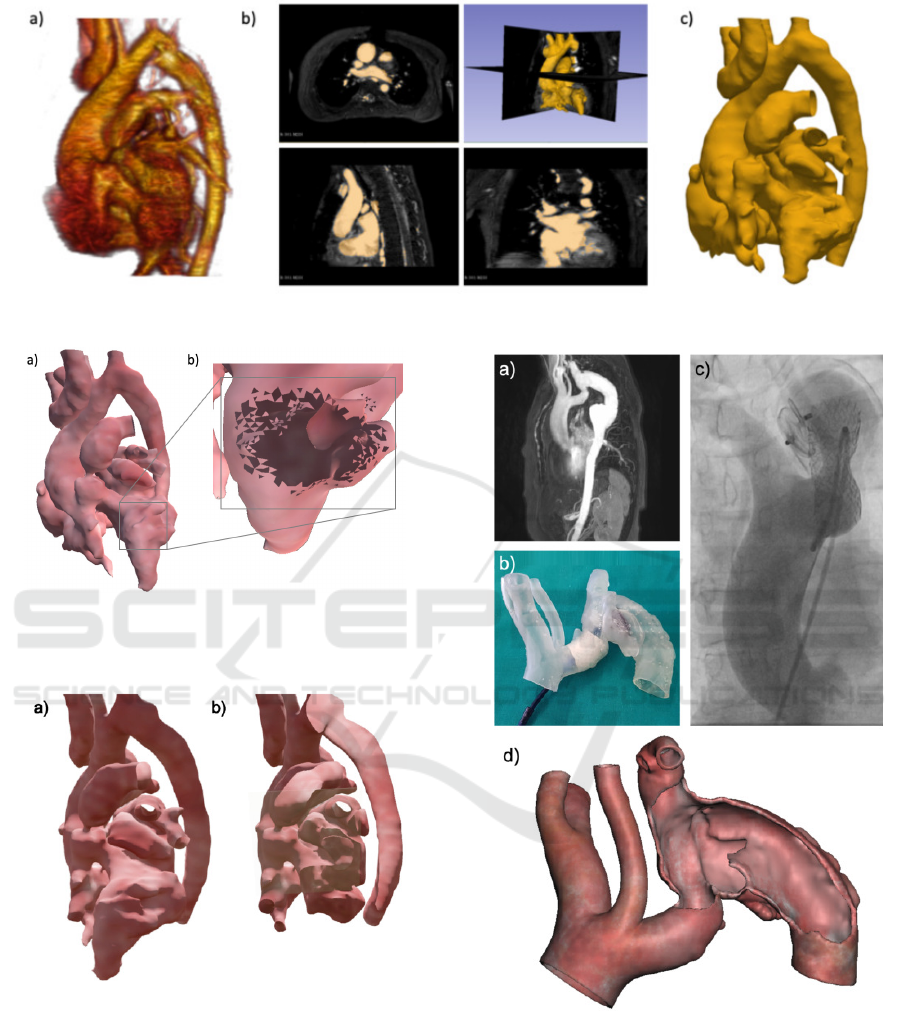
Figure 2: An example of standard volume rendering (a), images segmentation (b) and the final 3D model (c).
Figure 3: Rendering in VR of a virtual heart model with a
custom texture (a) and example of cut applied on the mesh
enabling the display of internal structures (b).
Figure 4: Virtual 3D model (a) and example of model ma-
nipulated through the use of a clipping plane (b).
Due to the complexity of this CHD, two differ-
ent devices were required (Figure 5b): one to occlude
a pseudo-aneurysm at the aortic arch level and one
to recover the lumen of the CoA. Figure 5c depicts
the intra-operative image according to the planning
procedure. In this case the 3D virtual model (Figure
5d) was used to take the measurements to define the
proper size of devices.
Figure 5: Case report of CoA from images to intervention:
MR image (a), simulation of endovascular treatment on 3D
printed model (b), angiographic image acquired during pro-
cedure (c) and 3D virtual model (d).
