
Classification of Optical Coherence Tomography using Convolutional
Neural Networks
A. A. Saraiva
2,6 a
, D. B. S. Santos
1 b
, Pimentel Pedro
1 c
, Jose Vigno Moura Sousa
1 d
,
N. M. Fonseca Ferreira
3,4 e
, J. E. S. Batista Neto
6
, Salviano Soares
3 f
and Antonio Valente
2,5 g
1
UESPI - University of State Piaui, Piripiri, Brazil
2
University of Tr
´
as-os-Montes and Alto Douro,Vila Real, Portugal
3
Coimbra Polytechnic, ISEC, Coimbra, Portugal
4
Knowledge Engineering and Decision-Support Research Center (GECAD) of the Institute of Engineering,
Polytechnic Institute of Porto, Portugal
5
INESC-TEC Technology and Science, Porto, Portugal
6
University of S
˜
ao Paulo, S
˜
ao Carlos, Brazil
Keywords:
OCT, CNN, Classification, K-fol, Labeled Optical Coherence Tomography.
Abstract:
This article describes a classification model of optical coherence tomography images using convolution neural
network. The dataset used was the Labeled Optical Coherence Tomography provided by (Kermany et al.,
2018) with a total of 84495 images, with 4 classes: normal, drusen, diabetic macular edema and choroidal
neovascularization. To evaluate the generalization capacity of the models k-fold cross-validation was used.
The classification models were shown to be efficient, and as a result an average accuracy of 94.35% was
obtained.
1 INTRODUCTION
An examination known as optical coherence tomog-
raphy (OCT) has gained ground in the latest comple-
mentary clinical tests for the diagnosis of retinal and
vitreous disease (Preti et al., 2018).
This technology was developed by Fujimoto at the
Massachusetts Institute of Technology, applied in the
ophthalmological diagnosis by Puliafito. The use of
this examination has become fundamental in the diag-
nosis, on evolution and postoperative control of mul-
tiple macular conditions (Dimitrova et al., 2017).
According to (Swanson and Fujimoto, 2017) ap-
proximately 30 million procedures of optical coher-
ence tomography (OCT) images are performed per
year, the analysis and interpretation of these images,
a
https://orcid.org/0000-0002-3960-697X
b
https://orcid.org/0000-0003-4018-242X
c
https://orcid.org/0000-0002-5291-0810
d
https://orcid.org/0000-0002-5164-360X
e
https://orcid.org/0000-0002-2204-6339
f
https://orcid.org/0000-0001-5862-5706
g
https://orcid.org/0000-0002-5798-1298
consumes a significant amount of time. OCT helped
patients prevent or minimize vision loss by detecting
retinal diseases in the early stages of treatment (Swan-
son and Fujimoto, 2017).
According to (Sivaprasad and Moore, 2008), the
growth of new choroidal blood vessels is known as
choroidal neovascularization (CNV). These new ves-
sels come from a rupture in the Bruch membrane that
is located in the subretinal pigment epithelium. Ac-
cording to (Baxter et al., 2013) CNV occurs in about
2 to 3% of cases of posterior uveitis.
Diabetic macular edema (DME) is a complication
of diabetes caused by fluid accumulation in the mac-
ula, or central portion of the eye, that causes the mac-
ula to swell (Wells et al., 2016). The macula is filled
with cells responsible for direct vision that aid in read-
ing and directing (Wells et al., 2016).
When the macula begins to fill with fluid and
swell, the capacity of these cells is impaired, caus-
ing blurred vision (Bressler et al., 2016). The DME
is diabetic retinopathy, in which the blood vessels of
the eye are damaged, allowing the fluid to escape,
this type of disease can also be diagnosed through the
168
Saraiva, A., Santos, D., Pedro, P., Sousa, J., Ferreira, N., Neto, J., Soares, S. and Valente, A.
Classification of Optical Coherence Tomography using Convolutional Neural Networks.
DOI: 10.5220/0009091001680175
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 3: BIOINFORMATICS, pages 168-175
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

OCT (Gill et al., 2017).
Drusen are small accumulations of yellow or
white extracellular material that accumulate between
Bruch’s membrane and the retinal pigment epithelium
of the eye, which can be detected by OCT (Gaier
et al., 2017). The presence of small Drusen is normal
with advancing age, and most people over 40 have
some Drusen (Alten et al., 2017). However, the pres-
ence of larger and more numerous Drusen in the mac-
ula is an early sign of age-related macular degenera-
tion (DMRI) (Schlanitz et al., 2017).
In this way, he inspired the design an OCT image
classifier, in order to classify three types of patholo-
gies visible in OCT: CNV, DME, and DRUSEN, in
an automated and fast way. The method chosen and
implemented constitutes in the classification of OCT
images of retinas of living patients, from this, to iden-
tify whether or not they have any of the diseases
mentioned above. For the classification was used
the dataset Labeled Optical Coherence Tomography
with total 84,495 images provided by (Kermany et al.,
2018).
