
Comparison of Ex-vivo Perfused and Non-perfused Porcine Liver
Ablations using Uncooled Microwave Applicators
Mattia Dimitri
1,*
, Fabio Staderini
2
, Sara Aquino
1
, Lucrezia Mazzantini
1
, Andrea Corvi
1
and Guido Biffi Gentili
3
1
Department of Industrial Engineering, University of Florence, via di Santa Marta 3, Florence, Italy
2
Department of Experimental and Clinical Medicine, University of Florence, S. Marco Square 4, Florence, Italy
3
Department of Information Engineering, University of Florence, via di Santa Marta 3, Florence, Italy
Keywords: Perfusion, Ex-vivo, Liver, Thermal Ablation, Microwave.
Abstract: The paper compares thermal ablation on perfused and not perfused porcine liver produced by 17 and 14
Gauge non internally cooled (NIC) microwave (MW) applicators. To the knowledge of the authors this
comparison, already made using cooled applicators has not so far been carried out with uncooled applicators
that have a very different thermo-kinetic behavior when they operate inside a biological tissue. The purpose
of this preliminary study is to explore the possibility of using ex vivo perfused liver in order to define an
optimal protocol to allow more reliable translation of the experiments to the clinical practice and compare
the obtained results with those of previous studies that used similar energy delivery.
1 INTRODUCTION
A typical MW system consists of a generator and
almost one minimally invasive applicator with its
cable, however there are no two equal MW systems,
in particular as regards the geometry of the
applicator.
There are several technological approaches that
every doctor should adopt to plan and perform a
microwave thermal ablation ensuring that the tumor
is completely treated with sufficient ablative
margins (> 0.5 cm).
Thanks to continuous advances in microwave
technology, manufacturers of MW systems are
proposing increasingly advanced minimally invasive
technologies (Ruiter 2019, Yung 2017, Meloni
2017) with the valuable support of experienced
clinical interventionists.
To minimize costs and the need for multiple
insertions, the current tendency of most
manufacturers is to use a single high powered (up to
140W) water or gas cooled applicator to create a
pseudo-spherical ablation with up to 5 cm diameter
in less than 10 minutes (Kodama 2018).
Corresponding Author: Mattia Dimitri Address: Via di
Santa Marta 3, Florence (FI), Italy. Phone Number:
+39 3407024067. e-Mail: mattia.dimitri@unifi.it
Moreover, this high-energy approach is not
without risks, especially when the treatment involves
tumors that are very close to delicate organs, which
must be preserved from thermal damage.
A possible alternative to this method is to
subdivide the energy needed to destroy the tumor
among multiple applicators (Biffi Gentili 2014). In
this case the input power of each applicator can be
reduced to the level that make the use of cooling
unnecessary.
Non Internally Cooled applicators are
structurally simpler and robust than the Internally
Cooled ones (IC), and also more reliable because
they operate at limited power (40 W maximum) and
are free from cooling system failures that can cause
serious damage to healthy tissues.
Shape and volume of the ablation zone after MW
treatment are depending on (De Cobelli 2017):
biophysical parameters as thermal conductivity and
perfusion rate of the liver parenchyma that can be
different in human liver tissue due to fibrosis,
cirrhosis or steatosis; effective MW power and
hyperthermic treatment duration; structure, gauge
and cooling mechanism (if present) of the applicator
(MW needle).
Planning for ablation is essentially based on
manufacturer algorithms or ablation charts in
combination with personal experience of the
118
Dimitri, M., Staderini, F., Aquino, S., Mazzantini, L., Corvi, A. and Gentili, G.
Comparison of Ex-vivo Perfused and Non-perfused Porcine Liver Ablations using Uncooled Microwave Applicators.
DOI: 10.5220/0008947501180123
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 1: BIODEVICES, pages 118-123
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

operator. These algorithms and charts, which try to
predict the three-dimensional diameter of the
ablation zone in relation to the amount of applied
energy, are often based on experiments which have
serious shortcomings, preventing a reliable
translation to daily clinical practice.
