
An Innovative Approach towards Incorporating the End User to the NMES
Wearable System Development
Anelise Ventura
1a
, João Marcos Peron Bataglia
2b
, Leonardo Mendes Ribeiro Machado
4c
,
Jorge Vicente Lopes da Silva
4d
, Renato Varoto
2,3 e
and Alberto Cliquet Jr.
1,2,3 f
1
Bioengineering Post Graduate Course, University of São Paulo (USP),
Avenida Trabalhador São Carlense 400, São Carlos, Brazil
2
Electrical & Computer Engineering Department, University of São Paulo (USP),
Avenida Trabalhador São Carlense 400, São Carlos, Brazil
3
Orthopedics & Traumatology Department, Faculty of Medical Sciences, State University of Campinas (UNICAMP),
Cidade Universitária Zeferino Vaz, Campinas, Brazil
4
Renato Archer Information Technology Center, Dom Pedro I Highway (SP-65) Km 143.6, Campinas, Brazil
Keywords: Parametric Design, NMES, Tetraplegia, Upper Limbs, Wearable System Design.
Abstract: This work presents a portable and customized wearable system design towards applying Neuromuscular
Electrical Stimulation (NMES) to tetraplegics´ upper limbs patients, from creation to production, with users’
participation into the design process. The rehabilitation system protocol for reach and grasp movements
developed by an academic research group, currently applied to patients, has already proven to be effective.
However, the current system and recently published researches, demonstrate proposals distancing from those
who will use and manipulate it, with limitations and failures evidenced. The propose wearable system
integrates electrodes and electronic components activated by a smartphone app to improve the performance
of upper limb movements and optimize the system, making it more functional for your users. The
methodology includes (1) Design Thinking process, (2) Parametric Design process and three dimensional
production, (3) Reduction of the electronic circuits, (4) Development of Android application for setting NMES
protocols and (5) Workbench tests and users experimentation. The methodology in this new approach of
development proved to be feasible and effective. Results have shown that including the end users and health
professionals in the design process to develop wearable system is a promising strategy to overcome the
limitations of the NMES systems.
1 INTRODUCTION
According to the World Health Organization (World
Health Organization, 2013), Spinal Cord Injury (SCI)
affects between 250,000 and 500,000 people
worldwide every year. It is a complex dysfunction
that has major physical, psychological and social
repercussions. Apart from the negative impact on the
quality of life for many individuals, SCI makes it
difficult to perform many activities of daily living
(ADL) and affects negatively on the motor and
a
https://orcid.org/0000-0002-8491-9959
b
https://orcid.org/0000-0001-7596-2296
c
https://orcid.org/0000-0002-3531-9471
d
https://orcid.org/0000-0002-2347-5215
e
https://orcid.org/0000-0001-5333-7123
f
https://orcid.org/0000-0002-9893-5204
sensory functions of the upper and lower limbs of
tetraplegic and paraplegic patients. Paralysis at
different levels and parts of the body, as well as
changes in sensitivity and comorbidities (Eng &
Miller, 2009), are the consequences of SCI, which is
divided into tetraplegia and paraplegia.
It is known that Neuromuscular Electrical
Stimulation (NMES) is an effective rehabilitation tool
applied to tetraplegics’ upper limbs, to perform
reaching and grasping movements (Varoto,
Barbarini, & Cliquet, 2008) (Peckham et al, 1988).
192
Ventura, A., Bataglia, J., Machado, L., Lopes da Silva, J., Varoto, R. and Cliquet Jr., A.
An Innovative Approach towards Incorporating the End User to the NMES Wearable System Development.