The classification stage consists of two sub-steps,
where the first is carried out to the training of a deep
learning known as Convolutional Neural Networks
(CNN), the second sub-step is the validation of the
model, that is, the tests with unknown images by CNN
(Saraiva. et al., 2019b), (Saraiva. et al., 2019a). The
method covered ensures a robust coverage in image
recognition, under certain assumptions that will be
clarified throughout the text.
The paper is divided into 5 sections, in which
section 2 is characterized by the description of the
methodology applied, followed by the validation met-
rics in section 3. The results after the application of
the proposal and the final conclusions are presented in
sections 4 and 5, respectively.
The data set is organized into 4 categories: NOR-
MAL, CNV, DME, DRUSEN, totaling 84,495 OCT
images. The OTC images were selected from retro-
spective cohorts of adult patients at the Shiley Eye
Institute of the University of California at San Diego,
the California Retina Research Foundation, the Med-
ical Center Ophthalmology Associates First People’s
Hospital and the Beijing Tongren Eye Center between
July 1 2013 and March 1, 2017.
Each image underwent a step-by-step classifica-
tion system consisting of several layers of trained
classifiers with experience for checking and correct-
ing image labels. Each image in the data set began
with a label that matches the patient’s most recent di-
agnosis. The first level of classifiers consisted of un-
dergraduate medical students who had completed and
passed a review of the OCT course of interpretation.
This first level of classifiers conducted initial qual-
ity control and excluded OCT images containing
noises or significant reductions in image resolution.
The second level of classifiers consisted of four oph-
thalmologists who independently classified each im-
age that passed the first level. Finally, a third level of
two senior independent retinal specialists, each with
more than 20 years of experience in clinical retina,
checked the labels for each image (Kermany et al.,
2018).
1.1 CNV
CNV generally reaches individuals under 50 years of
age, and its early diagnosis is extremely important for
the prompt institution of treatment, which may pre-
vent the occurrence of fibrosis and consequent perma-
nent central visual acuity in this economically active
population (Roy et al., 2017).
The main symptoms are central scotoma and
metamorphopsia, but the patient may be asymp-
tomatic, especially when the affected eye already has
low visual acuity prior to the presence of a central or
pericentral scar or granuloma. In the figure 1 it is pos-
sible to visualize the OCT image, divided into normal
and CNV.
(a) Image of CNV (b) Normal image
Figure 1: Example of OCT images divided into normal and
with CNV.
1.2 DME
In DME, an accumulation of liquid and proteins oc-
curs in the macula region (Parhi et al., 2017). The
retina becomes swollen, and the vision is greatly im-
paired. This accumulation of liquid and proteins be-
gins because of the excess of blood sugar in a pro-
longed way, which damages the blood vessels (Parhi
et al., 2017).
DME, defined as a retinal thickening involving
or approaching the center of the macula, is the most
common cause of vision loss in patients affected by
diabetes. In the figure 2 you can view the OCT im-
age, divided into Normal and DME.
Classification of Optical Coherence Tomography using Convolutional Neural Networks
169

(a) Image of DME (b) Normal image
Figure 2: Example of OCT images divided into normal and
with DME.
1.3 DRUSEN
Optical disc drusen are calcified deposits of extruded
mitochondria that appear in the upper part of the optic
nerve in approximately 2% of the population (Gaier
et al., 2017). In the figure 3 you can view the OCT
image, divided into normal and DRUSEN.
(a) Image of DRUSEN (b) Normal image
Figure 3: Example of OCT images divided into normal and
with DRUSEN.
2 MATERIALS AND METHODS
In this section, the structure of the adopted systems
will be presented to solve the classification of OCT
images, classifying them as, CNV, DME, NORMAL,
DRUSEN, will also be presented the entire structure
of the algorithms as well as the evaluation metrics.
2.1 Structure of the System
The system was divided into three stages, the first
consisting of the division and normalization of the
dataset, according to the figure 4. In the second one
the training was carried out and finally the data vali-
dation was done. Pre-processing consists of normaliz-
ing the data, the images are in grayscale, all pixels are
divided by 255, to convert them into floating points.
Represented in the figure 4 in yellow.
In figure 4 you can see the process of construc-
tion and training of artificial neural network model.
In the test data prediction step, the test images that
were separated by the k-fold algorithm are entered,
so accuracy is collected. The process is repeated 5
times, changing the test and training images after the
k-fold calculation.
Figure 4: Construct, training and validate of the models.
2.2 CNN
CNNs are similar to traditional neural networks, both
are composed of neurons that have weights and bias
that need to be trained. Each neuron receives some in-
puts, applies the scalar product of inputs and weights
in addition to a non-linear function (Chen et al.,
2017).
A CNN assumes that all inputs are images, which
allows you to encode some properties in the archi-
tecture. Traditional neural networks are not scalable
for images, since they produce a very high number of
weights to be trained (Esteva et al., 2017).