These shortcomings are the result of studies
performed in non-perfused ex vivo bovine or porcine
livers (as opposed to perfused in vivo human livers
with variable arterial and portal blood flow).
These differences affect the way in which the
applied energy is transferred into heat, resulting in
highly unpredictable ablation zone dimensions and
volumes.
Despite several individual papers reporting on
these shortcomings, a systematic review on this
topic is lacking.
A higher reproducibility of the experiments with
better predictability of the clinical results could be
achieved by defining a standardized procedure to be
shared among all manufactures, allowing to extract
the treatment parameters through ablation tests on
normothermic (body temperature) perfused ex-vivo
organs.
2 MATERIALS AND METHOD
Ablation procedures where performed using the
TATO thermal ablation system with 14 and 17
gauge NIC applicators.
TATO is a multi-applicator system that has been
developed by a small multidisciplinary team at the
University of Florence, in the framework of a
collaborative academic-industry agreement with
Biomedical Srl, Florence.
The experiment was performed in ex vivo fresh
porcine liver retrieved from animals in the food
chain. In this way important ethical implications can
be overcome and the economic burden is lower than
more common in vivo studies.
The Experimental platform for hepatic flow
simulation developed at the Industrial Engineering
Department (DIEF) of the University of Florence
(patent pending) was employed to maintain the
explanted porcine liver in a normothermic
physiological perfusion state.
Freshly taken porcine liver from adult animal
with intact in-and outflow vessels where obtained
from an abattoir and immediately connected to the
perfusion platform. Hepatic inflow was simulated
trough flexible plastic tubes sutured to the veins and
connected to a perfusion pump system. The hepatic
flow was established to emulate the average human
cardiac output. To acquire information on the
maximum temperature reached by the MW
applicators during thermo ablation procedures and
the divergences between active perfusion and
blocked perfusion, the test process was video
recorded with a Thermal-CAM A320 thermal
imaging camera (FLIR Systems, Inc., Wilsonville,
OR).
3 PERFUSION PLATFORM
The experimental platform for the simulation of the
hepatic flow is composed of 3 distinct sections, with
the possibility of independent activation /
management, which perform the following
functions:
1. pre-heating the blood and filtering any clots;
2. perfusion of the venous tree;
3. perfusion of the arterial tree, equipped with a
high efficiency oxygenator.
Each section is controlled by a dedicated processor
which, following the automatic learning approach,
processes the sensor signals (pressure, temperature,
flow) placed in strategic areas and performs the
actions necessary to maintain the normal
physiological conditions of the organ during the
extracorporeal perfusion process.
In the literature are described analogous systems
which perform similar functions but not in an
integrated and adaptive manner such as the current
system that was designed and built at the prototype
level at the DIEF of UNIFI (R. Ravikumar 2016).
and for which a patent application was filed.
4 THE ABLATION PROCEDURE
The ablation procedure was performed in
collaboration by a surgeon (FS) and an engineer
(MD).
As a first step, the perfusor was replenished by
filling the main tank with the perfusion fluid which
has the same characteristics as normal blood. In
order to evaluate the functioning of the system
avoiding the use of blood, a fluid was identified that
was able to simulate its behavior. The blood has a
dynamic viscosity of about 3 cP. To make a fluid
capable of reproducing the rheological properties of
blood in terms of viscosity and density, it is possible
to use distilled water, glycerol and cornstarch. Since
the blood has a physiological temperature of about
Comparison of Ex-vivo Perfused and Non-perfused Porcine Liver Ablations using Uncooled Microwave Applicators
119

37 °C, the percentages of the constituent elements of
the fluid must therefore be determined. With a
capillary viscometer, it is possible to measure the
kinematic viscosity [m2⁄s], from which it is possible
to calculate the dynamic viscosity [cP]. It was
estimated that at 37 °C the fluid with a viscosity as
close as possible to the blood is composed of:
70% distilled water; 30% vegetable glycerol.
Dynamic viscosity μ = 3.52 ± 0.01 cP
70% distilled water; 30% vegetable glycerol; 1%
cornstarch. Dynamic viscosity μ = 3.52 ± 0.15
cP.
