
Detection of Abnormalities in Electrocardiogram (ECG) using Deep
Learning
Jo
˜
ao Pestana
a
, David Belo
b
and Hugo Gamboa
c
LIBPHYS-UNL / FCT, New University of Lisbon, Portugal
Keywords:
Electrocardiogram, Signal Processing, Deep Learning, Artificial Intelligence, Arrhythmia Detection, Noise
Detection.
Abstract:
The Electrocardiogram (ECG) cyclic behaviour gives insights on a subject’s emotional, behavioral and car-
diovascular state, but often presents abnormal events. The noise made during the acquisition, and presence of
symptomatic patterns are examples of anomalies. The proposed Deep Learning framework learns the normal
ECG cycles and detects its deviation when the morphology changes. This technology is tested in two different
settings having an autoencoder as base for learning features: detection of three different types of noise, and
detection of six arrhythmia events. Two Convolutional Neural Network (CNN) algorithms were developed
for noise detection achieving accuracies of 98.18% for a binary-class model and 70.74% for a multi-class
model. The development of the arrhythmia detection algorithm also included a Gated Recurrent Unit (GRU)
for grasping time-dependencies reaching an accuracy of 56.85% and an average sensitivity of 61.13%. The
process of learning the abstraction of a ECG signal, currently sacrifices the accuracy for higher generalization,
better discriminating the presence of abnormal events in ECG than detecting different types of events. Further
improvement could represent a major contribution in symptomatic screening, active learning of unseen events
and the study of pathologies to support physicians in the future.
1 INTRODUCTION
In the context of medicine and healthcare in gen-
eral, physiological signals offer information about the
health state. For the ECG, in particular, the morpho-
logical and spectral components of each cycle pro-
vides hints of the emotional, behavioral and the health
state of the individual (Silipo and Marchesi, 1998;
Brown, 1999).
With a deeper understanding of pathologies and
the development of diagnostic methods and therapies
allied to the evolution of technology in the medical
field (e.g. wearables), greater healthcare expectations
emerged in terms of efficiency. The merged fields of
machine learning and the medical field provide tech-
nologies that are useful in assisting medical practi-
tioners not only by decision making processes, diag-
nosis and treatment, but also by continuous monitor-
ing (Johnson et al., 2018; Coiera, 2003; Faust et al.,
2018). On this account Deep Neural Networks (DNN)
a
https://orcid.org/0000-0002-1760-5255
b
https://orcid.org/0000-0002-5337-0430
c
https://orcid.org/0000-0002-4022-7424
present themselves as a tool that learns higher abstrac-
tions of data, while dealing with a large amount of
data and the decrease the need for feature engineer-
ing (Johnson et al., 2018; Faust et al., 2018).
We propose a framework that learns the abstrac-
tion of the default morphology of a normal ECG and
detects the divergence when it is modified, in two dif-
ferent scenarios: contamination due to noise and arti-
facts, and; the presence of symptomatic occurrences.
2 ELECTROCARDIOGRAM
The ECG measures the electrical activity generated
by the heart activity in relation to time by inserting
electrodes on the skin. This signal is used to diag-
nose the cardiac health state and can be accessed by
understanding the fundamentals of its cycle, i.e. the
morphological sequence its characteristic waves: P,
QRS complex, T and U (Acharya et al., 2007).
The P wave is due to the depolarization of the
atrial myocardium, the following QRS-complex, a
fast spiking wave that stimulates the ventricular con-
traction. The end of the cycle is made by the T wave
236
Pestana, J., Belo, D. and Gamboa, H.
Detection of Abnormalities in Electrocardiogram (ECG) using Deep Learning.
DOI: 10.5220/0008967302360243
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 4: BIOSIGNALS, pages 236-243
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

that indicates preparation to a new cycle with the re-
polarization of the ventricular myocardium. The U
may indicate the late depolarisation of the ventricular
myocardium (Acharya et al., 2007). The Signal-to-
Noise Ratio (SNR) comprises a heart rate between 60
and 100 bpm at rest while maintaining this sequence.
2.1 ECG Noise
Noise can be defined as any signal that is not related
to the heart’s electrical activity that obstructs the ECG
signal through interference (Rodrigues et al., 2017).
Usually, the signal is obtained in a controlled envi-
ronment, although the usage of ambulatory ECG is
more associated with certain variables that influence
the noise (Xiong et al., 2019).