A CNN consists of a sequence of layers as can
be seen figure 6, in addition to input layer, which is
usually composed of an image with width and height,
there are three main layers: convolutional layer, pool-
ing layer and fully connected layer. In addition, after
a convolutional layer it is common an activation layer,
normally a linear rectification unit function (ReLu)
equations 1, 2. These layers, when sequenced (or
stacked), form an architecture of a CNN (Salamon
and Bello, 2017).
f (x) = x
+
= max(0,x) (1)
f (x) =
(
0 for x < 0
x for x ≥ 0
(2)
2.2.1 Convolutional Layer
The convolutional layer is the most important layer of
the network, where it carries out the heaviest part of
computational processing. This layer is composed of
a set of filters (kernels) capable of learning according
to a training (Ustinova et al., 2017). The kernels are
small matrices that in this case was used the size 3x3
to obtain a better precision in the time to go through
the matrix of the images, composed by real values that
can be interpreted as weights.
Given a two-dimensional image, I, and a small ar-
ray, K of size h x w (kernel), the convoked image, I
* K, is calculated by overlapping the kernel at the top
of the image of all possible shapes, and recording the
BIOINFORMATICS 2020 - 11th International Conference on Bioinformatics Models, Methods and Algorithms
170
sum of the elementary products between the image
and the kernel equation 3.
(I ∗ K)
xy
=
h
∑
i=1
w
∑
j=1
K
i j
.I
x+i−1,y+ j−1
(3)
The kernels are convolved with the input data to
get a feature map. These maps indicate regions in
which specific features in relation to kernels. The ac-
tual values of the kernels change throughout the train-
ing, causing the network to learn to identify signif-
icant regions to extract characteristics from the data
set (Maggiori et al., 2017), in this way, each filter
results in an output of a three-dimensional array. In
the convolution results matrices the ReLU activation
function, equations 1, 2 are applied in each element
of the convolution result.
2.2.2 Pooling Layer
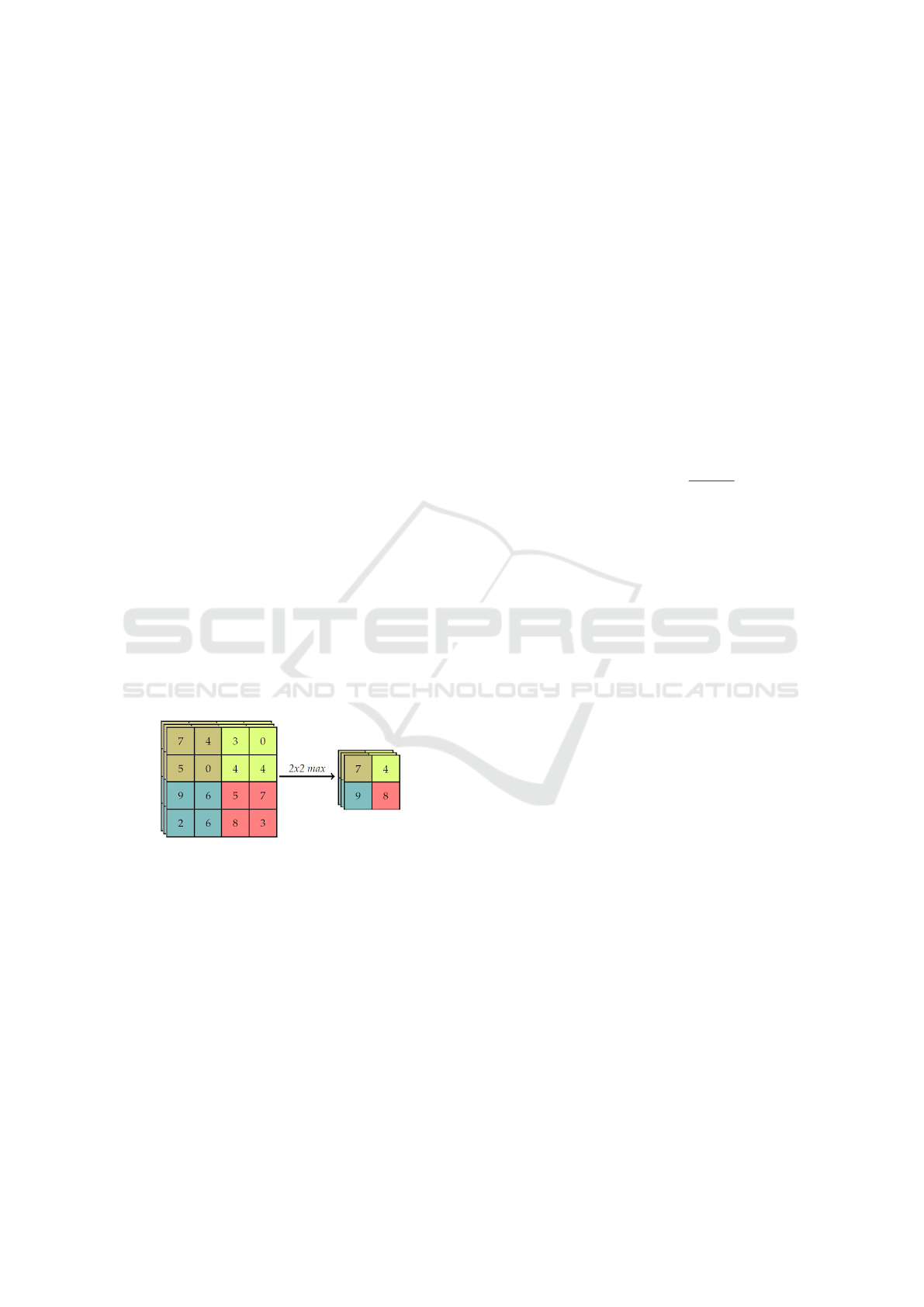
After convolution layer exists a pooling layer. The
pooling technique is used to reduce the spatial size of
the resulting convolution matrices, according to the
figure 5. Consequently, this technique reduces the
amount of parameters to be learned in the network,
contributing to the control of over-fitting, ie avoiding
the condition when a trained model works very well in
training data, but does not work very well in test data
(Yu et al., 2017). The pooling layers operate inde-
pendently on each of the channels of the convolution
result. In addition, you must first determine the size
of the filter to perform pooling.