DOI: 10.5220/0009095401920199
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 1: BIODEVICES, pages 192-199
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

The NMES employed to intact motor neurons allows
the resumption of movements that have been lost or
altered by the spinal cord injury and promote muscle
strengthening of the limbs (Varoto & Cliquet Jr,
2015). Nevertheless, stimulation parameters should
be carefully adjusted in consonance with the desired
goal, injury level, and muscle response under the
supervision of healthcare professionals (Benton,
1981). In biomedical systems, there are a number of
complexities that need to be addressed in an
integrated manner, including not only electrical and
electronic devices and disease-specific constraints,
but also the many users involved who need to deal
with the proposed product, such as patients, family
and health professionals. Including users early in
process development involves concerns about
ergonomics, appropriate materials used in the
product, user usability and acceptance. In this type of
integrated process, unforeseen problems can be
solved, leading the project to a better resolved final
product. Therefore, healthcare product development
involves a multidisciplinary team working with an
interdisciplinary way (Romero M., Perego P.,
Andreoni G., 2010)
Wearables are different in their nature and
application, so each of them is related to a different
type of a design and ergonomic criteria. The
ergonomics of a product or a system is related to
providing safety, health, comfort and performance to
the user. In this context, some rehabilitation
technology devices have been developed aiming at
upper limb motor rehabilitation, such as Handmaster
System (Snoek, Ijzerman, In ’t Groen, Stoffers, &
Zilvold, 2000), INTFES (Malesevic et al., 2012) and
the Wearable Multi-Site (Crema et al., 2018). This
work presents a new approach for the development of
wearable system towards applying NMES to
tetraplegic patients, including them from the
beginning of creation using the Design Thinking,
Parametric Design and File-to-Factory process to
achieve better results. A portable wearable system
was developed to be individually fitted to the upper
limb, to improve the performance of reach and grasp
movements for functional rehabilitation therapy. At
the same time makes the work of the health
professional more agile.
2 MATERIAL AND METHODS
The NMES rehabilitation for repetitive task training
of reach and grasp movement, currently applied to
patients with C5-C7 injury level, includes a computer
in which the stimulation signal parameters and the
protocol are defined for the rehabilitation program, a
4-channel electronic stimulator that creates stimuli
and commercial self-adhesive surface electrodes
(Fig. 1). Four active electrodes are manually
positioned on the upper limb to generate elbow
extension, extension and flexion of the fingers and
thumb opposition. Straps ensure adherence between
the electrode and the skin (Castro & Cliquet Jr.,
2001). Although the rehabilitation system and
program have already proven to be effective, self-
adhesive electrodes placement demands a longer
implementation time in each training session and
depends on the healthy professional experience for
finding the accurate position. The adhesive film on
the surface of the electrodes is highly adherent when
new, with frequent use this film loses its grip,
preventing its use. In addition, computer- based
system and cables that can interfere in the movements
impose certain limitations.
In order to minimize these inaccuracies and
benefit users, the NMES System currently applied to
tetraplegic patients has been completely redesigned
with a focus on end users and healthcare
professionals, since the actual system it only takes
into account the efficiency of electrostimulation, thus
presenting inaccuracies and failures regarding
ergonomics and the use itself.
Figure 1: The picture of the left shows the current NMES
system applied to the patient of the outpatient department
from the Clinic Hospital (HC), of the State University of
Campinas (Unicamp), Brazil versus the picture of the right
that shows the proposed novel wearable system.
This wearable system composes of three
integrated parts that cover the hand, forearm and arm
with commercial self-adhesive electrodes
incorporated, a smartphone application (app) and the
stimulation unit. The healthy professional can set
protocols for NMES via smartphone application. The
digital data are sent to the stimulation unit attached to
the wearable through Wi-Fi, which generates the
stimulation signal. Commercial surface electrodes
integrated to the wearable are used for applying
NMES to the patient.