Noise interference has many possible sources,
which can be classified as biological, like motion
artifact and muscle contraction, and environmental
or non-physiological noise, related to nearby electri-
cal devices and variables associated with the equip-
ment (Rodrigues et al., 2017).
baseline-wander (BW) artifacts are caused by
movement such as the contraction and relaxation of
the thoracic cage muscles, which is evident if the
event is periodic. Noise affected signals can also orig-
inate from the misplacement of electrodes and loss
of contact between the sensor and the skin, called
electrode motion (EM). Additionally, the activation of
the muscles cause the EMG signal interference in the
ECG signal, revealing motion artifact (MA).
2.2 Arrhythmias
Arrhythmias can be defined as rhythms that are
considered abnormal, considering the Normal Sinus
Rhythm (NSR), that are related to malfunctions in the
heart’s electrical activity and changes in the heart tis-
sue (arr, ). They can be detected in ECG by analysing
the heart rate, the presence or absence of certain
waves, the duration of intervals, wave overlap, ab-
normal timing of cardiac events, the shape, size and
direction of the waves, between others.
Common physiological arrythmias can be de-
tected from variations of the intervals between R
peaks (RR intervals). These changes are correlated
with the respiration rhythm and the sympathetic ner-
vous system. When RR interval is lower than 60 bpm
the arrhythmia is named tachycardia and higher than
100bpm bradycardia. (arr, ).
The pathophysiological arrhythmias may be
caused due to blocks in the electrical impulse
conduction, enlargement of the myocardium, peri-
carditis, electrolyte imbalance, respiratory diseases,
drugs, hypothermia and accessory conduction path-
ways(Acharya et al., 2007).
3 STATE OF THE ART
Over the years, various methods have been studied
for ECG noise and arrhythmia detection. The follow-
ing sections show an overview of the general devel-
opments in these areas.
3.1 Noise Detection in ECG
A decision rule-based algorithm is proposed by
(Satija et al., 2018) that calculates the maximum ab-
solute amplitude, number of zero crossings and local
maximum peak amplitude of the autocorrelation func-
tion are calculated, after using a modified ensemble
empirical mode decomposition. This work was able
to discriminate between six signal groups with an ac-
curacy of 98.93%. (Ansari et al., 2018) proposed a
16 layer Convolution Neural Networks (CNN) which
predicts once per second on a 10 second inputs. An
AUC of 0.977 was reached for this binary classifi-
cation model with a 88,7% sensitivity (John et al.,
2018). Ansari et al. (2018) also developed a CNN
to detect usable and unasable ECG segments, in terms
of calculating the heart rate variability (HRV). The fil-
tering using the CNN resulted in an area under curve
(AUC) of 0.96 for the classification of noise affected
segments, compared to an AUC of 0.87 for a Support
Vector Machine model.
3.2 Pathological Event Detection in
ECG
More recently, (Acharya et al., 2017) used a CNN
composed of 11 layers that analyse with 2 or 5 sec-
onds for detection of atrial fibrillation, atrial flutter,
and ventricular fibrillation. This study achieved ac-
curacy, sensitivity and specificity values of 92.50%,
98,09% and 93,13%, respectively, were achieved,
and for the two seconds ECG segments and 94,90%,
99.13% and 81.44% in the same order.
Also, a combination of CNN with Recurrent Neu-
ral Networks (RNN) was developed by Andersen et
al. (2018) in order to distinguish between atrial fibril-
lation and normal segments for ECG recordings of 24
hours. The extracted features using the CNN module
were processed by the RNN achieving a sensitivity of
98.98% and a specificity of 96.95%, making predic-
tions for 24 hours of ECG signal in under one second.
This algorithm was sensible to noise, reducing signif-
Detection of Abnormalities in Electrocardiogram (ECG) using Deep Learning
237

icantly its results when present (S. Andersen et al.,
2018).
Recently, Hannun et al. (2019) developed a 34
layer DNN that could detect between 10 different
arrhythmias and NSR using raw signal, while also
detecting noise corrupted segments, constituting 12
prediction classes in total. The results were higher
than average cardiologists in terms of sensitivity and
matching specificity values. The average AUC was
of 0.97 and the confusion matrices were similar, ac-
centuating the same problematic rhythm classes for
both (Hannun et al., 2019).