Figure 5: Example max-pooling with a 4x4 image.
The maximum pool operation reduces the size of
the resource map, this operation can be described by
the equation 4. Let S be the value of the passed and
Q × Q the shape of the feature map before the maxi-
mum grouping and p determines the clustering max-
pooling size (Havaei et al., 2017). The output of the
max-pooling operation would be D × D size.
D = (Q − p)/S + 1 (4)
2.2.3 Fully Connected Layer
The fully connected layer comes after a convolutional
or pooling layer, it is necessary to connect each ele-
ment of the convolution output matrices to an input
neuron. The output of the convolutional and pooling
layers represent the characteristics extracted from the
input image. The purpose of fully-connected layers is
to use these characteristics to classify the image in a
pre-determined class.
The last two layers of the network use the sigmoid
function as the activation function, equation 5. This
function takes a real value and ”transforms” it into
the interval between 0 and 1. In particular, large neg-
ative numbers become 0 and large positive numbers
become 1 (Zaheer and Shaziya, 2018). The sigmoid
function has seen frequent use historically since it has
a good interpretation like the firing rate of a neuron:
from not firing (0) to a fully saturated firing at a pre-
sumed maximum frequency (1) (Zaheer and Shaziya,
2018).
f (x) = sigmoid(x) =
1
1 + e
−x
(5)
The technique known as dropout is also used in
the fully connected layer to reduce training time and
avoid over-fitting. This technique consists in ran-
domly removing a certain percentage of neurons from
a layer at each training iteration, re-adding them to the
next iteration (Kov
´
acs et al., 2017).
2.2.4 CNN Architecture
In the figure 6 the CNN architecture is displayed, it
has 12 layers, where the first ten convolutionals layers
and the last two without convolution with the sigmoid
activation function. The input of the network receives
a 150x150 pixel image, each the convolutional layer
has the activation function ReLUs. For the convolu-
tion kernel, the 3x3 size was adopted, because this
way it is possible to have a greater precision in the
time to go through the entire image.
After two convolutional layers a Max-pooling
layer is used, this reduces the size of the matrices re-
sulting from the convolution. With this layer it is pos-
sible to reduce the amount of parameters that will be
learned by the network, this way it is done over fitting
control.
In the latter two the sigmoid activation function
is used, this function is responsible for making the
probabilistic distribution of the input image belong to
each of the classes in which the network was trained.
To reduce the training time and to avoid over-fitting is
used dropout in the layer, ie it is randomly removed at
each training interaction, a certain percentage of the
neurons of a layer, re-adding them in the following
iteration.