Therefore, since about 3.5 L of fluid is circulated in
the system in question, it is possible to simulate
blood using 2450 mL of distilled water and 1050 mL
of glycerol (and 35 mL of cornstarch).
The main pump was then activated and the
heating system was switched on to gradually
increase the fluid temperature up to 38 °. Gradual
heating of the fluid is necessary to avoid thermal
shock and clot formation. The temperature was then
monitored through the RP thermocouple and the RA
thermocouple and kept constant until the liver
cannulation was completed.
The liver was removed immediately after
slaughtering the pig. The hepatic hylum was
carefully dissected. The main hepatic artery was
isolated and cannulated with a flexible 8Fr Nelaton
catheter. The choledocic duct was isolated and
sutured. The main portal vein was isolated and
cannulated with a 16 fr Nelaton catheter. The lower
cave vein has been identified and sutured. The upper
cave vein was cannulated with a 45fr plastic tube.
The suprahepatic accessory vein was isolated,
cannulated and connected to the upper cave vein
tube with a Y-shaped joint.
Before the connection to the perfusor, the hepatic
vessels were washed with a heparinized
physiological solution to prevent the formation of
clots.
The hepatic artery and the portal vein cannulas
were connected to the respective pumps and the
upper vena cava tube was connected to the main
reservoir. At the end of this procedure the perfusor
was turned on and the correct functionality was
checked.
Fig. 1 shows the final installation immediately
before the thermal ablation tests.
Figure 1: Final set up immediately before the thermal
ablation tests.
4.1 Thermal Ablation Tests
For the microwave thermal ablation tests two NIC
applicators of 14 and 17 G were used respectively.
In order not to influence the results the two needles
were inserted in two distant hepatic lobes. The
TATO system microwave generator was then set to
supply 40 W to the 14 G needle and 30 W to the 17
one. The TATO microwave generator was switched
on for 10 minutes immediately after the perfusor
reached the normothermia state.
At the end of this first procedure the perfusion
was stopped and the needles were repositioned to
repeat the same procedure but in the absence of
perfusion.
At the end of both procedures the parenchyma