An Innovative Approach towards Incorporating the End User to the NMES Wearable System Development
193
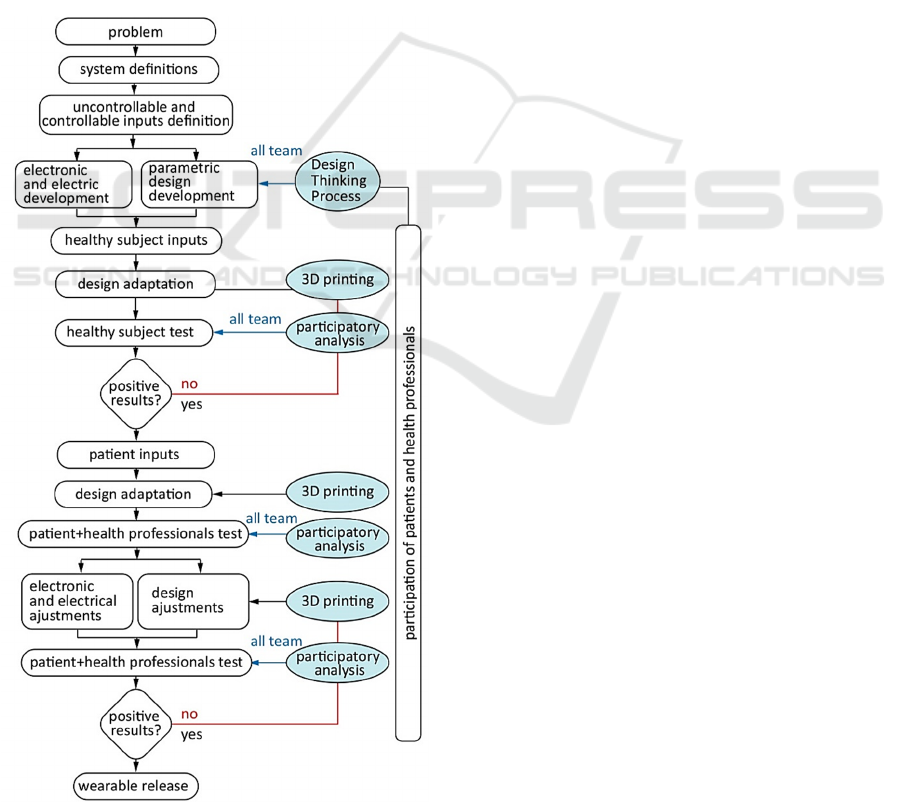
2.1 Wearable System Design
Interdisciplinary Design Process
This project approved by the ethics committee was
devised by researchers from the Bioengineering Post
Graduate Course and Electrical & Computer
Engineering Department of University of São Paulo
(USP), composed of architect, designer and
engineers. The team that participated in all stages of
the development of this project is composed of that
researchers, the patients and the health professionals,
these last two from the outpatient department of the
Clinic Hospital, of the Unicamp.
From flowchart
presented by Maximiliano Romero (Romero M.,
Perego P., Andreoni G., 2010), an adapted version
was created to clarify the interdisciplinary process
that involves the wearable system development
(Ventura, Varoto, & Cliquet Jr, 2018) (Fig. 2).
Figure 2: Interdisciplinary proposed flowchart.
The proposed flowchart shows that the
participation of the entire team occurs at the
beginning of the design process and at various times
throughout product development. Team integration is
the moment when everyone observes, evaluates and
discusses what was produced.
To get closer to the realities of the rehabilitation
routine and improving the current system, the Design
Thinking process was applied. The major goal is to
explore possibilities and to create options in a
divergent process of ideas without judgments or
limits, and then make choices, through the convergent
process (Brown, 2009), resulting in three-
dimensional models and prototypes.
After the Design Thinking stage, the parametric
design methodology was developed. In parallel, a
scanning protocol of the upper limbs was elaborated
and the results of the 3D scanning of a healthy
individual and a patient were inserted in the
generative algorithm inputs, for verification and
adjustments of the created methodology. The patient
participated in the product development of the healthy
individual from the point of view of wearable shape,
aesthetic acceptance and material malleability. The
health team evaluated the placement of the electrodes
in the wearable, their handling and the ease of placing
the product on the upper limb.
With positive results, the healthy subject's inputs
will be replaced by the patient's inputs. The wearable
will be 3D printed, and quantitative movement and
qualitative comfort assessments will be performed.
Parametric Design and File-To-Factory
Process
Wearable are devices designed to fit different moving
bodies so that they can be controlled and operated
without interruption or limiting the movements of the
users (Mann, 1997). The wearable shape, their active
relationship with the human anatomy and other
components must act in an integrated way for proper
secondary functioning and use. Specifically, for this
work, the anthropometric characteristics of the upper
limbs are very different from one patient to another
due to deformities resulting from the SCI. Thus, it
arises the need for individually fitted wearables, and
design and ergonomics criteria based on Design
Wearability Guidelines (Gemperle, Kasabach,
Stivoric, Bauer, & Martin, 1998).