Classification of Optical Coherence Tomography using Convolutional Neural Networks
171
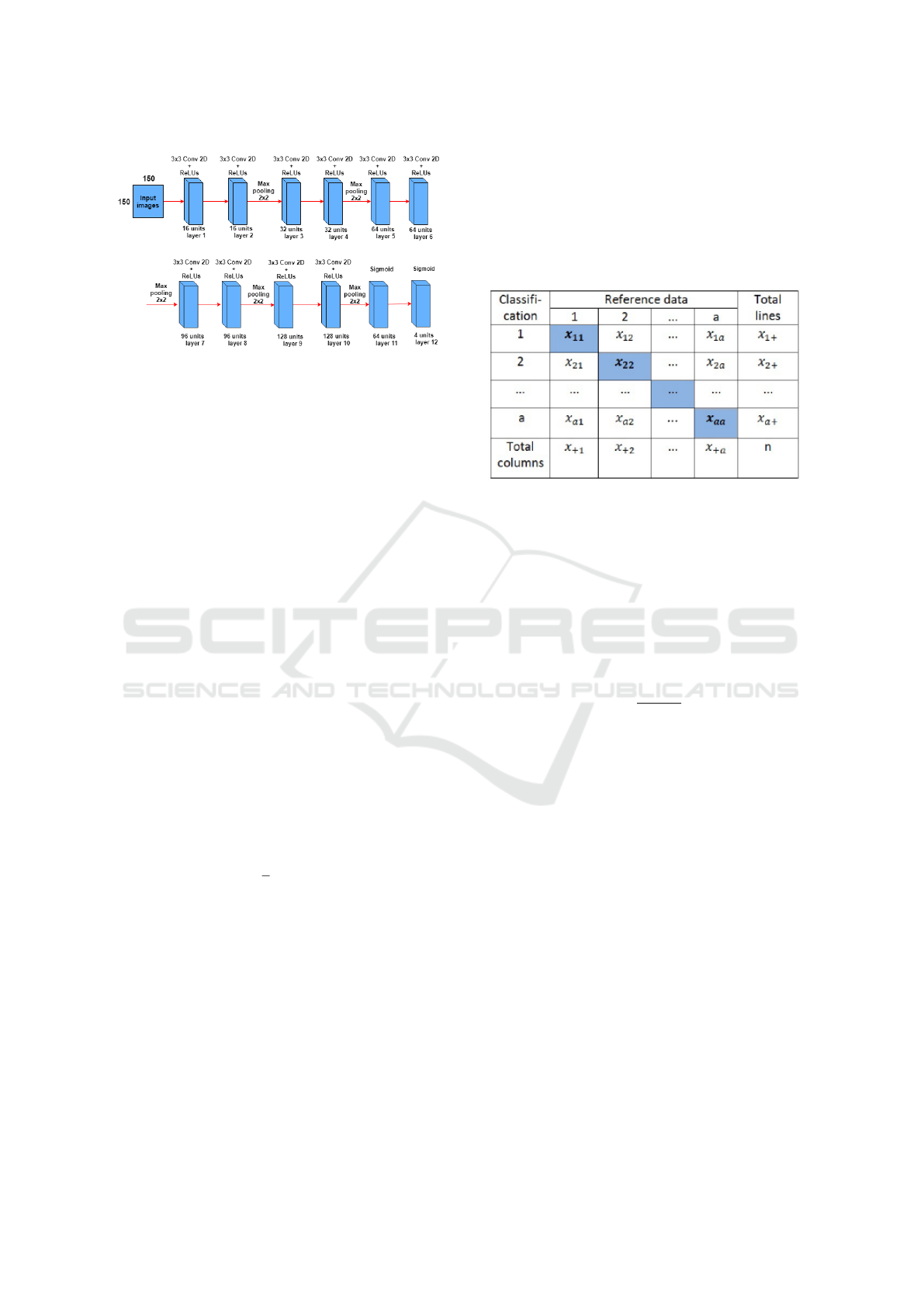
Figure 6: Construction of the CNN training mode.
3 METRICS OF THE
EVALUATION
3.1 Cross Validation
Cross-validation is an evaluation technique on the
ability of generalization models, from a dataset, is
widely used in problems where the object is the mod-
eling and prediction (Vehtari et al., 2017). With this it
is possible to estimate how precise the model is, that
is, its accuracy with data that it does not know.
The k-fold cross-validation method consists of di-
viding the total set into k subsets of the same size.
One subset is used for testing, and the other k-1 sub-
sets for training. This process is repeated by k times,
if circularly changing the subset of tests (Grimm et al.,
2017).
The final precision of the model is estimated by
equation 6, at where Ac
f
is the sum of the differences
between the actual value y
i
and the predicted value
ˆy
i
e k is the amount of k-fold divisions. With this it
is possible to infer the generalization capacity of the
network.
Ac
f
=
1
k
k
∑
i=1
(y
i
− ˆy
i
) (6)
3.2 Confusion Matrix
As a statistical tool we have the confusion matrix that
provides the basis for describe the accuracy of the
classification and characterize the errors, helping re-
fine the ranking (Saraiva et al., 2018).The confusion
matrix is formed by an array of squares of numbers
arranged in rows and columns that express the num-
ber of sample units of a particular category, inferred
by a decision rule, compared to the category current
field.
Usually below the columns is the set reference
data that is compared to the product data of the classi-
fication that are represented along the lines. The fig-
ure 7 shows the representation of an array of confu-
sion. The elements of the main diagonal in bold indi-
cate the level of accuracy, or agreement, between the
two sets of data.
Figure 7: Example matrix of confusion.
The measures derived from the confusion ma-
trix are: the total accuracy being that chosen by the
present work, accuracy of individual class, producer
precision, user precision and Kappa index, among
others. The total accuracy is calculated by dividing
the sum of the main diagonal of the error matrix x
ii
,
by the total number of samples collected n. Accord-
ing to the equation 7.
T =
∑
a
i
=
1
x
ii
n
(7)
4 RESULTS
In this section will be presented the classification per-
formance results of the training model. The metrics
used to evaluate the results are: The average accu-
racy of the cross validation, specificity and sensitivity,
given by the ROC curve.
In the table 1 the results obtained by the training
network are presented. through the table it is possible
to extract information such as: False positives, False
negatives, True positives, True Negative and accuracy
of each interaction of cross validation. The average
accuracy of the network was 94.35%.
