
Blue Light and Melanopsin Contribution to the Pupil Constriction
in the Blind-spot, Parafovea and Periphery
Tim Schilling
1
, Mojtaba Soltanlou
2,3
, Yeshwanth Seshadri
1
, Hans-Christoph Nuerk
2,3
and Hamed Bahmani
1,4,5
1
Dopavision GmbH, Berlin, Germany
2
Department of Psychology, University of Tübingen, Tübingen, Germany
3
LEAD Research Network, University of Tübingen, Tübingen, Germany
4
Max Planck Institute for Biological Cybernetics, Physiology of Cognitive Processes, Tübingen, Germany
5
Bernstein Center for Computational Neuroscience, Tübingen, Germany
Keywords: Melanopsin, Blind-spot, Optic Disc, Pupil.
Abstract: Retinal photoreceptors modulate the pupil diameter to regulate retinal illumination. At early stage the pupil-
response is formed by intrinsically-photosensitive-Retinal-Ganglion-Cells (ipRGCs) expressing melanopsin,
activated by blue light. ipRGCs’ axons pass through the optic nerve head, corresponding to the blind-spot. No
photoreceptors except melanopsin appear to exist in the blind-spot. Contributions of melanopsin to pupil
constriction in absence of classical photoreceptors in the blind-spot is not fully understood. We investigated
how blue light in the blind-spot changes melanopsin-pupil-response compared to parafovea and periphery.
The Post-Illumination-Pupil-Response (PIPR) amplitude reflecting melanopsin was analyzed for standardized
time windows (1s<1.7s, 1s>1.8s and 2–6s) and expressed as pupillary-change. Bayesian analysis showed a
BF>3 that PIPR>1.8s for blind-spot and periphery is not different. At times 2s–6s, a t-test comparison in the
blind-spot condition showed a significantly larger PIPR to blue compared to red light, confirming a
melanopsin-pupil-response in the blind-spot. Taken together, equivalent stimulation in the blind-spot and
periphery revealed comparable PIPR, although there are no rods and cones in the blind-spot. In absence of
classical photoreceptors in the blind-spot, melanopsin seems to be responsible for pupil constriction in similar
manner as in the periphery, which supports the presence of melanopsin on the axons of ipRGCs.
1 INTRODUCTION
In the human eye, the retina contains a peripheral
region without rods and cones where ganglion-cell
axons bundle in the optic nerve. The head of the optic
nerve is called the optic disc which corresponds to the
blind-spot. Although light illumination in the blind-
spot is reported to be invisible, but reduces the
brightness perception of a white light outside the
blind-spot (Saito, Miyamoto, Uchiyama, &
Murakami, 2018), this does not mean that the optic
disc is insensitive to light.
Melanopsin-expressing retinal ganglion cells
(RGCs) are intrinsically photosensitive (ipRGCs) and
receive extrinsic input from rods and cones in
primates (Dacey et al., 2005; Gamlin et al., 2007).
ipRGCs form the afferent pupil pathway to regulate
the pupil response (Gamlin et al., 2007).
Pupil size dynamics are controlled by melanopsin
containing ipRGCs (Fu et al., 2005; Hattar et al.,
2003; Lucas et al., 2003), whose axons pass through
the optic disc of the human eye. It has been shown in
rats that melanopsin is expressed in cell bodies,
dendrites, and proximal axonal segments of this
subset of RGCs (Hattar, Liao, Takao, Berson, & Yau,
2002). Melanopsin is sensitive to shorter wavelengths
centered around 480 nm (Berson, Dunn, & Takao,
2002; Hattar et al., 2002). In contrast to the short-
wave blue spectrum, red light consists of higher
wavelengths over 600 nm, which barely overlaps with
the sensitivity spectrum of melanopsin.
Light of different wavelengths have been shown
to modulate pupil response differently. When the
whole retina is stimulated, it has been shown that
short-wavelength blue light (467 ± 10 nm) induces a
larger change in pupil size, whereas global long-
482
Schilling, T., Soltanlou, M., Seshadri, Y., Nuerk, H. and Bahmani, H.
Blue Light and Melanopsin Contribution to the Pupil Constriction in the Blind-spot, Parafovea and Periphery.
DOI: 10.5220/0008972404820489
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 5: HEALTHINF, pages 482-489
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

wavelength red light (640 ± 17 nm) leads to a smaller
change (Park et al., 2011).
In the beginning of the 19th century, Hess found
a pupil contraction when he focused light on the
blind-spot (Hess, 1908). Later, stimuli of different
wavelengths were delivered to the blind-spot and the
spectral response curve was assumed to be a modified
rod curve originating from scattered light (Alpern &
Campbell, 1962). Alpern and Campbell speculated on
whether the deviation from the rod curve derives from
the activity of another photosensitive visual substance
in addition to rhodopsin. Their postulated visual
substance might be melanopsin, which was shown to
be present on ipRGCs’ axons a few decades later
(Hattar et al., 2002).