4 METHODS
Before the signals are fed to the deep learning algo-
rithms, preprocessing steps are implemented in order
to transform the raw data. These are imperative for
the models to learn the underlying patterns, to pro-
vide better generalization and overall quality. There-
fore, the signal is submitted to the decimation method
in order to reduce the number of samples, while miti-
gating the loss of information. This technique applies
a low pass anti-aliasing filter (order 8 Chebyshev type
I) that suppresses all frequencies that may cause alias-
ing, before the subsampling process.
In order to reduce the baseline noise that is con-
tained in the normal ECG, the clean signal was con-
volved with a Hanning window. All records were nor-
malized and the average was removed, in order to be
interpretable by the network, with the following rule:
x
0
=
x − x
max(x) − min(x)
(1)
where the normalized signal is x
0
and x denotes the
raw signal. Finally the signals are segmented in win-
dows of 64 samples, since the mean duration of a cy-
cle is of 1 second, most of the widows have one QRS
complex.
4.1 Architectures
In order to perform ECG classification according to
the two tasks at hand, two different architectures were
chosen and optimized to classify noise affected seg-
ments and to classify different types of arrhythmia
and NSR. Since autoencoders are good in learning
general features from the input, they were selected as
the base for the implementation of both detection al-
gorithms.
In a first stage, an autoencoder will lean the char-
acteristics of a normal ECG signal in the purest form
possible, i.e. with a good SNR and no symptomatic
events. Consequently, the latent vector will contain
the coded information of the input, and thus also
called ”feature vector”. When this structure is fed
with a modified ECG it will produce a different la-
tent space. As the state changes it can be analysed
and detected using a classifier.
The training of each algorithm was performed us-
ing batches of 256 shuffled windows, each without an
overlap for the noise detection experiment, and 50 %
for the arrhythmia. The testing was made with the
rest of each signal. Since the normal ECG cycle oc-
curs during one second it is expected that each win-
dow contains at least one QRS complex.
4.1.1 Autoencoder
CNN autoencoders are able to encode the input data,
as well as reconstruct it at the output layer with the
goal of reproducing the input signal as similar as pos-
sible. As the convolutional layers are capable learning
feature maps, which extracts the essential features to
reconstruct the input, these algorithms are an unsuper-
vised feature learning mechanism (Mao et al., 2017).
The diagram for the encoder is depicted in Fig. 1,
which comprises three convolutional blocks, each
containing one convectional layer with Rectified Lin-
ear Unit (ReLU) activation and a max pool, producing
the final latent vector with 256 elements.
Figure 1: The encoder is composed by three blocks com-
posed by a convolutional (”Conv”) with a ReLU activation
and a max pooling ”MP” layers.
Even though the decoder won’t be used specifi-
cally for the classification of abnormal events in ECG,
this component is vital for the training phase. The
training, monitoring and optimization of the encoder
is made by training comparing the input and output
with the mean squared error and RMSProp as the op-
timizer (initial learning rate of 0.001).
4.1.2 Noise Detection Neural Network
Two different detection networks were developed: (1)
a binary noise detection model capable of classify-
ing between Noise Affected Signals (NAS) or Nor-
mal Signal (NS); (2) multi-class detection using three
BIOSIGNALS 2020 - 13th International Conference on Bio-inspired Systems and Signal Processing
238

types commonly found in ECG: BW, MA and EM
noise.
Both algorithms follow the same structure, as de-
picted in Fig. 2. The difference between the al-
gorithms is in the number of layers in the fully
connected (FC) network because of the number of
classes. As this network progresses toward the end,
each layer decreases by half the number of neurons,
starting in 256, with ReLU activations in the first lay-
ers and softmax in the last one, and since the last layer
contains the number of classes for each system, the
first settled with 9 while the second with 10 layers.
In both setups, a dropout of 50% is implemented in
the fifth layer. The training was also made with RM-
SProp, but with cross entropy loss function.
Figure 2: Model architecture for the noise detection, with
C
D
number of classes.
4.1.3 Arrhythmia Detection Neural Network
The sequential approach for detection and prevention
of the arrythmia events is portrayed in the diagram in
Fig. 3. It is composed by an an encoder, FC layers and
a RNN comprising three sequential Gated Recurrent
Units (GRU). A RNN is a particular type of neural
network that can be described as a dynamic sequen-
tial data processing model where its current internal
state is affected by the previous one (Pascanu et al.,
2013). The GRU is a particular type that manages the
learning process using gates (Ghimes et al., 2018).