In the figure 9 it is possible to check the data re-
lating to iteration 5 of the table 1. In it is shown a
confluence matrix, a graph of the ROC curve and the
precision recall curve. Where it is possible to visual-
ize the sensitivity and specificity of each class. It is
possible to visualize in the figure 8 the graphs of pro-
portions, of each disease and of the test and training.
BIOINFORMATICS 2020 - 11th International Conference on Bioinformatics Models, Methods and Algorithms
172
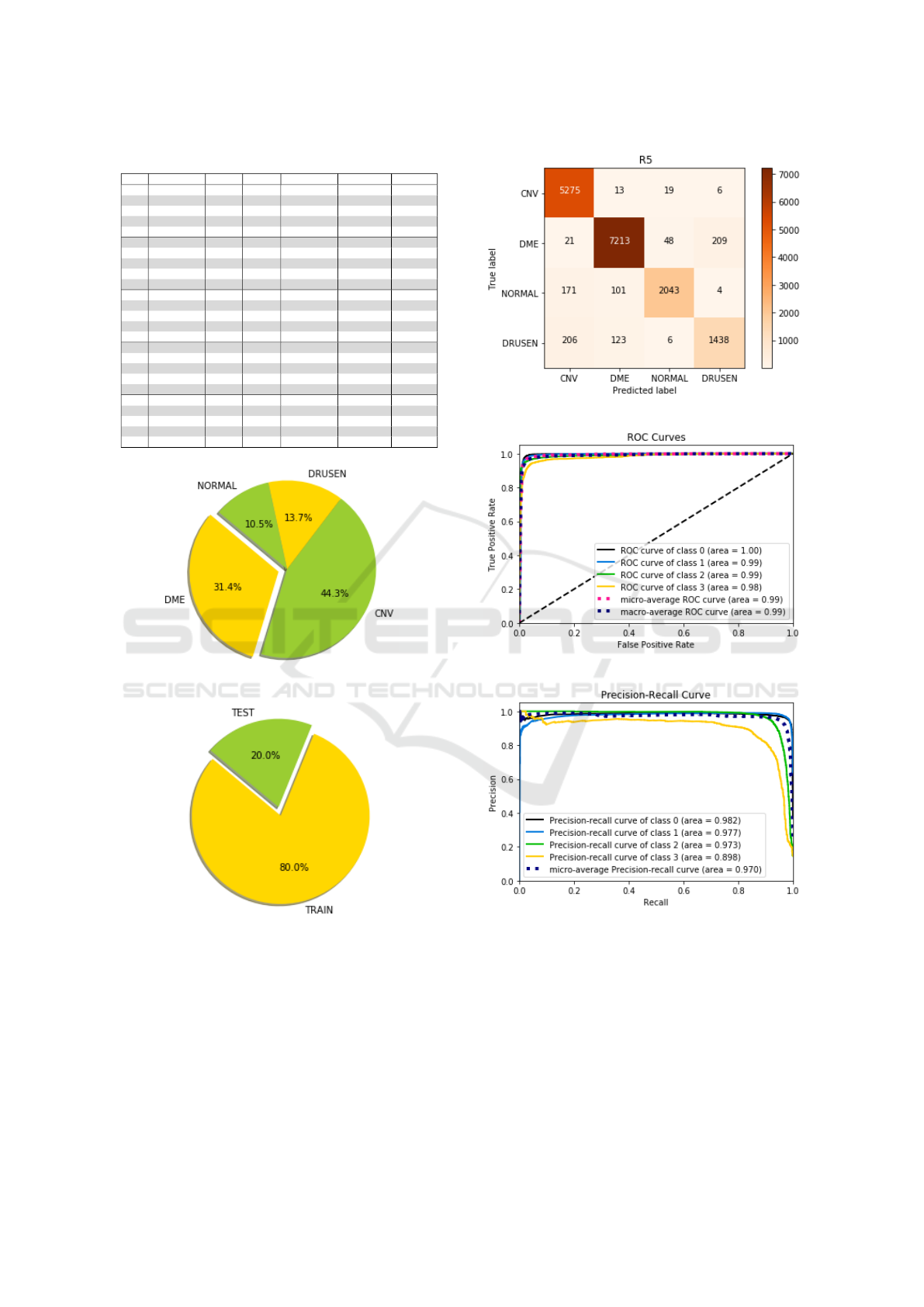
Table 1: Table of iterations and classifications.
** CNV DME NORMAL DRUSEN ACC
1 CNV 5274 10 19 10
1 DME 38 7247 77 83
1 NORMAL 2017 63 2045 5
1 DRUSEN 193 229 9 1343
1 ACC 94.44%
2 CNV 5087 14 168 44
2 DME 9 7329 47 106
2 NORMAL 7 96 2148 6
2 DRUSEN 126 212 22 1413
2 ACC 94.55%
3 CNV 5252 12 22 27
3 DME 17 7348 19 107
3 NORMAL 176 118 2014 12
3 DRUSEN 138 172 2 1461
3 ACC 95.13%
4 CNV 5253 9 8 43
4 DME 58 7130 56 247
4 NORMAL 390 71 1831 27
4 DRUSEN 160 79 7 1527
4 ACC 93.16%
5 CNV 5275 13 19 06
5 DME 21 7213 48 209
5 NORMAL 171 101 2043 4
5 DRUSEN 206 123 6 1438
5 ACC 94.51%
(a) Proportion of the dataset between training and validation
(b) Demographics Proportion Chart
Figure 8: Demographics Proportion Chart.