Melanopsin-expressing ipRGCs have been
documented as playing a role in the pupil light
response, contributing to it a sluggish recovery
component; an extended response primarily mediated
by melanopsin activation that persists for some time
after stimulus light termination - referred to as Post-
Illumination or Post-Stimulus Pupillary Response
(PIPR or PSPR respectively; Münch, Léon, Crippa, &
Kawasaki, 2012). There is evidence that melanopsin
drives the PIPR, which is a sustained pupil
constriction after stimulus offset, produced by the
intrinsic response of ipRGCs (Adhikari, Zele, &
Feigl, 2015; Dacey et al., 2005; Gamlin et al., 2007).
While both rods and ipRGCs have been shown to
provide input to the pupil light response, hence
rhodopsin and melanopsin contribute largely to PIPR
<1.7 s (Adhikari, Feigl, & Zele, 2016), it was reported
that melanopsin dominates all phases of PIPR and
solely contributes to the PIPR after 1.7 s (Adhikari et
al., 2016).
Miyamoto and Murakami studied the effects of
light stimulation inside the blind-spot on pupillary
light reflex with additional stimulus outside the blind-
spot (Miyamoto & Murakami, 2015). A sole
stimulation of the blind-spot and its effect on PIPR
has not been investigated to date, as far as we know.
We conducted experiments to examine the PIPR in
the blind-spot as well as periphery and parafovea to
have comparable conditions outside the blind-spot.
We tested the hypothesis that blue light constricts the
pupil more than red light in the blind-spot, when
elicited by melanopsin. The purpose of the study was
to compare melanopsin-induced PIPR when
illuminating the retina with a stimulus a) fitting in the
blind-spot and b) of equivalent size on parafovea and
periphery.
2 METHODS
2.1 Participants
The study contains data from 15 participants with
normal or corrected to normal vision. Visual acuity
was assessed before the experiment with FrACT
(Bach, 2006) to be greater or equal to 0.0 logMAR.
All experiments were approved by the Ethics
Committee for Psychological Research at the
Department of Psychology of the University of
Tuebingen and conducted in accordance with the
tenets of the Declaration of Helsinki. After explaining
the experiment, written informed consent was
obtained from all participants.
2.2 Stimulus
The stimulus consists of red or blue circular discs
presented in three different locations of the retina: in
the parafovea, in the peripheral retina and in the
blind-spot. The monitor (FUJITSU Display B24-8 TS
Pro, Fujitsu Technology Solutions GmbH, Munich,
Germany) was placed 50 cm in front of the
participant’s head, which was stabilized in a chin rest.
The right eye was covered with an eye patch while
the left eye dynamics were captured by an eye tracker
(EyeLink 1000 Eye Tracking system, SR Research
Ltd., Ottawa, Ontario, Canada), after the inbuilt
calibration procedure was completed.
The blind-spot of the left eye was mapped using
the following calibration procedure: the participant
looked at a fixation target and adjusted a disc on the
screen within the position and radius of the blind-
spot. The fixation target consisted of four points
around the center - above, below, left, and right -
connected circularly with a thin line (see Fig. 1). The
stimulus size and stimulus position had to be adjusted
by the participant with a keyboard until the stimulus
was invisible for the participant when fixating at all
four surrounding fixation points and the centered
fixation target. For the parafovea condition the
stimulus was in the parafoveal region, meaning
outside the fixation target in the horizontal direction
towards the corresponding blind-spot, 1.2° inferior
and 3.4° lateral leftwards to the fixation resulting in a
total distance of 3.4° visual angle from the fixation
target. The peripheral stimulus was in the same
direction as parafoveal and blind-spot in the visual
field, 12.4° distant from the fixation target, located at
4.6° superior and 11.9° lateral leftwards to the
fixation target. Stimulus diameter varied among
individuals for the blind-spot condition and was 1.25°
for parafovea condition and periphery condition.
Blue Light and Melanopsin Contribution to the Pupil Constriction in the Blind-spot, Parafovea and Periphery
483
Blue stimulus was composed of short-wavelength
blue light with a peak at 450 nm (CIE color
coordinates: x = 0.15, y = 0.06) whereas the red
stimulus consisted of long-wavelength red light with
a peak at 610 nm (CIE color coordinates: x = 0.65,
y = 0.34) stimulus measured with i1 studio (x-rite
Incorporated, Kentwood, Michigan, USA) and the
software f.luxometer™ LLC (Los Angeles,
California, USA). Both stimuli had a luminance of
11.8 cd/m², measured with a luminance meter
(Konica Minolta LS-110, Konica Minolta, Inc.,
Tokyo, Japan). The background of the monitor was
set to black. Participants were asked to passively view
the fixation target while the stimuli were presented to
the target locations. Gaze position was monitored
during the experiment to ensure a fixation on the
target.