According to ergonomic criteria and because it is
a highly complex product, the creation of wearable
involved the development of a Parametric Design
Methodology using generative algorithms, a 3D
scanning protocol for patient's upper limb and a file-
to-factory 3D printed product.
BIODEVICES 2020 - 13th International Conference on Biomedical Electronics and Devices
194
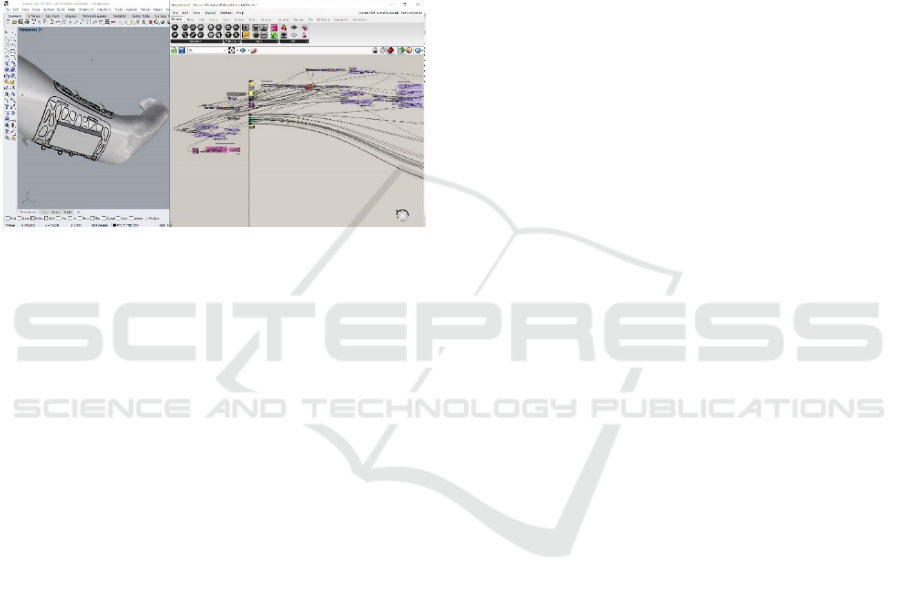
The production of complex geometries of this
order is associated with file-to-factory process, with a
direct relationship between design and production. It
involves direct transfer of data from a 3D modeling
software to a 3D printing machine (Kolarevic, 2009).
The wearable model was performed in Rhinoceros 3D
(TLM Inc., Seattle, WA, USA), which is a CAD tool,
and an algorithmic logic has been developed in
Grasshopper, which is a generative algorithm editor
for Rhinoceros, so that the design is completely
automated (Fig. 3). The input data changes according
to the individuality of each patient.
Figure 3: 3D model automated with generative algorithm.
According to the ergonomic criteria the inputs
required for the construction of wearable were
divided into primary (uncontrollable):
anthropometric data and the electrode points on the
muscle groups, and secondary (controllable): position
that the wearable will occupy on the arm, size of the
electrodes, tubes that will embed the cables,
stimulation unit position, wearable clearance, weight
of the wearable and ventilation. The resulting
wearable shape is achieved according to the
relationships of the primary and secondary
parameters.
The protocol scan created considerate the
wheelchairs and restricted upper limb movement of
the patient. It was simulated the environment and the
conditions of the patients to scan the dominant upper
limb of the healthy subject. The 3D scan of the
dominant upper limb resulted in an anthropometric
data, and it was collected with 3D iSense Scanner (3D
Systems, Rock Hill, SC, USA) with Skanect 3D
Scanning Software (Occipital, Inc., San Francisco,
CA, USA).
According to the developed model, the wearable
was printed by Fused Deposition Modelling (FDM)
process with flexible ABS material.
Electronic Apparatus and Smartphone
Application
The ESP32 DeviKit V1 Development Board
(Shenzhen Doctors of Intelligence & Technology Co.