was sectioned longitudinally to photograph the
obtained ablations and to measure their diameters
along the two main axes (Fig. 2, 3).
Figure 2: Post ablation sectioning.
5 PRELIMINARY RESULTS
The results of the ablation test are summarized in
Table 1.
Table 1: Preliminary results wit perfusion blocked.
Applicator
Gauge
Length
(cm)
Diameter
(cm)
Ablation
volume
(cm
3
)
Aspect
ratio
14 4.2 3.3 191 0.66
17 4 2.2 81 0.55
BIODEVICES 2020 - 13th International Conference on Biomedical Electronics and Devices
120
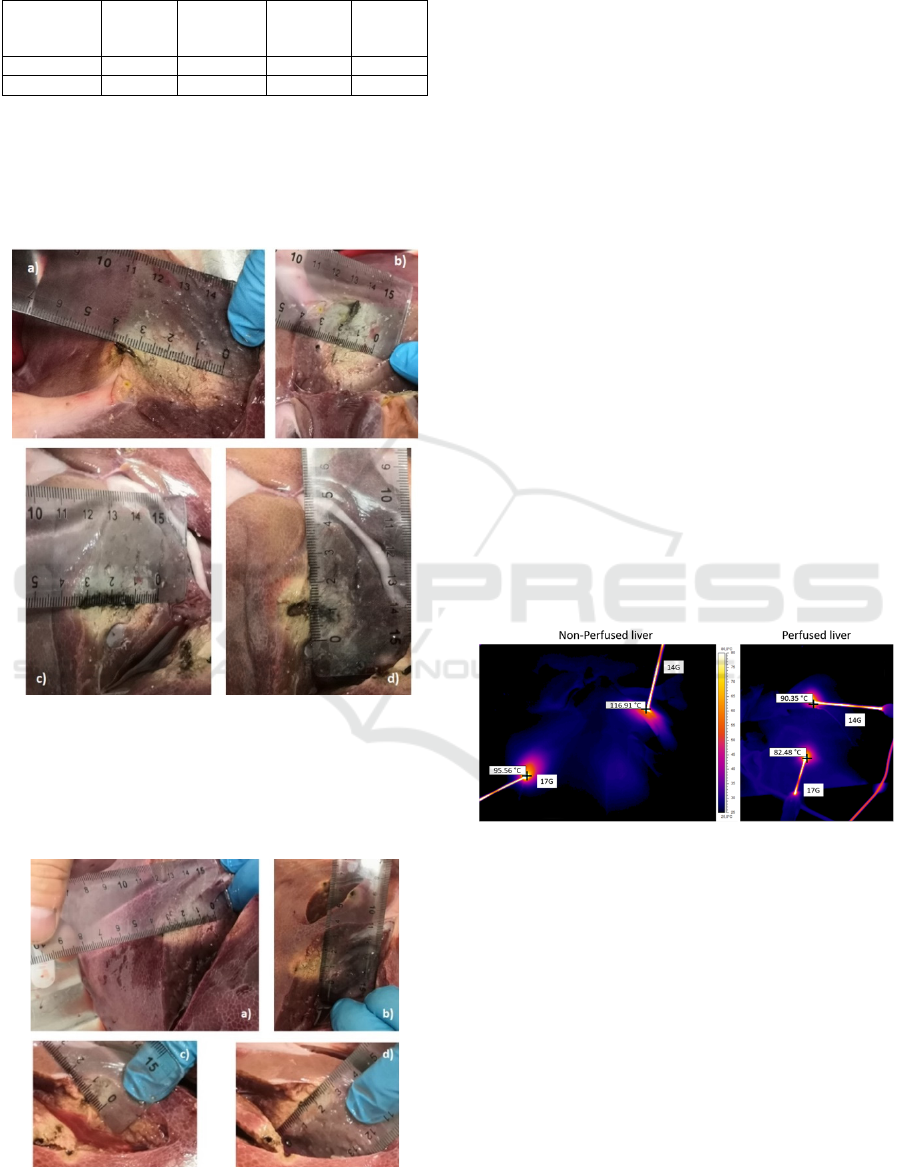
Table 2: Preliminary results with perfusion active.
Applicator
Gauge
Length
(cm)
Diameter
(cm)
Ablation
volume
(cm
3
)
Aspect
ratio
14 3.7 2.2 74 0.59
17 3.3 1.5 31 0.45
Without hepatic perfusion, the 14G needle
generated a 4.2x3.3 cm ablation (Fig. 3a and 3b)
while in perfused mode the ablation was smaller due
to the heat sink phenomenon, with dimensions of
3.7x2.2 cm (Fig. 3c and 3d).
Figure 3: Comparison between 14 Gauge ablation in non-
perfused (Fig. a and b) and in perfused (Fig c and d) liver.
The 17G needle produced an ablation of 4x2.2
cm in non-perfused mode (Fig. 4a and 4b) and
3.3x1.5 in perfused mode (Fig. 4c and 4d).
Figura 4: Comparison between 17 Gauge ablation in non-
perfused (Fig. a and b) and in perfused (Fig. c and d) liver.
The ablation volumes for the 14G needle were 24
cm3 and 9.4 cm
3
, in non-perfused and perfused
mode, with a volumetric ratio of 0.66 and 0.59
respectively.
The ablation volumes for the 17G needle were 10
cm3 and 3.4 cm
3
in non-perfused and perfused
mode, with a volumetric ratio of 0.55 and 0.45
respectively.
The data acquired with thermal cameras allowed
to measure with great accuracy the temperature
distribution on the entire liver and therefore to verify
in real time the correct perfusion state of the
parenchyma during the two ablation procedures with
active perfusion. Within the limits of the small
number of ablations performed, the preliminary
results indicate that an isolated spherical tumor with
a diameter up to 1.5 cm can be thermally ablated
with an adequate margin using a single NIC 14 G.
applicator.
If the tumor diameter exceeds 1.5 cm for its
complete thermal ablation it will be necessary to use
multiple NIC applicators or a single adequately
cooled high power IC device.