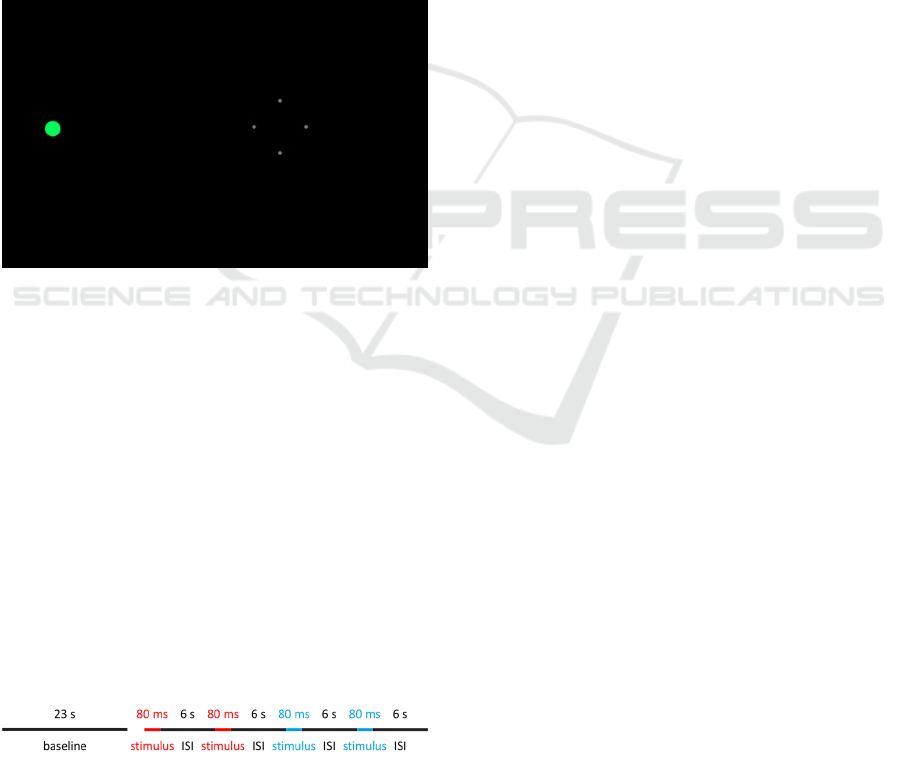
Figure 1: A screenshot of the display during the calibration
phase. Stimulus color was green for calibration phase.
The experiment consisted of one block of four
trials with two red and blue stimuli, separately (see
Fig. 2). The red disc was always shown first to
account for the bi-stability factor (Mure et al., 2009).
Each block was presented separately for blind-spot
condition, parafovea condition, and periphery
condition. One block (47.32 s) began with a 23 s
baseline, followed by an 80 ms stimulus four times
and a 6 s inter-stimulus-interval (ISI). The stimulus
length of 80 ms was chosen as it is over the minimal
duration for a pupillary light reflex (Webster, 1969)
and secondly, to reduce the chance that eye fixation
was outside the fixation target.
A 6 s window is recommended when using short
pulse (Adhikari et al., 2015).
Figure 2: One block consists of a 23 s baseline and an 80 ms
stimulus four times followed by a 6 s inter-stimulus-interval
(ISI).
2.3 Analysis
Data was preprocessed by down-sampling the signal
to 10 Hz, removing blinks and interpolating the
signal. Pupil response was corrected to the 500 ms
pre-stimulus baseline. Furthermore, the pupillary
change was calculated as a percentage relative to the
pre-stimulus baseline, as recommended by Kelbsch
and colleagues to obtain relative pupil constriction
amplitude (Kelbsch et al., 2019). For the Area Under
the Curve (AUC) analysis, the pupil response values
were calculated to a 100% pre-stimulus baseline,
whereas for pupillary change, the baseline was
adapted to 0% by subtracting 100%.
The pupillary change was analyzed along
different time windows. Following the standards of
pupillography, combined rhodopsin and melanopsin
contribution was analyzed in a 1 s time window
before 1.7 s, and sole melanopsin contribution was
analyzed in a 1 s time window after 1.8 s (Kelbsch et
al., 2019). Additionally, the AUC from 2 s to 6 s PIPR
was calculated, which is until the end of the ISI,
which was adapted from the standard AUC of 2 s to
10 s (Kelbsch et al., 2019) because our ISI was not
longer than 6 s.
For statistical analysis repeated measure ANOVA
for the factor stimulus location and color with post-
hoc test Tukey correction was conducted.
Additionally, to test the a priori hypothesis, a paired
one-sided t-test was performed on AUC.
Furthermore, Bayesian inference statistics was used
in order to test the absence of a difference (null
hypothesis). Therefore, the Bayes factors were
calculated to evaluate evidence in favor of the null
hypothesis (BF
01
). Bayes factors were categorized to
the degrees of evidence by Kass and Raftery (Kass &
Raftery, 1995) i.e. BF
01
= 1-3 is interpreted as weak
evidence, BF
01
= 3-20 is interpreted as positive
evidence, BF
01
= 20-150 is interpreted as strong
evidence and BF
01
> 150 is interpreted as very strong
evidence.