It is worth noting that the test and training data were
separated proportionally to their total quantity.
Neural network training was performed using an
NVIDIA GTX 1060 video card, which features 1280
CUDA cores (processors), 6 GB of dedicated mem-
ory, 12 GB of RAM and a fourth-generation Core i5
Processor with time of training of 29 minutes.
(a) Confusion matrix
(b) ROC curves
(c) Precision recall curve
Figure 9: Interaction test 5 table 1, Precision recall curve
and ROC curves.
5 CONCLUSION
In this work, a model was presented to classify visi-
ble pathologies in OCT, the classes are CNV, DME,
DRUSEN and NORMAL. For the validation of the
models, cross validation was performed, where it is
Classification of Optical Coherence Tomography using Convolutional Neural Networks
173

possible to verify the generalization capacity. The
classification model evaluated in this work was shown
to be efficient, obtaining an average of 94.35 % ac-
curacy. Being able to reach a high accuracy, even
with the unbalanced dataset and with the iterations
obtained in the cross validation being proxies.
ACKNOWLEDGMENTS
The elaboration of this work would not have been
possible without the collaboration of the Engineering
and DecisionSupport Research Center (GECAD) of
the Institute of Engineering, Polytechnic Institute of
Porto, Portugal and FAPEMA.
REFERENCES
Alten, F., Lauermann, J., Clemens, C., Heiduschka, P.,
and Eter, N. (2017). Signal reduction in chorio-
capillaris and segmentation errors in spectral domain
oct angiography caused by soft drusen. Graefe’s
Archive for Clinical and Experimental Ophthalmol-
ogy, 255(12):2347–2355.
Baxter, S. L., Pistilli, M., Pujari, S. S., Liesegang, T. L.,
Suhler, E. B., Thorne, J. E., Foster, C. S., Jabs,
D. A., Levy-Clarke, G. A., Nussenblatt, R. B., et al.
(2013). Risk of choroidal neovascularization among
the uveitides. American journal of ophthalmology,
156(3):468–477.
Bressler, S. B., Glassman, A. R., Almukhtar, T., Bressler,
N. M., Ferris, F. L., Googe Jr, J. M., Gupta, S. K., Jam-
pol, L. M., Melia, M., Wells III, J. A., et al. (2016).
Five-year outcomes of ranibizumab with prompt or
deferred laser versus laser or triamcinolone plus de-
ferred ranibizumab for diabetic macular edema. Amer-
ican journal of ophthalmology, 164:57–68.
Chen, Y.-H., Krishna, T., Emer, J. S., and Sze, V. (2017).
Eyeriss: An energy-efficient reconfigurable acceler-
ator for deep convolutional neural networks. IEEE
Journal of Solid-State Circuits, 52(1):127–138.
Dimitrova, G., Chihara, E., Takahashi, H., Amano, H., and
Okazaki, K. (2017). Quantitative retinal optical co-
herence tomography angiography in patients with dia-
betes without diabetic retinopathy. Investigative oph-
thalmology & visual science, 58(1):190–196.
Esteva, A., Kuprel, B., Novoa, R. A., Ko, J., Swetter, S. M.,
Blau, H. M., and Thrun, S. (2017). Dermatologist-
level classification of skin cancer with deep neural net-
works. Nature, 542(7639):115.
Gaier, E. D., Rizzo III, J. F., Miller, J. B., and Ces-
tari, D. M. (2017). Focal capillary dropout asso-
ciated with optic disc drusen using optical coher-
ence tomographic angiography. Journal of Neuro-
ophthalmology, 37(4):405–410.
Gill, A., Cole, E. D., Novais, E. A., Louzada, R. N., Carlo,
T., Duker, J. S., Waheed, N. K., Baumal, C. R., and
Witkin, A. J. (2017). Visualization of changes in the
foveal avascular zone in both observed and treated di-
abetic macular edema using optical coherence tomog-
raphy angiography. International journal of retina and
vitreous, 3(1):19.
Grimm, K. J., Mazza, G. L., and Davoudzadeh, P. (2017).
Model selection in finite mixture models: A k-fold
cross-validation approach. Structural Equation Mod-
eling: A Multidisciplinary Journal, 24(2):246–256.
Havaei, M., Davy, A., Warde-Farley, D., Biard, A.,
Courville, A., Bengio, Y., Pal, C., Jodoin, P.-M., and
Larochelle, H. (2017). Brain tumor segmentation
with deep neural networks. Medical image analysis,
35:18–31.
Kermany, D., Zhang, K., and Goldbaum, M. (2018). La-
beled optical coherence tomography (oct) and chest
x-ray images for classification. Structural Equation
Modeling: A Multidisciplinary Journal.