Moreover, as shown in figure 4, the thermal
behavior highlights the differences produced by the
perfusion in terms of maximum temperature reached
by the metallic shaft and consequently by the tissue
in direct contact with the MW tools.
Figure 5: Maximum Temperature comparison between
ablation in non perfused and in perfused liver at the end
of the termoablation (10 min).
6 DISCUSSION
With the exception of special situations such as a
neoplasia localized in unresectable positions, a high
number of lesions, inadequate hepatic reserve or
multiple comorbidities that contraindicate
anesthesia, percutaneous thermal ablation techniques
(TPA) are not yet considered the gold standard in the
treatment of liver neoplasms. This is mainly due to
the fact that liver transplantation and hepatic
resection have shown their superiority in the
Comparison of Ex-vivo Perfused and Non-perfused Porcine Liver Ablations using Uncooled Microwave Applicators
121

treatment of primary and secondary liver neoplasms
demonstrating higher long-term disease-free survival
rates than TPA (F. Romano 2012). In the
oncological TPA one of the main problems facing
doctors is the uncertainty about the actual size of the
ablations, mainly as a direct consequence of the
well-known "heat sink effect".
The prediction of this effect and of the 3D
dimensions of the ablation zone in relation to the
amount of applied microwave energy is a crucial
aspect since an incorrect prediction can lead to an
insufficient volume of ablation and a relapse of the
neoplasm. Since it is very hard to predict in practice
the exact size of the ablation for each combination of
time, power and size of the needle before the
execution of the procedure, these data are often
supplied to the doctor by the manufacturers of
thermal ablation systems in the form of ablation
charts or algorithms, but in our opinion these
information, together with the operator's personal
experience does not always provide the expected
results because they are often based on experiments
on ex-vivo organs at ambient temperature that
present serious deficiencies, preventing reliable
translation into the clinical practice.
In order to overcome these limitations, the
Department of Industrial Engineering (DIEF) of the
University of Florence has developed an
experimental platform (patent pending) to keep the
explanted liver in a state of normal physiological
thermal perfusion, capable to simulate the actual
heat sink effect during a TPA procedure. We need to
underline that the platform can simulate
physiological liver perfusion but is not able to keep
the liver cells alive and this aspect has been
evaluated by the authors before performing
experimental tests. Recent studies have underlined
that a warm ischemia time up to 60min does not
generate any irreversible cellular change and is
acceptable even for hepatic transplant
(Kalisvaart,
2018).As a consequence of that, if the ablation
experiment is performed in 60min beginning from
the liver explant, the effect of liver warm ischemia
on ablation shape and dimensions is negligible or
even absent. The aim of the study was therefore to
extract preliminary data to be compared with the
literature data in vivo. In our opinion, the ex-vivo
pig perfused liver test should represent the gold
standard for the definition of truly reliable
algorithms and ablation charts for the following
reasons:
1. The test is easily reproducible and allows a
definitive evaluation of the ablation volume in
the presence of the heat sink effect;
2. The proposed approach could allow the
standardization of the experimental procedure to
extract reliable algorithms and ablation charts;
3. The economic burden is lower than the costly in
vivo animal procedures.
4. Important ethical implications can be overcome.
In order to confirm experimentally this belief, the
ablation experiments where made in two different
perfusion inflow states: active and blocked using the
UniFi Hepatic Flow platform.
Ablation results of the two inflow state are
depicted in Tab1.
Preliminary results obtained by comparing
ablation performed in blocked and active perfusion
states show a reduction of about 30% in the ablation
radius from one to the other state, regardless of the
size of the applicator.
The ablation zones in this study where
commensurate with those of previous studies (M.
Dimitri 2018) obtaining results that confirm the
substantial equivalence between in-vivo and ex-vivo
normothermic perfused liver for the same energy
delivery.
It is important to note that the electromagnetic
and thermal properties of the ex vivo liver at
ambient temperature normally used by
manufacturers to extract the ablation chart, are very
different from those of the ex vivo normothermic
perfused liver.