After the ECG features from each widow are ex-
tracted using the previous CNN encoder, N number
of feature vectors are stacked, resulting in matrices of
size (N × F
D
), where F
D
is the dimension of the fea-
ture vector. Since the RNN module has its own set of
hidden units (H
D
), the FC layer, with linear activation,
makes the connection between both modules. The pa-
rameters used for this experiment were F
D
= 256 and
H
D
= 128 and N = 32
After the three GRU layers the output is carried
into a FC layer, classifying into one of the seven
classes (C
D
= 7). The final module starts with a batch
normalization layer but is similar to the previous FC
layers used for classification of the noise algorithms
from H
D
= 128, until the size of C
D
= 7.
The training of this algorithm was made with RM-
SProp with the initial learning rate of 0.001, which
was automatically adjusted during its training phase.
The input data is separated into batches of 256 sig-
nal windows ensuring that it was within the computa-
tional limitations.
4.2 Datasets
All the datasets used are public and can be accessed
through Physionet, a biosignal database that was cre-
ated under the auspices of the National Institutes of
Health (Goldberger et al., 2000).
The Fantasia dataset was acquired from twenty
young (21-34 yr) and twenty elderly (68-81 yr) while
exposed to 120 min of continuous supine resting ECG
recordings (250 Hz sampling rate) while watching the
Disney’s movie Fantasia (Iyengar et al., 1996). The
signals collected by this database are mostly clean and
free from symptoms, which reflects a good baseline
for training the autoencoder.
For the noise detection model, both Fantasia
and MIT-BIH Noise Stress Database were consid-
ered (Moody et al., 1984). The last includes 12 half-
hour ECG recordings and 3 half-hour recordings typ-
ical noise in ambulatory ECG recordings with 250Hz.
For the last task, the MIT-BIH Arrhythmia
Database was used, displaying a sampling frequency
of 360 Hz. The chosen 6, out of 14, had enough
number of episodes to provide a good balance be-
tween classes while training the algorithm: Atrial
Fibrillation (AFIB), Atrial Flutter (AFL), Ventricular
Bigeminy (B), Paced Rhythm (P), Wolff-Parkinson-
White Syndrome, or Pre-excitation (PREX), and Si-
nus Bradycardia (SBR) (Moody and Mark, 2001).
5 RESULTS
All models were developed, trained and tested using
Keras API on top of the Tensorflow library. The hard-
ware included a NVIDIA GeForce GTX 960.
5.1 Autoencoder
The autoencoder was trained and tested using all 40
individuals in the Fantasia Database, being that 70%
were used for training and 30% for testing. After
approximately 2100 epochs of training the minimum