Ltd, Shenzhen, Guangdong, China) provides an
interface between the smartphone app and the
stimulation unit, presenting Wi-Fi and Bluetooth Low
Energy (BLE) connectivity.
Powered with 6V by one 18650 rechargeable
lithium ion battery (4.2V, 9.8Ah) connected to an
adjustable booster power supply module with
XL6009 DC-DC step up module (Shanghai Xinlong
Semiconductor Technology Co. Ltd, Pudong,
Shanghai, China), the ESP32 operates as a custom-
made monophasic square waveform generator,
creating the stimulation signal with following
parameters: number of pulses for each burst: 4; pulse
width: 100s; period between pulses: 100s and burst
frequency: 25Hz.
The microcontroller module was programmed
using language and Integrated Development
Environment (IDE) of Arduino (Arduino S.r.l,
Scarmagno, TO, Italy) with extension packages for
ESP32. Ports D13, D12, D14, D27, D26, D25, D33
and D32 were configured as outputs, corresponding
to the stimulation channels 1 to 8, respectively.
In the stimulation unit, the generated signal is
transmitted to the isolation circuitry, which uses
optical coupling. In order to adequate the signal for
the amplification circuitry, an inverter buffer operates
at the optocoupler output.
A potentiometer, an N-Channel Power MOSFET
and a pulse transformer compose the amplification
circuitry, designed for output up to 100V.
The same strategy - previously described - is used
to power the stimulation unit, but the booster module
output has been set to 12V.
The stimulation unit is electrically coupled to the
patient via self-adhesive surface electrodes.
Communication between the microcontroller and
the Android app occurs using a Wi-Fi router (there is
no need of internet access). In relation to the router, a
static local IP address and a port number were
assigned for microcontroller connection, which
works as a network server. The app connects to the
same Wi-Fi router as a network client, requesting
permission to exchange data with the microcontroller.
The smartphone app written in Java Programming
Language was designed using Android Studio
(Google LLC, Mountain View, CA, USA). The user
interface was done through FIGMA online design
tool (Figma Inc., San Francisco, CA, USA) and
presents three tabs.
An Innovative Approach towards Incorporating the End User to the NMES Wearable System Development
195

Four strings are written to adjust the channels and
to define the NMES protocol. The first one is
dedicated to the stimulation channels tuning; the
stimulation signal is provided on the selected channel
for one minute. This period allows the health
professional to adjust the signal amplitude - via
stimulation unit - according to the patient's motor
responses.
NMES protocol has up to 12 phases that
correspond to the planned movements of the upper
limb. For each phase, up to three channels can be
activated during the specified period (up to 12s with
resolution of 400ms). This information is gathered in
the second string. In the rehabilitation program, this
protocol can be repeated. Thus, the third string
determines the number of cycles (up to 300) along the
program. The fourth string contains an indicator to
initiate the process.
Workbench Tests
The workbench tests were done to verify the
electronic apparatus performance, including the
temperature variation of some components. Using a
TDS2024 Digital Storage Oscilloscope (Tektronix
Inc, Beaverton, OR, USA), the stimulation signal was
characterized in terms of time and amplitude at the
output of ESP32, optocoupler, inverter buffer and
amplification circuitry. A resistor played a role of
electrodes and biological tissue (impedance
equivalent to 1k). Battery autonomy was estimated
for the 100V amplitude stimulation signal. Thus, a
channel powered by a fully charged battery was
activated uninterrupted until the amplitude reached at
least 90% of this voltage.
Users Experimentation
The health professional inserted the electrodes into
the wearable in the space designed for them; new and
reused electrodes were tested. Then, the wearable was
placed in the healthy subject's hand, forearm and arm,
and the channels were adjusted according to their
motor response to perform effective wrist and elbow
extension movements and effective flexion and
extension of the fingers. The amplitude was gradually
increased until reaching the excitability threshold of
each muscle group. The NMES protocol was adapted,
since the stimulus sensitivity of the healthy individual
is higher.
3 RESULTS
The relevant and unanimous concepts extracted as a
result of Design Thinking dynamics for wearable
design were safety and confidence in the movement,
agility of the process during NMES therapy and the
possibility to do it at home with healthy professional
supervision.