For statistical analysis JASP (Version 0.10.2.0,
JASP Team, 2019) was used. The rest of the pupil
signal processing and preprocessing was done with
Octave (John W. Eaton, David Bateman, Søren
Hauberg, 2018).
3 RESULTS
We analyzed PIPR for melanopsin contribution in
different stimulus locations using three standardized
time windows, separately: times < 1.7 s for rhodopsin
and melanopsin contribution, and times > 1.8 s for
HEALTHINF 2020 - 13th International Conference on Health Informatics
484
melanopsin contributions, and 2 s – 6 s for
melanopsin contributions, see shaded gray box for
different time windows in Fig. 3.
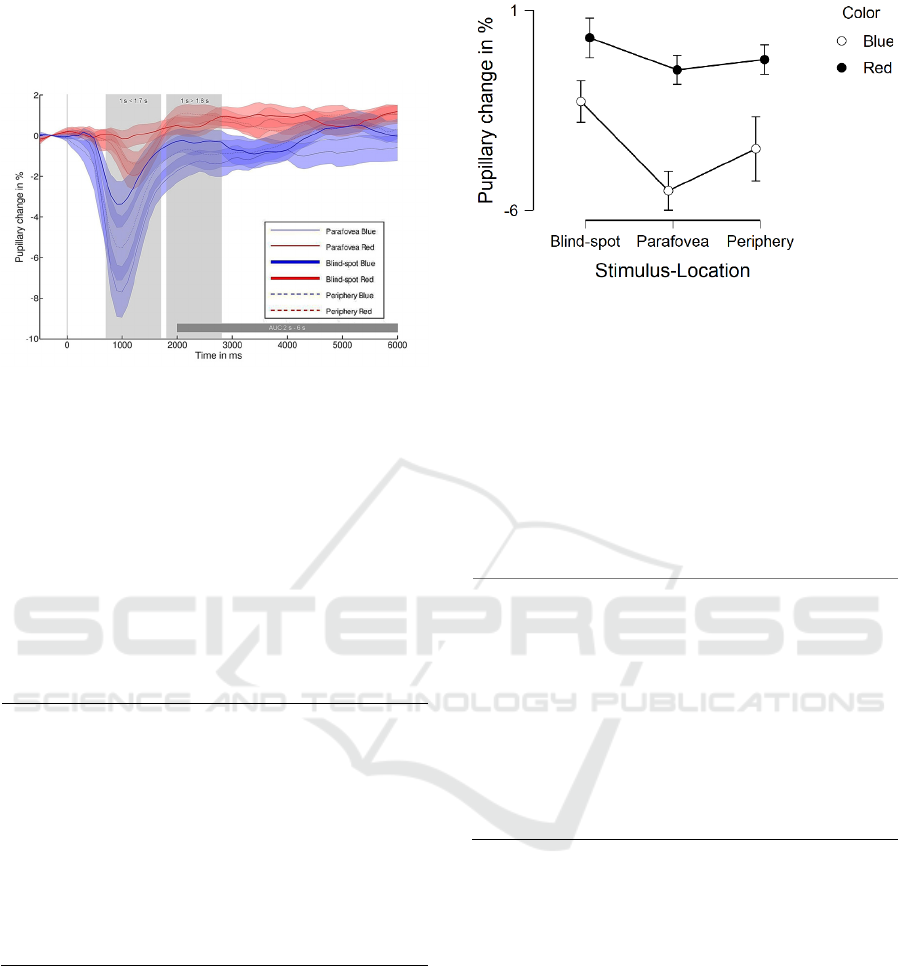
Figure 3: Mean and standard error of the mean (SEM) of
pupillary change in % to blue and red stimulus for blind-
spot, parafovea and periphery over time in ms. Stimulus
onset is at 0 ms.
3.1 Melanopsin and Rhodopsin at
Times < 1.7 s
A repeated ANOVA revealed a significant main
effect for color (p < 0.001) and stimulus location
(p < 0.05), but not for their interaction (p = 0.20), see
Table 1.
Table 1: ANOVA of PIPR at times < 1.7 s in detail.
df F p η²
p
Stimulus
location
2.28 3.60 0.04 0.06
Color
1.14 17.42 < 0.001 0.19
Stimulus
location
✻ Color
2.28 1.71 0.20 0.01
Regarding stimulus location, post-hoc tests
corrected with Tukey showed that pupil response to
the parafovea is larger than blind-spot condition
(p < 0.05), but no significant difference appeared
between the blind-spot condition and periphery
condition (p = 0.25) and the parafovea condition and
periphery condition (p = 0.50), see Fig. 4.
Regarding color, post-hoc tests corrected with
Tukey showed that pupil response to blue light is
significantly larger than the red light condition
(p < 0.001).