reached loss was 0.0001. For the testing phase, the
mean squared error was calculated for each signal
reaching a mean value of 0.0026 and a standard devi-
ation of 0.0012 during ±140 minutes. The results for
Detection of Abnormalities in Electrocardiogram (ECG) using Deep Learning
239
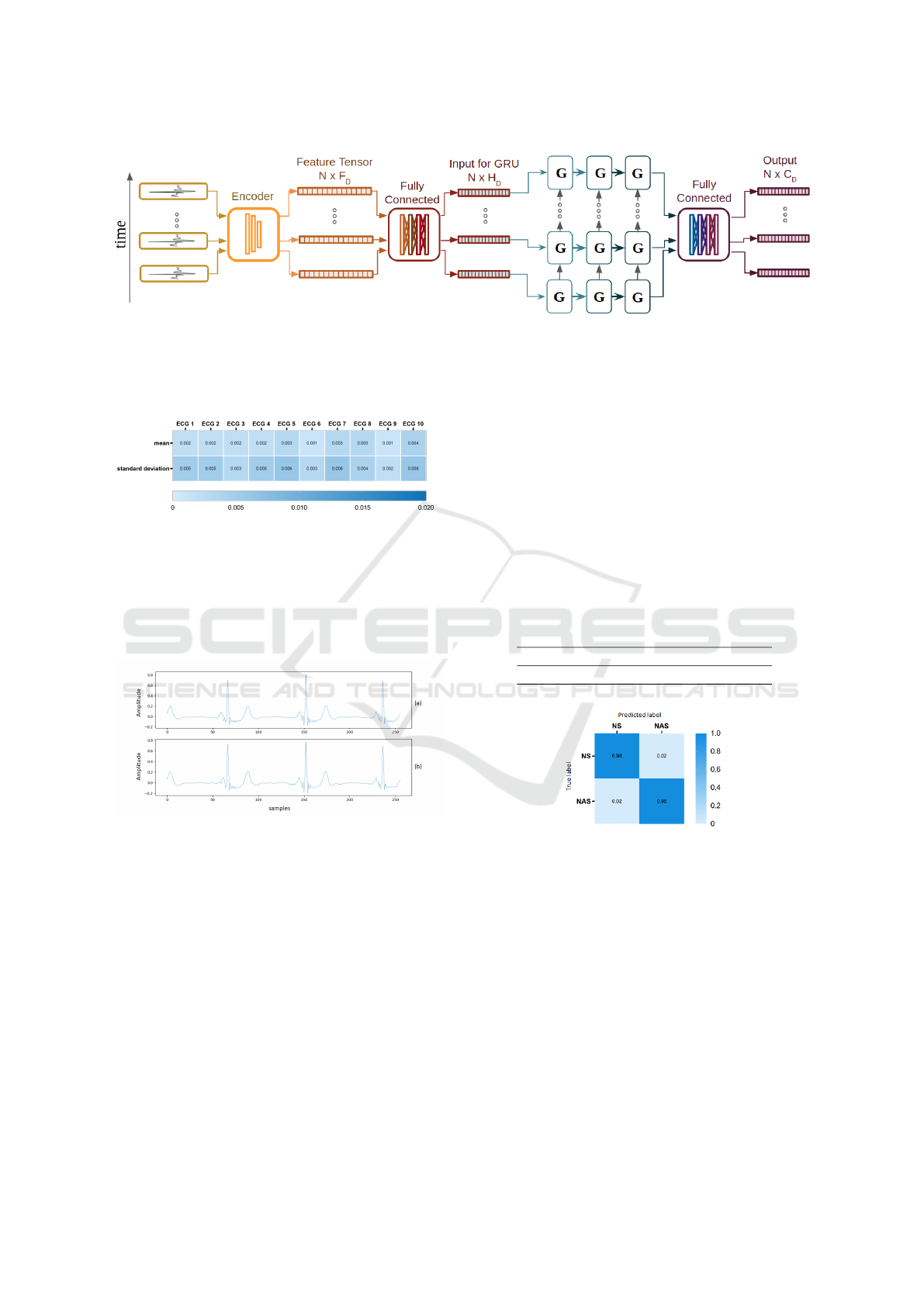
Figure 3: Model architecture for the arrhythmia detection, with C
D
= 7 classes.
the first 10 individuals of the dataset, which have ages
between 21 and 34 years old, are depicted in Fig. 4.
Figure 4: Mean and standard deviation values for the first
10 subjects within the age group of 21 to 34 years old.
The low values exhibited by the results suggest
that the model was able reconstruct the main charac-
teristics of the signal, that can be seen in the example
shown in Fig. 5.
Figure 5: Portion of the signal ECG 9, from the Fantasia
Database (a) and reconstruction of the same signal (b).
After analyzing the results, it was concluded that
the encoder could be applied for the following classi-
fication models.
5.2 Noise Detection Neural Network
The training set for both noise detection models the
data was separated in 70% for training and 30% for
testing. Furthermore, the testing set had three indi-
viduals that the training set did not contain. Both al-
gorithms took ±120min to train.
5.2.1 Binary Noise Detection Model
As stated before, the binary noise detection model
classifies ECG segments according to two classes: NS
or NAS. The training proceeded until 600 epochs,
where an abrupt increase of error was observed,
reaching a minimum cross entropy of 0.51 with an
accuracy of 98,56%.
The classification results, shown in Table 1, pro-
vides evidence that the model was able to successfully
detect the presence of noise in the ECG. Further sup-
port for this claim is presented in the confusion matrix
exhibited in Fig. 6.
Table 1: Binary noise detection model: classification per-
formance (%).
Accuracy Sensitivity Specificity
98,18 98,21 98,15
Figure 6: Normalized confusion matrix of the dataset used
for the training phase, where NS is the positive label and
NAS is the negative label.
The encoded data for both classes were submitted
to t-Distributed Stochastic Neighbour Embedding (t-
SNE) in order to visualize the proximity between the
feature vectors. Therefore, each point in the result-
ing graph represents the tensor created by the encoder
originated by a single window input. Both classes are
clearly separated in two clusters when each time win-
dow is (equally distributed by label) submitted to t-
SNE(Fig. 7). This confirms that the encoder extracts
different values for each feature represented by the
two classes.