Case of Complex CHD - In Figure 6 the planning for
a heart CHD is reported. The surgeon tested the se-
lected plan on the 3D printed model by cutting in the
defined region from the same view that he would have
in the operating room and he explored the heart inside
IVAPP 2020 - 11th International Conference on Information Visualization Theory and Applications
284
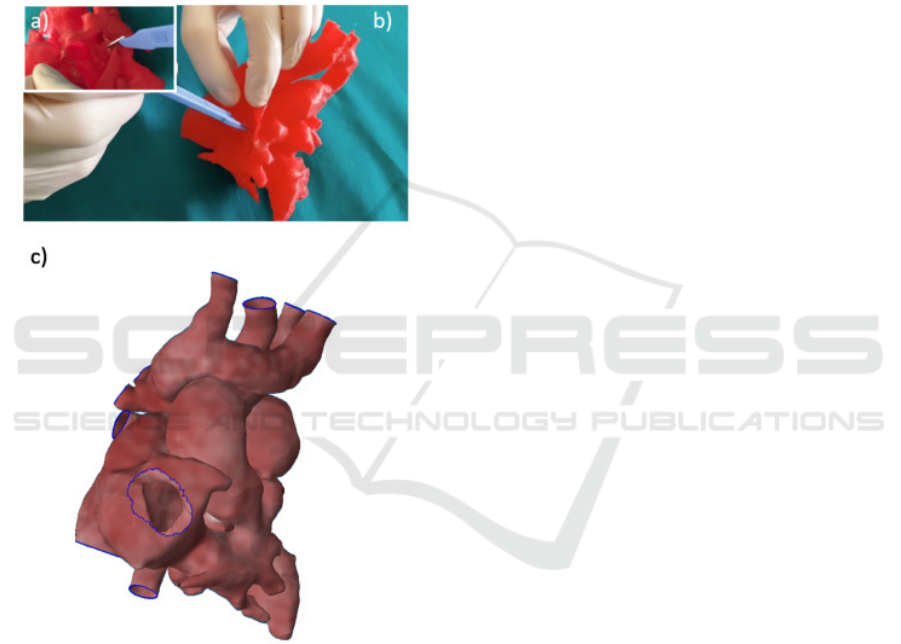
(Figure 6a-b) to better understand which parts could
be seen and reached thank to the possibility to inter-
act with the real patient dimensions. Also the possible
surgical strategies were investigated by implementing
a virtual planning (Figure 6c).
Regarding the 3D printing techniques, it has al-
ways been possible to obtain 3D models with high
accuracy by using the FDM approach. This technique
permitted to have 3D deformable heart models to sim-
ulate the operative incisions by the surgeons as re-
ported in Figure 6.
Figure 6: Surgical planninig on printed model (a-b) and on
3D virtual model (c).
The models printed with SLA were required in
case of device insertion and expansion (Figure 5).
In Figure 7 an example of VR application is re-
ported. A user can visualize the organic tissues in 3D
within an HMD, thus exploring the model in the first
person. This enables changing the viewpoint in a nat-
ural manner reproducing an immersive experience for
the practitioner. Special tools enable the user to visu-
alize both the surface properties and the internal cav-
ities, thanks to the virtual culling of the 3D mesh.
The preliminary results of clinicians’ feedback
showed that the use of the 3D model in a virtual en-
vironment is the better choice to investigate the eli-
gible CHDs repair strategies due to the possibility to
investigate more sites of access for the operation by
reversibly editing the model. The 3D printed models
allowed the in vitro surgical and percutaneous plan-
ning simulations in time with clinical timing.
4 CONCLUSIONS
Despite recent advances in current imaging tech-
niques for the diagnosis and management of CHDs,
several limitations in 3D visualization remain. The
3D printing technique and the deployment of a virtual
environment for the CHDs models improve the surgi-
cal and percutaneous planning for the pathology treat-
ment. 3D virtual models have proven to be a useful in-
strument to assist the clinical team and the surgeon in
the decision-making procedure for the patient-specific
best intervention strategy in the case of surgical ap-
proach.
The 3D printed model is used to test the feasibility
of the determined surgical technique by giving clini-
cians the appropriate level of confidence, also related
to the real dimensions of patient anatomic structures,
not reached with biomedical images visualization and
3D virtual models (Batteux et al., 2019).