Kov
´
acs, G., T
´
oth, L., Van Compernolle, D., and Ganapathy,
S. (2017). Increasing the robustness of cnn acoustic
models using autoregressive moving average spectro-
gram features and channel dropout. Pattern Recogni-
tion Letters, 100:44–50.
Maggiori, E., Tarabalka, Y., Charpiat, G., and Alliez, P.
(2017). Convolutional neural networks for large-scale
remote-sensing image classification. IEEE Transac-
tions on Geoscience and Remote Sensing, 55(2):645–
657.
Parhi, K. K., Reinsbach, M., Koozekanani, D. D., and Roy-
chowdhury, S. (2017). Automated oct segmentation
for images with dme. In Medical Image Analysis and
Informatics, pages 115–132. CRC Press.
Preti, R. C., Govetto, A., Aqueta Filho, R. G., Zacharias,
L. C., Pimentel, S. G., Takahashi, W. Y., Monteiro,
M. L., Hubschman, J. P., Sarraf, D., et al. (2018). Op-
tical coherence tomography analysis of outer retinal
tubulations: sequential evolution and pathophysiolog-
ical insights. Retina, 38(8):1518–1525.
Roy, R., Saurabh, K., Bansal, A., Kumar, A., Majum-
dar, A. K., and Paul, S. S. (2017). Inflammatory
choroidal neovascularization in indian eyes: Etiology,
clinical features, and outcomes to anti-vascular en-
dothelial growth factor. Indian journal of ophthalmol-
ogy, 65(4):295.
Salamon, J. and Bello, J. P. (2017). Deep convolutional neu-
ral networks and data augmentation for environmental
sound classification. IEEE Signal Processing Letters ,
24(3):279–283.
Saraiva, A., Melo, R., Filipe, V., Sousa, J., Ferreira, N. F.,
and Valente, A. (2018). Mobile multirobot manipula-
tion by image recognition.
Saraiva., A. A., Ferreira., N. M. F., de Sousa., L. L., Costa.,
N. J. C., Sousa., J. V. M., Santos., D. B. S., Valente.,
A., and Soares., S. (2019a). Classification of images
of childhood pneumonia using convolutional neural
networks. In Proceedings of the 12th International
Joint Conference on Biomedical Engineering Systems
and Technologies - Volume 2: BIOIMAGING,, pages
112–119. INSTICC, SciTePress.
Saraiva., A. A., Santos., D. B. S., Costa., N. J. C., Sousa.,
J. V. M., Ferreira., N. M. F., Valente., A., and Soares.,
BIOINFORMATICS 2020 - 11th International Conference on Bioinformatics Models, Methods and Algorithms
174

S. (2019b). Models of learning to classify x-ray im-
ages for the detection of pneumonia using neural net-
works. In Proceedings of the 12th International Joint
Conference on Biomedical Engineering Systems and
Technologies - Volume 2: BIOIMAGING,, pages 76–
83. INSTICC, SciTePress.
Schlanitz, F. G., Baumann, B., Kundi, M., Sacu, S., Barat-
sits, M., Scheschy, U., Shahlaee, A., Mitterm
¨
uller,
T. J., Montuoro, A., Roberts, P., et al. (2017). Drusen
volume development over time and its relevance to the
course of age-related macular degeneration. British
Journal of Ophthalmology, 101(2):198–203.
Sivaprasad, S. and Moore, A. (2008). Choroidal neovascu-
larisation in children. British Journal of Ophthalmol-
ogy, 92(4):451–454.
Swanson, E. A. and Fujimoto, J. G. (2017). The ecosystem
that powered the translation of oct from fundamental
research to clinical and commercial impact. Biomedi-
cal optics express, 8(3):1638–1664.
Ustinova, E., Ganin, Y., and Lempitsky, V. (2017). Multi-
region bilinear convolutional neural networks for per-
son re-identification. In Advanced Video and Signal
Based Surveillance (AVSS), 2017 14th IEEE Interna-
tional Conference on, pages 1–6. IEEE.
Vehtari, A., Gelman, A., and Gabry, J. (2017). Prac-
tical bayesian model evaluation using leave-one-out
cross-validation and waic. Statistics and Computing,
27(5):1413–1432.
Wells, J. A., Glassman, A. R., Ayala, A. R., Jampol, L. M.,
Bressler, N. M., Bressler, S. B., Brucker, A. J., Fer-
ris, F. L., Hampton, G. R., Jhaveri, C., et al. (2016).
Aflibercept, bevacizumab, or ranibizumab for diabetic
macular edema: two-year results from a comparative
effectiveness randomized clinical trial. Ophthalmol-
ogy, 123(6):1351–1359.
Yu, S., Jia, S., and Xu, C. (2017). Convolutional neural
networks for hyperspectral image classification. Neu-
rocomputing, 219:88–98.
Zaheer, R. and Shaziya, H. (2018). Gpu-based empiri-
cal evaluation of activation functions in convolutional
neural networks. In 2018 2nd International Confer-
ence on Inventive Systems and Control (ICISC). IEEE.
Classification of Optical Coherence Tomography using Convolutional Neural Networks
175