BIOSIGNALS 2020 - 13th International Conference on Bio-inspired Systems and Signal Processing
240

Figure 7: Binary noise detection algorithm - t-SNE repre-
sentation of normal signal (NS) (red) and Noise affected
signal (NAS) (green) encoded data windows.
5.2.2 Multi-class Noise Detection Model
During the training phase, a minimum cross entropy
error of 0.85 was reached after 600 epochs, result-
ing in a training accuracy of 86,17% after ±180min.
However, testing accuracy was only able to reach
70,74%.
The evaluation of this model for each class are
conveyed in Table 2.
Table 2: Multi-class noise detection model: classification
performance for each class (%)
Class Sensitivity Specificity
NS 89,77 96,63
BW 72,70 84,26
EM 65,19 91,35
MA 59,11 89,47
By inspecting the normalized confusion matrix in
Fig. 8, it is can be stated that even though the algo-
rithm is able to correctly identify normal signal most
of the times, it is harder to correctly separate the iden-
tity of each type of noise. However, the model was
able to discern between EM and MA characteristics.
Figure 8: Multi-class Noise detection algorithm - Confu-
sion matrix for the classes: Normal Signal (NS), baseline-
wander (BW), electrode motion (EM) and motion artifact
(MA).
Comparing these results with the previous exper-
iments, we come to the conclusion that even though
it is possible to detect noise corrupted ECG with high
accuracy, but differentiating between the several types
of noise reveals to be a harder task. This may be due
the fact that most of the times signals are affected by
different types of noise sources at once and because
the encoder was not trained to deal with these differ-
ences.
5.3 Arrhythmia Detection Model
Cross-validation was performed with 90% for training
the data and the rest for testing. The minimum cross
entropy error of 0.65 was reached after 1750 epochs,
ending this training phase with a 90,74% accuracy.
However, for the test data, the accuracy was only of
56,85%.
The confusion matrix, displayed in Fig. 9, shows
that the model was able to correlate between certain
characteristics with the correspondent arrhythmia,
but underperforming between distinguishing some of
them. This DNN architecture had a high classification
rate for the P in contrast with the detection of AFIB,
often confusing its morphology with AFL, since both
of them are quite similar.
Figure 9: Arrithmia detection algorithm - Confusion matrix
for the classes of Atrial Fibrillation (AFIB), Atrial Flutter
(AFL), Ventricular Bigeminy (B), Paced Rhythm (P), Pre-
excitation (PREX), and Sinus Bradycardia (SBR).
These results are promising and reveal enough
facts to embrace the development of this algorithm by
fine-tuning its parameters and have some considera-
tions in the training phase of the autoencoder. Unfor-
tunately at this current stage it cannot be used in a real
life context, since the model often considers patho-
logical ECG segments as NSR, giving too may false
positives and negatives. By analysing the classifica-
tion performance of each class presented in Table 3, it
is possible to conclude that the algorithm often strug-
gles to correctly identify ECG segments with B, AFL
and AFIB, reaching an average sensitivity and speci-
Detection of Abnormalities in Electrocardiogram (ECG) using Deep Learning
241

ficity for this model of 61.13% and 93.10%, respec-
tively. But we are confident that these results can be
improved with more powerful tools. Options for these
improvement are the increase of the window size, that
would help the algorithm understand the differences
of AFL and AFIB that, between other aspects, change
the distance between peaks and extending the period
of time, including more cycles, would make these eas-
ier to perceive. In a future work, this algorithm will
be tested with a more powerful hardware setting to be
able to encompass this parameter change.
Table 3: Arrhythmia detection algorithm: classification per-
formance for each class (%).
Class Sensitivity Specificity
NSR 63,35 85,49
P 97,12 99,73
B 36,31 93,67
SBR 62,60 96,33
PREX 81,33 94,45
AFL 38,14 91,88
AFIB 49,06 90,12
AFIB+AFL 84,80 90,91
Hannun et al (2019) were able to reach an average
sensitivity of 75.22% for a 12 class model, compared
to the average sensitivity of 61.13% for the proposed
model. However, is it important to note that in the
mentioned study, AFIB and AFL classes were com-
bined into one unique class (Hannun et al., 2019).
