
Photoinactivation of Methicillin-Resistant S. Aureus Biofilm using a
New Chlorin as Photosensitizer
L. S. Amaral
1a
, I. A. P. Linares
2b
and J. R. Perussi
1,2 c
1
Programa de Pós-Graduação Interunidades em Bioengenharia EESC/FMRP/IQSC, Universidade de São Paulo,
Av. Trabalhador Sãocarlense, 400, 13566-590, São Carlos - SP, Brazil
2
Instituto de Química de São Carlos, Universidade de São Paulo,
Av. Trabalhador Sãocarlense, 400, 13566-590, São Carlos - SP, Brazil
Keywords: Bacterial Biofilm, Membrane, Resistant Bacteria, Photoinactivation, Photosensitizer, Chlorin.
Abstract: Due to the increase in bacterial resistance to antibiotics, the development of new drugs and technologies for
the eradication of microorganisms is a priority. Photodynamic Therapy (PDT) depends on the interaction
between a light-sensitive compound (photosensitizer), light, and molecular oxygen. The reaction generates
reactive oxygen species (ROS), which induce cell death by oxidative stress. Antimicrobial Photodynamic
Therapy (A-PDT) may be a promising alternative for microbial infections since its action occurs by multiple
targets, which hinders the development of resistance. The main goal of this study was the evaluation of the
potential of a newly synthesized chlorin derivative sterically prevented from self-aggregation as a
photosensitizer to photoinactivation Methicillin-Resistant S. aureus (MRSA) biofilm and to investigate the
membrane integrity after the treatment. The results showed a high potential of this chlorin for
photoinactivation of MRSA biofilms reducing the survival index more than 5 log CFU mL
-1
leading to the
unstructured membrane and consequent cell death by photooxidation of membrane components after A-PDT.
1 INTRODUCTION
Due to the increasing worldwide resistance of
bacteria to antibiotics, the development of new drugs
and technologies for the eradication of
microorganisms is a priority (Sobotta et al., 2019).
The biggest problem with using antibiotics is that
bacteria have different resistance mechanisms, which
can result in the formation of biofilms that are even
more refractory to treatments. Antimicrobial
Photodynamic Therapy (A-PDT) may be a promising
alternative for microbial infections since its action
occurs by multiple targets, which hinders the
development of resistance (Stanislaw et al., 2018).
PDT involves the combination of a photosensitizer
(PS), molecular oxygen, and visible light of adequate
wavelength to produce reactive oxygen species
(ROS), causing the cell to die through the oxidation
of its constituent biological molecules (Fig. 1).
PS is a substance that induces light sensitivity to
chemical, physical, or both processes usually
insensitive to light. Most photosensitizers have a
a
https://orcid.org/0000-0003-3796-0032
b
https://orcid.org/0000-0002-4660-5166
c
https://orcid.org/0000-0001-7098-0647
heterocyclic ring similar to chlorophyll and the
hemoglobin heme group. Photons are absorbed in the
band of the electromagnetic absorption spectrum
characteristic of photosensitizers and can transfer this
energy to other molecules, especially to molecular
oxygen, which will result in the release of short-lived
energy species, leading to damage to the biological
system involved (Hamblin et al., 2008).
Chlorins are molecules of high abundance and
importance in nature, present in most plants that make
photosynthesis (De Oliveira et al., 2014). CHL-Ph-A
is a new chlorin derivative sterically prevented from
aggregation due to the structural shape in “L”. Diels-
Alder reaction was used to synthesize CHL-Ph-A
from protoporphyrin IX (Linares et al., 2017).
The objective of this study was to photoinactivate
Methicillin-Resistant S. aureus (MRSA) biofilm
using a new chlorin (CHL-Ph-A) with the aid of Full
Factorial Design 2
3
and microscopy techniques to
evaluate the integrity of the bacterial membrane.
92
Amaral, L., Linares, I. and Perussi, J.
Photoinactivation of Methicillin-Resistant S. Aureus Biofilm using a New Chlorin as Photosensitizer.