Figure 4: Mean and SEM of pupillary change in % at times
< 1.7 s for blind-spot, parafovea and periphery stimulus
location separated into blue and red light conditions.
3.2 Melanopsin at Times > 1.8 s
The repeated ANOVA revealed a significant main
effect for color (p < 0.01), but not for stimulus
location (p = 0.48) and their interaction (p = 0.39),
see Table 2.
Table 2: ANOVA of PIPR at times > 1.8 s in detail.
df F p η²
p
Stimulus
location
2.28 0.75 0.48 0.02
Color
1.14 11.93 < 0.01 0.12
Stimulus
location ✻
Color
2.28 0.96 0.39 0.01
Regarding color, post-hoc tests corrected with
Tukey showed that pupil response to blue light is
significantly larger than for the red light condition
(p < 0.01), see Fig. 5.
In the red light condition for the parafovea
condition and periphery condition, an overshoot after
pupil constriction shortly before 2 s can be observed,
which is not in the blind-spot condition, see Fig. 3.
Therefore, PIPR at times 1 s > 1.8 s was analyzed
only in blue light conditions in the following analysis.
In order to test the hypothesis for no difference
between blind-spot and periphery conditions,
Bayesian analysis was conducted. Positive evidence
(i.e., BF
01
= 3.2, error = 0.003%) was observed
comparing blind-spot and periphery at times
1 s > 1.8 s. An overlapping PIPR at times after 1.8 s
is visible in Fig. 6 between blind-spot and periphery.
Blue Light and Melanopsin Contribution to the Pupil Constriction in the Blind-spot, Parafovea and Periphery
485
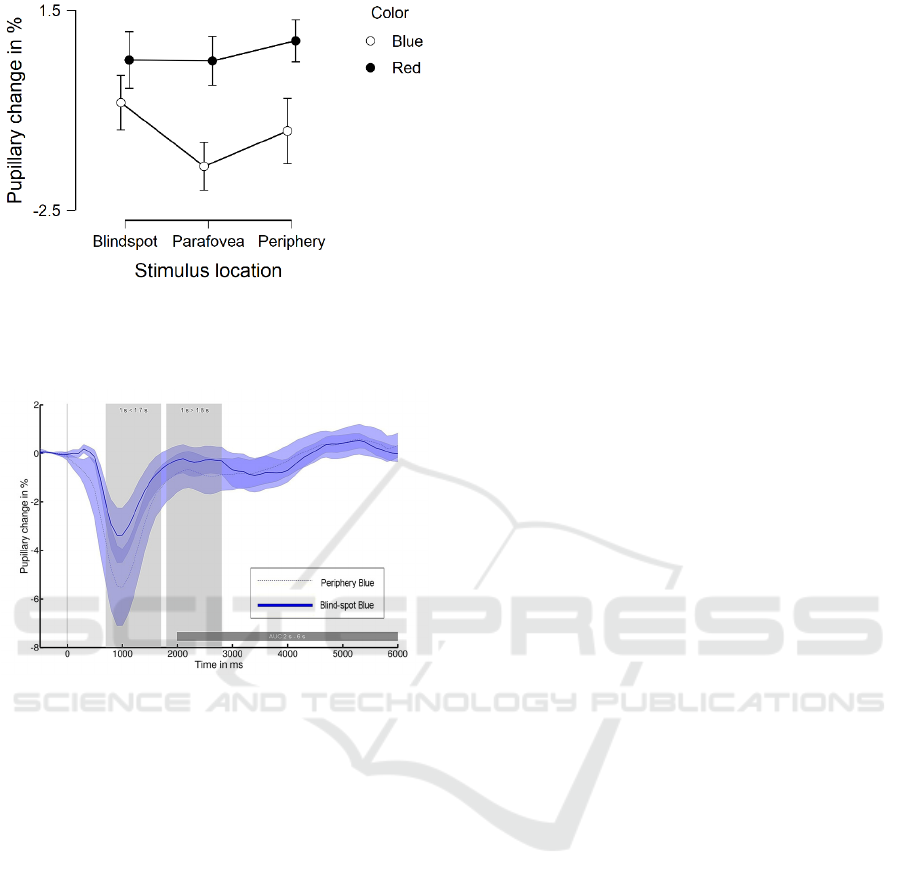
Figure 5: Mean and SEM of pupillary change in % for
sustained pupillary response Melanopsin at times > 1.8 s for
blind-spot, parafovea and periphery stimulus location
separated into blue and red light conditions.
Figure 6: Pupillary change for blind-spot (solid line) and
periphery (dotted line) condition for blue light condition.
Stimulus onset is at 0 ms.
3.3 Melanopsin at Times 2 s – 6 s
For the AUC at times 2 s – 6 s, Bayesian analysis was
conducted in order to test for no difference in PIPR
after blue light stimulus between the blind-spot and
periphery. Positive evidence (i.e., BF
01
= 3.81,
error = 0.003% was observed when comparing the
blind-spot and periphery conditions.