As a result of the methodology developed with the
Grasshopper plug-in the full automation of the project
was achieved and the developed method confirmed to
be efficient regarding the substitution of the
parameters from one individual to the other, although
the generative algorithm editor had limitations
regarding the modeling flexibility over double
curvature geometries, such as topographic features of
the upper limbs.
The solution found for the loss of electrode
contact with the user's skin over time due to decreased
electrode film adhesion and its precise location on
muscle groups was the print of the wearable with
embossed markings, positioned exactly at the
locations of the muscle groups to be stimulated, with
the shape and size of the electrodes. The high relief
promoted the integral contact of the surface of the
electrodes to the individual's skin, so that the reach
and grip functions were achieved even with the loss
of the adherent film. In addition, the solution found
for the fixation of the electrodes on the werarable
were double sided tapes. The double-sided tape was
suitable for its fixation to the werarable and for the
fixation of the electrodes to it.
Figure 4: Left wearable inside with the electrodes and right
without the electrodes.
Regard file-to-factory process the printing was
faithful to the 3D modeling data. The characteristics
of the flexible ABS material allow the wearable
surfaces to have flexibility without losing their
original shape and allow the health professional to
easily place the product on the patient. In addition,
BIODEVICES 2020 - 13th International Conference on Biomedical Electronics and Devices
196

flexible ABS features a comfortable texture to the
touch.
In relation to users’ experimentation, appropriate
results were achieved with new and reused electrodes.
The wearable system design resulted in efficiency
of the projected areas for the electrodes, and its
position and emboss surface provided better adhesion
to the electrodes on the skin surface, resulting in
effective NMES (Fig. 4). The extension and flexion
movements of the wrist, fingers and elbow were
efficient performed with stimuli of approximately
10V (for 1k equivalent impedance). The electronic
circuit was designed to provide up to 100V. However,
10V was sufficient for the healthy subject.
The physical access and manipulation of the
wearable by the health professional was intuitive. The
anthropometric characteristics of the 3D model were
faithful to the real ones, facilitating the wearable
placement in the correct position. There was no
readjustment in the wearable position to perform
efficient movements.
Figure 5: Printed wearable and stimulation unit.
The tubes that embed the electrode cables were
strategically positioned in the wearable avoiding their
disconnection and flow close to the stimulation unit
solving the problems with the dimension of the
cables.
Regard the Android app, called “Stimulator”, on
the “Setting Channels Up” tab, each channel can be
selected to tune the stimulation intensity. The “NMES
Protocol” tab is dedicated to configure the phases.
When the phase button is tapped, the “Phase Settings”
screen pops up, so up to three channels can be
selected and a slider allows adjustment of the
activation period. The last tab- “Rehabilitation
Activities”-summarizes the phases configuration and
shows the slider to select the number of cycles of the
rehabilitation activity, indicating the total time
(Fig. 5). Tapping the send button on this tab, a status
screen of the communication process appears, and
then the rehabilitation activity is started.
Figure 6: Stimulator android app.
The generated signal achieved the timing
characteristics (pulse width, period between pulses
and burst frequency) and provided four pulses for
each burst, from ESP32 to amplification circuitry
output. General-purpose input/output (GPIO) of
ESP32 operates with analog voltage ranging from 0
to 3.3V. Thus, the stimulation signal was generated
with amplitude of 3.24V. On the analog circuit, this
signal was inverted, reaching -4.72V according to the
5V power supply. Amplified to 8.08V at the inverter
buffer output, the stimuli range from 0 to about 100V
for 1k impedance. Battery autonomy was
approximately 65 minutes.
4 DISCUSSION
It is considered that one of the major obstacles in the
development of the wearable system in this work is
the product to meet the varied anthropometries of the
upper limb of the spinal cord injured patient and the
system to function properly. Current computing
technologies have proven to be able to handle these
complexities.
Using parametric design produced with the file-
to-factory process, including end-user participation
throughout the product development process and
being faithful to upper limb anthropometry, has
allowed preliminary testing to be performed on a
healthy individual. It contributed to verify the
strengths and weaknesses of the proposed wearable.