DOI: 10.5220/0008938100920096
In Proceedings of the 8th International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS 2020), pages 92-96
ISBN: 978-989-758-401-5; ISSN: 2184-4364
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
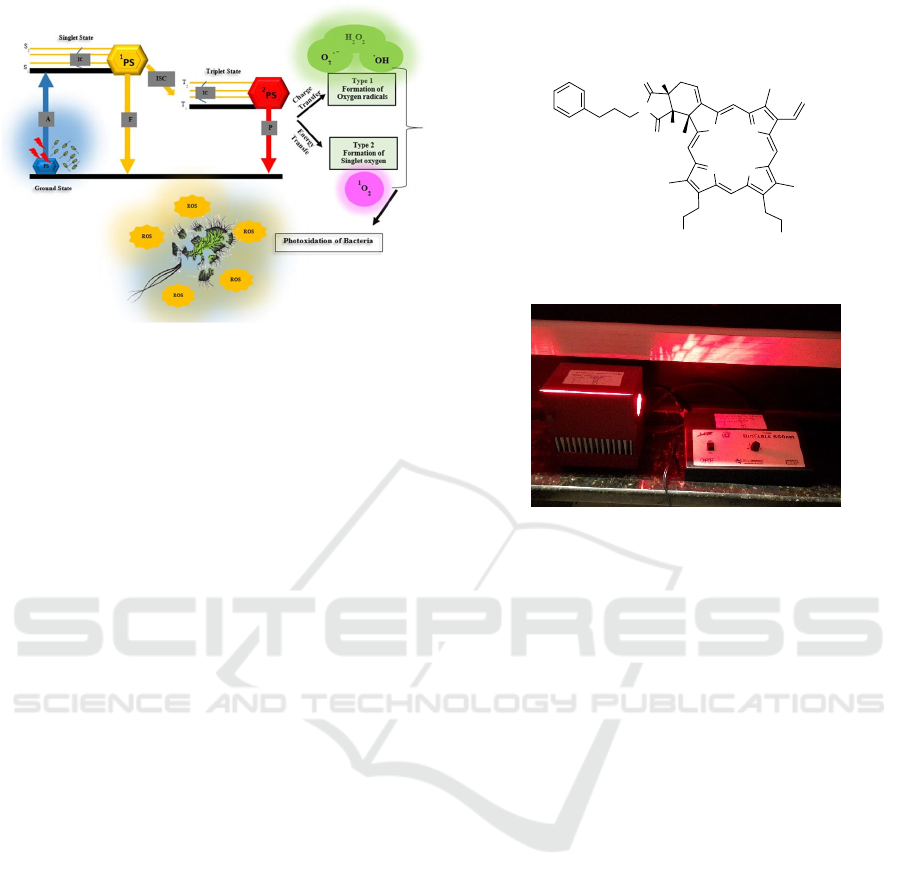
Figure 1: Mechanisms of oxygen reactive species production:
when the photosensitizer (PS) is incubated with the bacteria,
it absorbs energy from light and goes to the excited singlet
state (S
0
S
1
or S
2
decaying to S
1
by Internal Conversion −
IC). The relaxation can occur by fluorescence (F) or by
intersystem crossing (ISC). In the triplet state, the PS
molecule may lose energy by phosphorescence (P) or energy
transfer to molecular oxygen, generating singlet oxygen (
1
O
2
)
by type II mechanism. When charge transfer occurs
generating free radicals (type I), death of the bacteria may
happen due to the oxidation of their cellular components.
2 METHODS
2.1 Bacterial Strain and Biofilm
Culture Conditions
Methicillin-Resistant Staphylococcus aureus –
MRSA (ATCC® 33591 ™) was grown of the
planktonic form in Brain Heart Infusion (BHI)
medium at 37° C for 18 h with orbital agitation at 250
rpm. After planktonic cultivation, the optical density
of each bacterium was standardized to OD 600 in
phosphate-buffered saline (PBS), using a HITACHI
U-2800 spectrophotometer. For biofilm formation,
150 μL of the suspension was deposited on the 96-
well plate and supplemented with 150 μL of the BHI
medium. The plates were kept in an incubator for 48
h at 37° C.
2.2 Photosensitizer and Light Source
CHL-Ph-A (Fig. 2) was synthesized in our research
group, and the procedure and full characterization are
described in Linares et al., 2018.
The light source used for photoinactivation of
MRSA was an illumination platform called Biotable
(Fig. 3) developed by the LAT at the Instituto de
Física de São Carlos, Brazil, composed by 40 red
LEDs (660 ± 10 nm).
HN
N
NH
N
CO
2
Me
CO
2
Me
N
O
O
H
H
Figure 2: Molecular structure of chlorin CHL-Ph-A.
Figure 3: Red biotable 660 ± 10 nm used in irradiation
procedures.
2.3 Photodynamic Inactivation of
Bacteria in Biofilm Form
The MRSA biofilm photoinactivation was performed
with the aid of Full Factorial Design 2
3
( FFD 2
3
= 8
experiments), using three parameters with two levels
and a central point allowing to perform a smaller
number of experiments, less time consuming and
lower expenses to obtain the results. The three
parameters used were CHL-Ph-A concentration (C
PS
:
5; 7.5 and 10 µmol L
-1
) incubation time (IT: 20; 30
and 40 min), and light dose (LD: 15; 22 and 30 J cm
-
2
) at 660nm combined among them in a multivariate
form, submitting the results to Two-way ANOVA.
2.4 Determination of the Bacterial
Viability
MRSA biofilm was slowly homogenized, removing a
100 µL aliquot from each well of the 96-well plate
(before and after A-PDT) and diluting from 10
-1
to 10
-
8
. Four aliquots of 15 µL were taken from each
dilution and deposited on BHI agar plates and
incubated at 37° C for 18 h. Each BHI agar plate was
divided into four quadrants, each assigned to a
dilution. Quantification was performed by counting
the colonies at the dilution in which they had 5 to 50
colony forming units (CFU). The number of survivors
Photoinactivation of Methicillin-Resistant S. Aureus Biofilm using a New Chlorin as Photosensitizer
93
present in the sample was determined by the average
number of colonies, multiplied by the dilution, and
the number of CFU per milliliter of the solution was
obtained.