Lastly, to test our hypothesis, due to no overshoot
in blind-spot red light condition, a paired one-sided t-
test comparison in the blind-spot condition showed a
significantly larger pupillary change to blue light as
compared to red light (t(14) = -1.9, p = 0.043,
d = -0.48).
4 DISCUSSION
We characterized pupil responses after blue light
stimulation in the blind-spot to investigate
melanopsin contributions in the blind-spot compared
to parafovea condition and periphery condition.
Miyamoto and Murakami found that stimulation
inside the blind-spot enhances, but does not trigger,
the pupillary light reflex (Miyamoto & Murakami,
2015). A photo-sensitive mechanism inside the optic
disk, which most likely involves melanopsin, has
been suggested to provide a reference for calibrating
the perceived brightness of visual objects (Saito et al.,
2018). In this study, we further explored the presence
of melanopsin in the blind-spot by investigating the
sustained response of pupil constriction regarding
melanopsin contribution to PIPR when stimulating
the blind-spot solely.
At both times < 1.7 s and times > 1.8 s pupillary
change in blue light conditions were larger than in the
red light conditions independent of the stimulus
location. This independency was indicated by the
absence of a significant interaction between the two
factors color and stimulus location. Therefore, we
assume that melanopsin contributes to the sustained
pupil response in the blind-spot, parafovea and
periphery. This is consistent with previous work that
described sustained pupil constriction by the spectral
sensitivity of melanopsin (Gamlin et al., 2007).
However, the difference between the blue and red
light could also be explained by greater than a 0%
pupillary change in the red light conditions, which
could come from a dilating pupil, while the pupillary
change was only slightly below 0% in blue light
conditions.
The time window of 1 s before times < 1.7 s is
reported to be influenced by the contribution of
rhodopsin and melanopsin (Adhikari et al., 2016).
Due to the absence of rods and cones in the optic disc,
blind-spot stimulation is influenced less by rhodopsin
than parafovea region is, which could explain the
observed difference between the blind-spot condition
and parafovea condition at times < 1.7 s.
Furthermore, this difference between blind-spot and
parafovea can be explained by the finding that red
light does not trigger the pupil constriction in the
blind-spot because of the absence of cones in the
blind-spot, as shown before (Miyamoto & Murakami,
2015).
For the blind-spot condition, we would mainly
expect the pupillary response to be influenced by
melanopsin, whereas the periphery would be
influenced by both rhodopsin and melanopsin.
Even when the light is focused exactly on the
optic disc, there could be light hitting the retina on
other locations than the target area due to light
scattering. Small particles in the compartments of the
eye, such as cornea and crystalline lens, and the
HEALTHINF 2020 - 13th International Conference on Health Informatics
486

spectacles - if worn - can scatter light away from the
target (van den Berg, Franssen, Kruijt, & Coppens,
2013), which would activate photoreceptors on the
retina. Therefore, we implemented two control
conditions in the periphery and parafovea. The
scattered light profile is similar in blind-spot and
periphery due to comparable location and similar
stimulus characteristics. If scattering was the reason
for blind-spot PIPR, we would have expected a larger
PIPR in the periphery as compared to the blind-spot
due to the extra rhodopsin contribution which is not
present in the blind-spot; but both conditions have
similar PIPRs. Earlier works have provided further
arguments to rule out a substantial influence by
scattered light when stimulating the blind-spot (Saito
et al., 2018). Still, we cannot completely exclude that
the remaining scattered light may stimulate rods to
modulate the pupil response in our experiment.
There are a few reasons for choosing the
parafoveal location. First, in the central fovea there
are no RGCs between the entering light and the
photoreceptor layer. To have comparable condition to
the blind-spot and periphery, the stimulus was placed
at the outer edge of the parafoveal region, where
RGCs project away from the fovea. Secondly,
potential filtering by the macular pigment is expected
to be reduced when moving from the center towards
the periphery as compared to the central fovea.
Finally, no cells containing melanopsin have been
reported in the central retina (Dacey et al., 2005; Liao
et al., 2016; Nasir-Ahmad, Lee, Martin, & Grünert,
2019); therefore, to target melanopsin containing
cells, we stimulated near the fovea in the parafovea
region.
Previous studies have shown that pupil response
can be induced by purely activating melanopsin, for
example see (Woelders et al., 2018). Furthermore,
pupil response is intact in cone-less and rod-less mice
with a peak sensitivity at 479 nm (Lucas et al., 2001).
Nevertheless, there was no difference between
periphery and blind-spot, which could be explained
by the presence of melanopsin in the blind-spot,
because the pupil response in the periphery should be
driven by both rhodopsin and melanopsin. To date,
we are not aware of any systematic comparison
between melanopsin concentration in the blind-spot
and other regions, but it has been shown that the
concentration of melanopsin cells is higher near the
human fovea when compared to the periphery (Nasir-
Ahmad et al., 2019). Such a difference between
parafovea and periphery was not evidenced in our
data, probably because our peripheral condition was
not far enough in the periphery. We would expect a
decrease in the sustained pupil response with a more
eccentric peripheral condition that can be subject to
future studies.