Problems were minimized during product
development without the patient being overwhelmed
in the preliminary phase of the patient's wearable
impression.
According to the concepts discussed in Design
Thinking process - safety, confidence and agility- the
wearable shape provided robustness, towards a safety
physical constitution, although light (177,21 g),
without limiting the range of motion. In contrast to
the current system the stimulation unit has
An Innovative Approach towards Incorporating the End User to the NMES Wearable System Development
197

considerably decreased its size. However, it was
heavy to be coupled to the wearable (401,6 g).
The inconvenient of the double-sided tape for
attaching the electrodes to the wearable was that it left
material residue on the product when removed from
it.
The wearable was designed to be produced by the
file-to-print method with 3D printing, without having
to be finalized by the industry. This optimizes the
design and production process.
Filament deposition printing (FDM) showed
flaws mainly in the final shape of the fastening parts
and the wearable finish. However, its malleability and
tactile comfort characteristics were achieved.
According to the literature review the commercial
product Handmaster (Snoek et al., 2000), in terms of
performance, presented functional benefits to
patients. In terms of product design, it did not present
ergonomic for all sizes of forearms tested, and among
ten product units, three did not serve patients because
of its small size. Among the three examples
presented, both Handmaster as INTFES (Malesevic et
al., 2012) and The Wearable Multi-Site System
(Crema et al., 2018) proved to be effective in relation
to NMES functionality, although they have
anthropometric constraints. Furthermore, the last two
examples do not suggest the end-user and health team
participation until the presented stage of
development, which may have generated the
mentioned results in relation to the particularities of
each patient. This means that if users are ignored
early in the stimulation system development process,
incompatibilities between this system and the end
product tend to occur more easily. In addition,
recurring failures in ergonomics, usability, handling
and user acceptance of the product will be detected
and new issues will need to be fixed. Thus, users,
product design, electronics, and assembly
manufacturing must be developed simultaneously by
an interdisciplinary team.
An advantage of using an app and the
microcontroller other than a fixed apparatus (such as
a computer) to set up the NMES, is the fact that a
single device containing the app is capable, with
minor adjustments, to be used for multiple patients at
the same time. After the stimulation has been sent
from the app to the microcontroller, the app is no
longer in charge of the stimulation, been possible to
operate in another person. This feature has the
potential to increases the amount of patients that can
receive the treatment at a time with the same amount
of health professionals, therefore creating a more
accessible treatment to the public.
The possible limitations of the present study are
that a designer with programming knowledge it is
necessary to alter the parameters for the development
of a new wearable to a new patient, using the same
method and the product has been designed for a
particular stimulation protocol, if new muscle groups
need to be stimulated, the inputs must be reformulated
and a new wearable printed.
5 CONCLUSIONS
The results demonstrated that including the end user
and the health professionals from the development of
the concept design to the wearable production can be
a promising alternative to reconciling the
complexities involved in a NMES wearable system to
overcome its shortcomings and limitations. To
achieve these results, the use of Design Thinking,
Parametric Design and File-to-Factory processes
proved to be feasible and effective.
The next steps for this work include system
development for the patient and performance analysis
with the motion imaging system.
ACKNOWLEDGEMENTS
The authors would like to thank the support by grants
from São Paulo Research Foundation (FAPESP),
Brazilian Federal Agency for Support and Evaluation
of Graduate Education (Capes) and National Council
for Scientific and Technological Development
(CNPq).
REFERENCES
Benton, L. A.; Baker, L. L.; Bowman, B. R.; Waters, R. L.
(1981) Functional Electrical Stimulation - A Practical
Clinical Guide, 2 ed., Rancho Los Amigos
Rehabilitation Engineering Center, California.
Brown, T. (2009). Change by Design. How Design
Thinking Transforms Organizations and Inspires
Innovation. HarperBusiness.
Castro, M. C. F. De, & Cliquet Jr., A. (2001).
Neuromuscular Electrical Stimulation and Electron-
tactile Stimulation in Rehabilitation of Artificial
Prehension and Proprioception in Tetraplegic Patients.