Figure 10 represents the merged results for a direct
comparative study Figure 9, and when the sensitivity
and specificity values are re-calculated (Table 3) the
first mean increases to 84,80%, but the second aver-
age decreases 70,91%, a value that only differs from
the study mentioned by 4,31%.
6 CONCLUSIONS
As for the last example of application of DNN archi-
tectures, the objective was to develop a framework
that detects abnormalities in the normal pattern of the
ECG signal, by creating a model that learns the key
characteristics of a normal ECG cycle. The resultant
models were capable of detecting two types of abnor-
malities: noise and pathological events.
A machine-learned extractor was developed in or-
der to be included in the architectures for noise and
arrhythmia detection, by using the encoder module
of a trained autoencoder. From the shown results it
is suggested that by successfully reproduce the input
signals, the autoencoder learns the key characteristics
of a normal ECG cycle.
Figure 10: Arrhythmia detection algorithm -Normalized
confusion matrix of the dataset used for the testing phase,
with classes: Atrial Fibrillation and Atrial Flutter (AFIB +
AFL), Ventricular Bigeminy (B), Paced Rhythm (P), Pre-
excitation (PREX), and Sinus Bradycardia (SBR).
For the noise detection models, reaching state-of-
the-art performance in the case of binary classifica-
tion, despite achieving lower results in discriminating
different types of noise. In the case of the arrhythmia
decision system, the model did not perform as well as
the compared state-of-the-art accuracy, but the sensi-
tivity and specificity showed to be promising.
The improvement of the system could rely on the
need to increase the power for the encoder by sup-
plying it with more ECGs. As the autoencoder learns
and replicates an increasing quantity of different seg-
ments of asymptomatic signals, the more this algo-
rithm will embrace the abstract notion of what is an
ECG. The presence of more noisy and symptomatic
episodes would also contribute to the classification
blocks, since the dataset was limited in the number
of provided segments.
In sum, the performance of these algorithms
proves that it is possible to detect generic abnor-
mal events in ECG using the proposed architectures.
Therefore, the implementation of an encoder for clas-
sification algorithms proves to be effective in the de-
tection of abnormal events by learning the signals key
characteristics.
As for the detecting each type of abnormal events
the model did not reach state-of-the-art results as ex-
pected since the algorithms developed sacrifice accu-
racy to reach better generalizations for all ECG sig-
nals to be applicable in real life. Applications that
understand the deviation from the normal signal into
unseen pathologies and the possibility of creating new
classes that did not exist before, could be extremely
beneficial for future not only for ECG, but also other
physiological signals.
BIOSIGNALS 2020 - 13th International Conference on Bio-inspired Systems and Signal Processing
242

One example is the application of this framework
to active learning mechanisms as most of the available
algorithms are specific to specific conditions, while
not detecting others. The ability to detect similar ab-
normalities in the ECG signal, the classifier could ac-
tively create new labels for patterns that diverge from
the normal ECG signal, while increasing new diag-
nostic possibilities. That said, this technique could
not only aid in the diagnostic of arrhythmias, but also
contribute to the study of these pathological anoma-
lies, by finding correlations between the different ex-
pressions of the ECG morphology, and also by ex-
ploring these manifestations with different variables
in mind such as the patient’s gender, age, medications,
among other factors. Another example is by analysing
billions of data points in wearable data, the medical
doctor could focus only in the parts which contained
different aspects of the signal, so that he could diag-
nose without wasting hours or days of analysis.
This method helps to increase the possibilities, not
only for detection, but also for studying what exactly
is a normal cycle and which are the deviation patterns.
By having early detection procedures, one could early
seek for medical help without developing a symp-
tomatic episode. This preventive point of view could
represent a major change in perspective in how medi-
cal care should be delivered in the future.
REFERENCES
Arrhythmia — National Heart, Lung, and Blood Institute
(NHLBI).
Acharya, R., Krishnan, S. M., Spaan, J. A., and Suri,
J. S. (2007). Advances in cardiac signal processing.
Springer.
Acharya, U. R., Fujita, H., Lih, O. S., Hagiwara, Y., Tan,
J. H., and Adam, M. (2017). Automated detection
of arrhythmias using different intervals of tachycar-
dia ECG segments with convolutional neural network.
Information Sciences, 405:81–90.
Ansari, S., Gryak, J., and Najarian, K. (2018). Noise de-
tection in electrocardiography signal for robust heart
rate variability analysis: A deep learning approach.