Looking only at blue light conditions, the
Bayesian analysis provided support for the null
hypothesis that there is no difference between blind-
spot and periphery PIPR; indicating an equal
contribution of melanopsin in both conditions. One
explanation for the similarity in the sustained signal
among all stimulus location for the blue light
condition could be that blind-spot contains as much
melanopsin as the parafovea and periphery.
The limitation of this comparison among the blue
light conditions is that BF
01
was uncorrected and no
strong or very strong evidence was found, however it
showed positive evidence for the absence of a
difference in PIPR between the blind-spot and
periphery. One possible explanation would be that
only two trials per stimulus condition were recorded
and no trials were excluded. Furthermore, we looked
closer at the blue light conditions only at times
1 s > 1.8 s for the following reason: the red light
condition in blind-spot seems to increase pupil size
continuously, because no input triggered the pupil
size change and the pupil was not dark adapted and
therefore not stable. Moreover, an overshoot after
pupil constriction was noticeable in the red light
conditions, except in the blind-spot condition, likely
because there was no pupil constriction. This
overshoot could affect the calculation of the
melanopsin response at times 1 s > 1.8 s, if the red
light condition was the reference.
However, this overshoot in the red light condition
was not visible for the blind-spot. Therefore, it allows
a comparison between blue and red light in the blind-
spot. This comparison confirmed our hypothesis that
blue light constricts the pupil more than red light
when shone in the blind-spot. To our knowledge, this
sustained pupil constriction in the blind-spot is a
novel finding and suggests the presence of
melanopsin in the blind-spot.
Previous work found that pupil response is
enhanced by blue light as compared to red light inside
the blind-spot, when outside blind-spot was also
illuminated (Miyamoto & Murakami, 2015).
Miyamoto and Murakami speculated that this is
modulated by melanopsin in the blind-spot. Our
results provide further support for the presence of
melanopsin in the blind-spot. Similarly, the AUC
between 2 s – 6 s showed that blue light stimulation
in the blind-spot keeps the pupil constricted for a
longer time as compared to red light; which suggests
a contribution of blind-spot melanopsin to the PIPR.
Blue Light and Melanopsin Contribution to the Pupil Constriction in the Blind-spot, Parafovea and Periphery
487

5 CONCLUSIONS
In conclusion blue light stimulation inside blind-spot
and outside blind-spot in the peripheral retina
revealed a comparable PIPR, although there are no
rods and cones in the optic disc. In the absence of
classical photoreceptors, melanopsin seems to be
responsible for pupil constriction when light is shone
in the blind-spot. This supports the presence of
melanopsin on the axons of ipRGCs at the head of
optic nerve, which can constitute potential
applications of stimulating melanopsin with visible
light, although invisible to the observer.
ACKNOWLEDGEMENTS
This work was supported by the Federal Ministry of
Education and Research, Industrie-in-Klinik-
Plattform Program BMBF, Germany (FKZ:
13GW0256). MS was supported by the DFG grant
[NU 265/3-1] to HCN. MS and HCN are members of
the LEAD Research Network [GSC1028], which is
funded within the framework of the Excellence
Initiative of the German federal and state
governments. We would like to thank Zoë Kirste for
language proofreading.
REFERENCES
Adhikari, P., Feigl, B., & Zele, A. J. (2016). Rhodopsin and
melanopsin contributions to the early redilation phase
of the post-illumination pupil response (PIPR). PLoS
One, 11(8), e0161175.
Adhikari, P., Zele, A. J., & Feigl, B. (2015). The post-
illumination pupil response (PIPR). Investigative
Ophthalmology & Visual Science, 56(6), 3838–3849.
Alpern, M., & Campbell, F. W. (1962). The spectral
sensitivity of the consensual light reflex. The Journal of
Physiology, 164(3), 478–507.
Bach, M. (2006). The Freiburg Visual Acuity Test-
variability unchanged by post-hoc re-analysis. Graefe’s
Archive for Clinical and Experimental Ophthalmology,
245(7), 965–971.
Berson, D. M., Dunn, F. A., & Takao, M. (2002).
Phototransduction by retinal ganglion cells that set the
circadian clock. Science, 295(5557), 1070–1073.
Dacey, D. M., Liao, H.-W., Peterson, B. B., Robinson, F.
R., Smith, V. C., Pokorny, J., … Gamlin, P. D. (2005).
Melanopsin-expressing ganglion cells in primate retina
signal colour and irradiance and project to the LGN.
Nature, 433(7027), 749.