Regarding the time costs, the segmentation pro-
cess affected all approaches and ranging from less
than an hour for CT datasets with contrast medium to
several hours for some MR datasets. The 3D virtual
simple model is available from the 3D shell model
without any additional times except for the texture
assignment, if required. The VR implementation al-
lowed us to navigate in an immersive context with
an intuitive manipulation of the 3D model. The in-
clusion of 3D model in a virtual reality environment
made possible the model rotation in the 3D space,
measurements on the model to get the real distance
and relationships between the different regions of the
heart or arterial models, the variation of model di-
mensions and the option to cut a specific portion of
surface model to analyze internal cavities in a non-
destructive manner. This last aspect is particularly
important due to several surgical cuts can be tested
on the same model. Even if a simple texture has been
adopted, this approach reveals an increase of confi-
dence from the clinical team with respect to a single
color model. This same comment has been reported
by clinician comparing the VR model with respect to
the 3D printed one. In fact, it is worth to point out that
the 3D printed model were in a single color with a low
range of color available from the 3D printer manufac-
3D Printing and 3D Virtual Models for Surgical and Percutaneous Planning of Congenital Heart Diseases
285

Figure 7: Example of VR application.
turer. Regarding the 3D printing approach, the time
cost for the 3D printing technique included the print-
ing time and the supports removal time and increases
with the model size and complexity ranging from ten
to twenty-five hours. The SLA technique presented
the main drawback of internal supports removal that
it resulted in impossible in the cases of the whole
heart model. The supports removal was feasible for
the artery models and in these cases, a simulation of
endovascular procedure for the CHDs treatment was
easily performed. 3D printed models with FDM was
more suitable for a surgical procedure, while the use
of the elastic resin turned out to be the most suitable
to simulate endovascular procedures involving device
expansion.
Moreover, the VR platform and 3D printing mod-
els seemed suitable for medical students’ education
thanks to the possibility to navigate inside the model
and better visualize CHDs (Figure 7). With specific
attention to the VR environment, usability assessment
of different interaction metaphors is the focus of fu-
ture work, testing different VR setups including a
classical monitor visualization, an immersive simula-
tion trough an HMD and the use of a tablet for navi-
gation and display of 3D virtual content.
ACKNOWLEDGEMENTS
This work is supported by the ”3D VIRTUAL BABY
HEART” project (2018-2020), founded by the Ital-
ian Ministry of Health (grant number: GR-2016-
02365072).
REFERENCES
Batteux, C., Haidar, M., and Bonnet, D. (2019). 3d-printed
models for surgical planning in complex congenital
heart diseases: a systematic review. Frontiers in pe-
diatrics, 7:23.
Burchill, L. J., Huang, J., Tretter, J. T., Khan, A. M., Crean,
A. M., Veldtman, G. R., Kaul, S., and Broberg, C. S.
(2017). Noninvasive imaging in adult congenital heart
disease. Circulation Research, 120(6):995–1014.
Cantinotti, M., Valverde, I., and Kutty, S. (2017). Three-
dimensional printed models in congenital heart dis-
ease. The international journal of cardiovascular
imaging, 33(1):137–144.
Capellini, K., Vignali, E., Costa, E., Gasparotti, E., Bian-
colini, M. E., Landini, L., Positano, V., and Celi, S.
(2018). Computational fluid dynamic study for ataa
hemodynamics: An integrated image-based and radial
basis functions mesh morphing approach. Journal of
Biomechanical Engineering, 140(11).
Celi, S. and Berti, S. (2013). Three-dimensional sensitivity
assessment of thoracic aortic aneurysm wall stress: a
probabilistic finite-element study. European Journal
of Cardio-Thoracic Surgery, 45(3):467–475.
Celi, S., Martini, N., Pastormerlo, L. E., Positano, V., and
Berti, S. (2017). Multimodality imaging for interven-
tional cardiology. Current Pharmaceutical Design,
23(22):3285–3300.
Celi, S., Vaghetti, M., Palmieri, C., and Berti, S. (2013).
Superficial coronary calcium analysis by oct: Look-
ing forward an imaging algorithm for an automatic 3d
quantification. International Journal of Cardiology,
168(3):2958–2960.
Cooper, J. and Taqueti, V. (2008). A brief history of the
development of mannequin simulators for clinical ed-
ucation and training. Postgraduate medical journal,
84(997):563–570.
IVAPP 2020 - 11th International Conference on Information Visualization Theory and Applications
286

El Seoud, M. S. A. and Mady, A. S. (2019). A compre-
hensive review on volume rendering techniques. In
Proceedings of the 2019 8th International Conference
on Software and Information Engineering, ICSIE ’19,
page 126–131, New York, NY, USA. Association for
Computing Machinery.
Gardner, H. E. (2008). Multiple intelligences: New horizons
in theory and practice. Basic books.