In 2018 40th Annual International Conference of the
IEEE Engineering in Medicine and Biology Society
(EMBC), pages 5632–5635. IEEE.
Brown, B. H. (cop. 1999). Medical physics and biomedi-
cal engineering. Medical Science Series. Institute of
Physics Publishing, Bristol.
Coiera, E. (2003). Guide to Health Informatics. Oxford
University Press, second edition.
Faust, O., Hagiwara, Y., Hong, T. J., Lih, O. S., and
Acharya, U. R. (2018). Deep learning for healthcare
applications based on physiological signals: A review.
Computer Methods and Programs in Biomedicine,
161:1 – 13.
Ghimes, A.-M., Avram, A.-M., and Vladuta, V.-A. (2018).
A character prediction approach in a security context
using a recurrent neural network. In 2018 Interna-
tional Symposium on Electronics and Telecommuni-
cations (ISETC), pages 1–4. IEEE.
Goldberger, A. L., Amaral, L. A., Glass, L., Hausdorff,
J. M., Ivanov, P. C., Mark, R. G., Mietus, J. E., Moody,
G. B., Peng, C.-K., and Stanley, H. E. (2000). Phys-
iobank, physiotoolkit, and physionet: components of
a new research resource for complex physiologic sig-
nals. Circulation, 101(23):e215–e220.
Hannun, A. Y., Rajpurkar, P., Haghpanahi, M., Tison, G. H.,
Bourn, C., Turakhia, M. P., and Ng, A. Y. (2019).
Cardiologist-level arrhythmia detection and classifi-
cation in ambulatory electrocardiograms using a deep
neural network. Nature Medicine, 25(1):65–69.
Iyengar, N., Peng, C., Morin, R., Goldberger, A. L., and
Lipsitz, L. A. (1996). Age-related alterations in the
fractal scaling of cardiac interbeat interval dynam-
ics. American Journal of Physiology-Regulatory, Inte-
grative and Comparative Physiology, 271(4):R1078–
R1084.
John, J. N., Galloway, C., and Valys, A. (2018). Deep con-
volutional neural networks for noise detection in ecgs.
arXiv preprint arXiv:1810.04122.
Johnson, K. W., Soto, J. T., Glicksberg, B. S., Shameer,
K., Miotto, R., Ali, M., Ashley, E., and Dudley, J. T.
(2018). Artificial intelligence in cardiology. Journal
of the American College of Cardiology, 71(23):2668 –
2679.
Mao, Z., Yao, W. X., and Huang, Y. (2017). Eeg-based
biometric identification with deep learning. In 2017
8th International IEEE/EMBS Conference on Neural
Engineering (NER), pages 609–612.
Moody, G. B. and Mark, R. G. (2001). The impact of the
mit-bih arrhythmia database. IEEE Engineering in
Medicine and Biology Magazine, 20(3):45–50.
Moody, G. B., Muldrow, W., and Mark, R. G. (1984). A
noise stress test for arrhythmia detectors. Computers
in cardiology, 11(3):381–384.
Pascanu, R., Gulcehre, C., Cho, K., and Bengio, Y. (2013).
How to construct deep recurrent neural networks.
Rodrigues, J., Belo, D., and Gamboa, H. (2017). Noise de-
tection on ecg based on agglomerative clustering of
morphological features. Computers in Biology and
Medicine, 87:322 – 334.
S. Andersen, R., Peimankar, R., and Puthusserypady, S.
(2018). A deep learning approach for real-time de-
tection of atrial fibrillation. Expert Systems with Ap-
plications, 115.
Satija, U., Ramkumar, B., and Manikandan, M. S.
(2018). Automated ecg noise detection and classifi-
cation system for unsupervised healthcare monitoring.
IEEE Journal of Biomedical and Health Informatics,
22(3):722–732.
Silipo, R. and Marchesi, C. (1998). Artificial neural net-
works for automatic ecg analysis. IEEE Transactions
on Signal Processing, 46(5):1417–1425.
Xiong, F., Chen, D., Chen, Z., and Dai, S. (2019). Cancel-
lation of motion artifacts in ambulatory ECG signals
using TD-LMS adaptive filtering techniques. Journal
of Visual Communication and Image Representation,
58:606–618.
Detection of Abnormalities in Electrocardiogram (ECG) using Deep Learning
243