Acta Ortop. Bras., 9(3), 19–28.
Crema, A., Maleševi, N., Furfaro, I., Raschellà, F.,
Pedrocchi, A., & Micera, S. (2018). A Wearable Multi-
Site System for NMES-Based Hand Function
Restoration. IEEE Transactions on Neural Systems and
BIODEVICES 2020 - 13th International Conference on Biomedical Electronics and Devices
198

Rehabilitation Engineering, 26(2), 428–440.
https://doi.org/10.1109/TNSRE.2017.2703151
Eng, J. J., & Miller, W. C. (2009). Rehabilitation: From
Bedside To Community Following Spinal Cord Injury
(SCI). SCIRE Spinal Cord Injury Rehabilitation
Evidence: Version 2.0, (1). Retrieved from
www.icord.org/scire C:%5CEMH%5CScannede art
ikler referanser%5CRefMan4265.pdf
Gemperle, F., Kasabach, C., Stivoric, J., Bauer, M., &
Martin, R. (1998). Design for Wearability. In Digest of
Papers. Second International Symposium on Wearable
Computers (Cat. No.98EX215) (pp. 116–122). IEEE.
Kolarevic, B. 2009. Architecture in the digital age: design
and manufacturing. New York; London: Taylor &
Francis.
Malesevic, N. M., Popovic Maneski, L. Z., Ilic, V.,
Jorgovanovic, N., Bijelic, G., Keller, T., & Popovic, D.
B. (2012). A multi-pad electrode based functional
electrical stimulation system for restoration of grasp.
Journal of NeuroEngineering and Rehabilitation, 9(1),
66. https://doi.org/10.1186/1743-0003-9-66
Mann, S. (1997). An historical account of the `WearComp’
and `WearCam’ inventions developed for applications
in `Personal Imaging’. In PISWC ’97 Proceedings of
the 1st IEEE International Symposium on Wearable
Computers (pp. 66–73). Cambridge, MA, USA: IEEE.
https://doi.org/10.1109/ISWC.1997.629921
Peckham, P.H., Keith, M.W.; Freehafer, A. A. (1988).
Restoration of functional control by electrical
stimulation in the upper extremity of the quadriplegic
patient. J Bone and Joint Surg 70-A(1):144-148.
Romero M., Perego P., Andreoni G., C. F. (2010). New
Strategies for Technology Products Development in
Health Care, New Trends in Technologies: Control,
Management, Computational Intelligence and Network
Systems. (Meng Joo E, Ed.). Meng Joo Er.
https://doi.org/DOI: 10.5772/293
Snoek, G. J., Ijzerman, M. J., In ’t Groen, F. A. C. G.,
Stoffers, T. S., & Zilvold, G. (2000). Use of the NESS
Handmaster to restore handfunction in tetraplegia:
Clinical experiences in ten patients. Spinal Cord, 38(4),
244–249. https://doi.org/10.1038/sj.sc.3100980
Varoto, R., Barbarini, E. S., & Cliquet, A. J. (2008). A
Hybrid System for Upper Limb Movement Restoration
in Quadriplegics. Artificial Organs. Thoughts and
Progress, 32(9). https://doi.org/10.1111/j.1525-
1594.2008.00597.x
Varoto, R., & Cliquet Jr, A. (2015). Experiencing
Functional Electrical Stimulation Roots on Education ,
and Clinical Developments in Paraplegia and
Tetraplegia With Technological Innovation. Artificial
Organs, 39(ii). https://doi.org/10.1111/aor.12620
Ventura, A., Varoto, R., & Cliquet Jr, A. (2018). Wearable
Technology: Healthcare Product Design For
Participation Of Tetraplegics In Society. In Blucher
Design Proceedings. https://doi.org/10.5151/sigradi
2018-1726
World Health Organization. (2013). Spinal cord injury.
Retrieved March 12, 2019, from https://www.who.int/
news-room/fact-sheets/detail/spinal-cord-injury
An Innovative Approach towards Incorporating the End User to the NMES Wearable System Development
199