7 CONCLUSION
The preliminary results obtained with this study are
too limited to have a statistical relevance but if this
result will be confirmed even in future tests this
model could constitute the best procedure to
evaluate the effectiveness of TPA without the use of
in vivo animal models. The availability of a
standardized ablation model based on ex-vivo
perfused liver opens the way to a more in-depth
investigation of the heat sink effect at peripheral and
central vessel locations. The UNIFI Perfusion
Platform is very versatile and it allows to easily
change the composition and flow rate of the
perfusion solution of the liver parenchyma.
Moreover this model could be used to emulate an
open surgical ablation, allowing the surgeon to
rotate the liver within its anatomical surrounding,
manually protect heat-sensitive organs (bowel) and
easily insert clustered applicators to treat large non
spherical tumors.
BIODEVICES 2020 - 13th International Conference on Biomedical Electronics and Devices
122

The preliminary results obtained with this study
are too limited to have a statistical relevance,
therefore the final validation of the proposed
approach will require a further and more in-depth
experimental activity.
8 FUTURE WORK
Future work will have the main focus trying to
statistically validate the actual results. If the new
tests will confirm them, a compact and autonomous
platform for the normothermic perfusion of porcine
livers will be engineered.
With this new device in the future we would like
to carry out:
the comparisons between TPA in a swine in-vivo
model and TPA in a swine ex-vivo perfused
model.
the comparison between ablations performed by
high power (120W) cooled applicator and low
power single or multiple uncooled applicator in
term of energetic efficiency and procedural
security;
the optimization of multi-applicator ablation
procedures and the editing of more realistic and
affordable ablation charts;
the study and optimization of a pulsed
microwave ablation technology (PMWA)
(
M.Bedoya 2012) in order to obtain an increase of
the ablation volume with the same input average
power.
REFERENCES
Ruiter S.J.S., Heerink W.J., De Jong K.P., (2019). Liver
microwave ablation: a systematic review of various
FDA-approved systems. Eur Radiology 29(8):4026-
4035
Meloni M.F. et alii, (2017). Microwave ablation in
primary and secondary liver tumours: technical and
clinical approaches. International Journal of
Hyperthermia 33(1): 15–24.
Yung J., Liang P., (2017). Status and advancement of
microwave ablation in China. International Journal of
Hyperthermia Vol. 33, 2017 - Issue 3
Kodama H., (2018). High power microwave ablation of
normal swine lung: impact of duration of energy
delivery on adverse event and heat sink effects.
International Journal of Hyperthermia 34(8):1186-
1193.
Biffi Gentili G, Ignesti C, Tesi V. (2014). Development of
a novel switched-mode 2.45 GHz microwave multi-
applicator ablation system. International Journal of
Microwave Science and Technology, Article ID
973736
De Cobelli F et alii, (2017). Microwave ablation of liver
malignancies: comparison of effects and early
outcomes of percutaneous and intraoperative
approaches with different liver conditions. Medical
Oncology 34(4)
R. Ravikumar, et alii, (2016). Liver transplantation after
ex-vivo normothermic machine preservation: a phase 1
(first-in-man) clinical trial. American Journal of
Transplantation, pp. 1779-1787.
F. Romano et alii, (2012). Bleeding in hepatic surgery:
sorting through methods to prevent it. HPB Surgery,
vol. 2012, 12pg, Article ID 169351.
Kalisvaart M, et alii, (2018).The impact of combined
warm ischemia time on development of acute kidney
injury in donation after circulatory death liver
transplantation: stay within the golden hour. P.
Transplantation. 102(5):783-793.
M. Dimitri et alii, (2018). A new microwave applicator for
laparoscopic and robotic liver resection. Internal
Journal of Hyperthermia, vol 36: 75-86.
M.Bedoya et alii (2012). Microwave ablation energy
delivery: Influence of power pulsing on ablation
results in an ex vivo and in vivo liver model. Medical
Physics 41 (12).
Comparison of Ex-vivo Perfused and Non-perfused Porcine Liver Ablations using Uncooled Microwave Applicators
123