Greil, G., Tandon, A. A., Silva Vieira, M., and Hussain, T.
(2017). Noninvasive imaging in adult congenital heart
disease. Frontiers in Pediatrics, 5(6):36.
Hegarty, M. (2014). Spatial thinking in undergraduate sci-
ence education. Spatial Cognition & Computation,
14(2):142–167.
Izard, S. G., Juanes, J. A., Pe
˜
nalvo, F. J. G., Estella, J. M. G.,
Ledesma, M. J. S., and Ruisoto, P. (2018). Virtual re-
ality as an educational and training tool for medicine.
Journal of medical systems, 42(3):50.
Konietschke, R., Tobergte, A., Preusche, C., Tripicchio, P.,
Ruffaldi, E., Webel, S., and Bockholt, U. (2010). A
multimodal training platform for minimally invasive
robotic surgery. In 19th International Symposium in
Robot and Human Interactive Communication, pages
422–427. IEEE.
Kumalasari, L., Yusuf Hilmi, A., and Priyandoko, D.
(2017). The application of multiple intelligence ap-
proach to the learning of human circulatory system.
In Journal of Physics Conference Series, volume 909.
Kurenov, S. N., Ionita, C., Sammons, D., and Demmy,
T. L. (2015). Three-dimensional printing to facilitate
anatomic study, device development, simulation, and
planning in thoracic surgery. The Journal of thoracic
and cardiovascular surgery, 149(4):973–979.
Maresky, H., Oikonomou, A., Ali, I., Ditkofsky, N.,
Pakkal, M., and Ballyk, B. (2018). Virtual real-
ity and cardiac anatomy: Exploring immersive three-
dimensional cardiac imaging, a pilot study in un-
dergraduate medical anatomy education. Clinical
Anatomy, 32.
Moore, R. A., Riggs, K. W., Kourtidou, S., Schneider,
K., Szugye, N., Troja, W., D’Souza, G., Rattan, M.,
Bryant III, R., Taylor, M. D., et al. (2018). Three-
dimensional printing and virtual surgery for congeni-
tal heart procedural planning. Birth defects research,
110(13):1082–1090.
Ong, C. S., Krishnan, A., Huang, C. Y., Spevak, P., Vricella,
L., Hibino, N., Garcia, J. R., and Gaur, L. (2018). Role
of virtual reality in congenital heart disease. Congen-
ital heart disease, 13(3):357–361.
Park, G., Lubinski, D., and Benbow, C. P. (2010). Recog-
nizing spatial intelligence. Scientific American.
Sajid, A., Ewy, G., Felner, J., Gessner, I., Gordon, M.,
Mayer, J., Shub, C., and Waugh, R. (1990). Cardiol-
ogy patient simulator and computer-assisted instruc-
tion technologies in bedside teaching. Medical educa-
tion, 24(6):512–517.
Silva, J. N., Southworth, M., Raptis, C., and Silva, J. (2018).
Emerging applications of virtual reality in cardiovas-
cular medicine. JACC: Basic to Translational Science,
3(3):420–430.
Standard, A. (2005). D2240,”standard test method for rub-
ber property—durometer hardness”. ASTM Interna-
tional, West Conshohoken, PA, USA.
Stout, K. K., Daniels, C. J., Aboulhosn, J. A., Bozkurt, B.,
Broberg, C. S., Colman, J. M., Crumb, S. R., Dearani,
J. A., Fuller, S., Gurvitz, M., Khairy, P., Landzberg,
M. J., Saidi, A., Valente, A. M., and Hare, G. F. V.
(2019). 2018 aha/acc guideline for the management
of adults with congenital heart disease: A report of the
american college of cardiology/american heart associ-
ation task force on clinical practice guidelines. Circu-
lation, 139(14):e698–e800.
Sun, Z., Lau, I., Wong, Y. H., and Yeong, C. H. Personalized
three-dimensional printed models in congenital heart
disease. Journal of Clinical Medicine, 8(522).
Triedman, J. K. and Newburger, J. W. (2016). Trends in
congenital heart disease. Circulation, 133(25):2716–
2733.
Vukicevic, M., Mosadegh, B., Min, J. K., and Little, S. H.
(2017). Cardiac 3d printing and its future directions.
JACC: Cardiovascular Imaging, 10(2):171–184.
3D Printing and 3D Virtual Models for Surgical and Percutaneous Planning of Congenital Heart Diseases
287