Fu, Y., Zhong, H., Wang, M. H., Luo, D., Liao, H., Maeda,
H., … Yau, K. (2005). Intrinsically Photosensitive
Retinal Ganglion Cells Detect Light With a Vitamin A–
Based Photopigment That is Most Likely Melanopsin.
Investigative Ophthalmology & Visual Science, 46(13),
2238.
Gamlin, P. D. R., McDougal, D. H., Pokorny, J., Smith, V.
C., Yau, K.-W., & Dacey, D. M. (2007). Human and
macaque pupil responses driven by melanopsin-
containing retinal ganglion cells. Vision Research,
47(7), 946–954.
Hattar, S., Liao, H.-W., Takao, M., Berson, D. M., & Yau,
K.-W. (2002). Melanopsin-containing retinal ganglion
cells: architecture, projections, and intrinsic
photosensitivity. Science, 295(5557), 1065–1070.
Hattar, S., Lucas, R. J., Mrosovsky, N., Thompson, S.,
Douglas, R. H., Hankins, M. W., … Foster, R. G.
(2003). Melanopsin and rod–cone photoreceptive
systems account for all major accessory visual
functions in mice. Nature, 424(6944), 75.
Hess, C. v. (1908). Untersuchungen zur Physiologie und
Pathologie des Pupillenspieles. Arch. f. Augenheilk, 60,
327–389.
John W. Eaton, David Bateman, Søren Hauberg, R. W.
(2018). GNU Octave version 4.4.1 manual: a high-level
interactive language for numerical computations.
Kass, R. E., & Raftery, A. E. (1995). Bayes factors. Journal
of the American Statistical Association, 90(430), 773–
795.
Kelbsch, C., Strasser, T., Chen, Y., Feigl, B., Gamlin, P. D.,
Kardon, R., … Szabadi, E. (2019). Standards in
pupillography. Frontiers in Neurology, 10.
Liao, H., Ren, X., Peterson, B. B., Marshak, D. W., Yau,
K., Gamlin, P. D., & Dacey, D. M. (2016). Melanopsin-
expressing ganglion cells on macaque and human
retinas form two morphologically distinct populations.
Journal of Comparative Neurology, 524(14), 2845–
2872.
Lucas, R. J., Douglas, R. H., & Foster, R. G. (2001).
Characterization of an ocular photopigment capable of
driving pupillary constriction in mice. Nature
Neuroscience, 4(6), 621.
Lucas, R. J., Hattar, S., Takao, M., Berson, D. M., Foster,
R. G., & Yau, K.-W. (2003). Diminished pupillary light
reflex at high irradiances in melanopsin-knockout mice.
Science, 299(5604), 245–247.
Miyamoto, K., & Murakami, I. (2015). Pupillary light
reflex to light inside the natural blind spot. Scientific
Reports, 5, 11862.
Münch, M., Léon, L., Crippa, S. V, & Kawasaki, A. (2012).
Circadian and wake-dependent effects on the pupil light
reflex in response to narrow-bandwidth light pulses.
Investigative Ophthalmology & Visual Science, 53(8),
4546–4555.
Mure, L. S., Cornut, P.-L., Rieux, C., Drouyer, E., Denis,
P., Gronfier, C., & Cooper, H. M. (2009). Melanopsin
bistability: a fly’s eye technology in the human retina.
PLoS One, 4(6), e5991.
Nasir-Ahmad, S., Lee, S. C. S., Martin, P. R., & Grünert,
U. (2019). Melanopsin-expressing ganglion cells in
human retina: Morphology, distribution, and synaptic
connections. Journal of Comparative Neurology,
527(1), 312–327.
HEALTHINF 2020 - 13th International Conference on Health Informatics
488

Park, J. C., Moura, A. L., Raza, A. S., Rhee, D. W., Kardon,
R. H., & Hood, D. C. (2011). Toward a clinical protocol
for assessing rod, cone, and melanopsin contributions
to the human pupil response. Investigative
Ophthalmology & Visual Science, 52(9), 6624–6635.
Saito, M., Miyamoto, K., Uchiyama, Y., & Murakami, I.
(2018). Invisible light inside the natural blind spot alters
brightness at a remote location. Scientific Reports, 8(1),
7540.
Van den Berg, T. J. T. P., Franssen, L., Kruijt, B., &
Coppens, J. E. (2013). History of ocular straylight
measurement: a review. Zeitschrift Für Medizinische
Physik, 23(1), 6–20.
Webster, J. G. (1969). Critical duration for the pupillary
light reflex. JOSA, 59(11), 1473–1478.
Woelders, T., Leenheers, T., Gordijn, M. C. M., Hut, R. A.,
Beersma, D. G. M., & Wams, E. J. (2018). Melanopsin-
and L-cone–induced pupil constriction is inhibited by
S-and M-cones in humans. Proceedings of the National
Academy of Sciences, 115(4), 792–797.
Blue Light and Melanopsin Contribution to the Pupil Constriction in the Blind-spot, Parafovea and Periphery
489