2.5 Membrane Integrity Analysis by
Fluorescence Microscopy
MRSA Biofilm before and after photoinactivation
was submitted to membrane integrity analysis.
Biofilm was cultivated in microscopy slides and
submitted to a mixture of SYTO
®
9 and Propidium
Iodide (PI) (mixture of the LIVE/DEAD™ kit,
Invitrogen Molecular Probes
®
) being finally analyzed
by a Fluorescence Microscope (Olympus BX41) with
100X objective, 500 nm dichromatic filter, excitation
at 460-490 nm and emission at 520 nm.
2.6 Morphostructural Analysis of
Photoxidized MRSA
For the morpho-structural analysis by Scanning
Electron Microscopy (SEM), the biofilm was
cultivated under polystyrene slides 1x1 cm arranged
in the bottom of 12 well plates. Then 1 mL of BHI
broth was added, keeping them in the oven for 48 h at
37° C. Alternatively, in place of the BHI, 1 mL of the
PS solution was placed in a previously defined
concentration and irradiated. Then the biofilms were
washed with PBS and fixed with 1 ml 2.5%
glutaraldehyde for 1 hour. Then, the sample was
dehydrated with ethyl alcohol in different
concentrations: 10, 25, 50, 75, 90, and 100% for 20
min each. After drying, the slides were metalized and
visualized in the LEO scanning electron microscope,
model 440, with a magnitude of 60.00 KX.
3 RESULTS
3.1 Biofilm Photoinactivation
The inactivation obtained using CHL-Ph-A by the
multivariate form presented in Table 1. The best
photoinactivation obtained was 53 % for the more
significant variation in the survival index (Δ log
10
) of
5.13 corresponding to nine assays. This decrease in
the survival index can be considered good (or
enough) when dealing with biofilm, which is very
difficult to inactivate. So, the best parameters were PS
concentration of 5 µmol L
-1
, IT of 40 min, and LD 30
J cm
-2
reaching maximum photoinactivation of 4,52 ±
0,02 log CFU.
In a biofilm, bacteria have the same genetics as in
planktonic culture, but their biochemical activities differ by
40%, presenting a greater difficulty to be eliminated due to
acquired resistance (Wiesch et al., 2011). Given this
difficulty, antimicrobial photodynamic therapy can be
employed as an option for indiscriminate use of antibiotics,
thus reducing the problem related to bacterial resistance.
The methodology does not entail resistance to bacteria due
to the vast number of possible targets that ROS can act in
preventing any bacterial adaptation/mutation. However,
according to the American Society of Microbiology, the
reduction must be more significant than required (> 3 log
CFU mL
-1
) for a new approach to be called antimicrobial
(ASM, 2015). Fortunately, photodynamic therapy using
CHL-Ph-A fulfills this requirement.
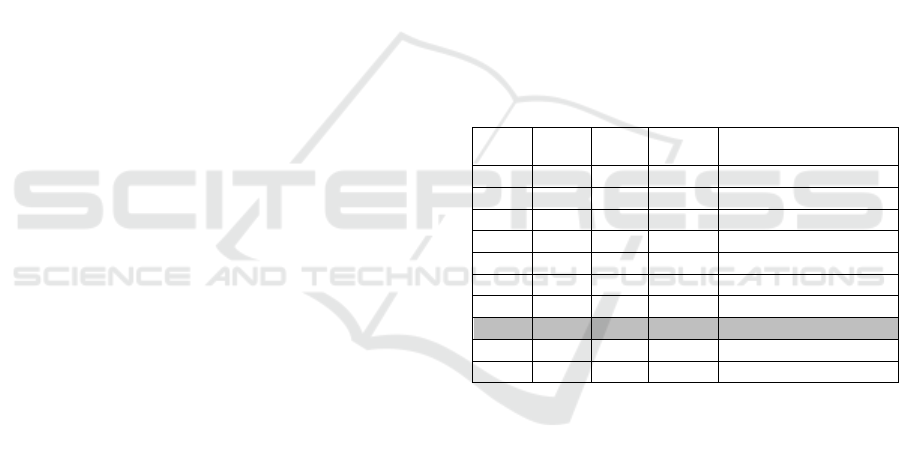
Table 1: Bacterial viability of MRSA biofilm after a-PDT
with CHL-Ph-A. The results of the experiments are
arranged according to the experimental matrix FFD 2
3
where IT: incubation time (min), LD: light dose (J cm
-2
),
and C: chlorin concentration (µmol L
-1
). Nine different
experiments were performed as described according to the
values of the parameters used. After the procedures, the
results of each assay (Colony Forming Unit – CFU) are
described and presented as average ± standard deviation
(SD) with n = 4 replicas.
Assay
IT
(min)
LD
(J cm
-2
)
C
(µmol L
-1
)
CFU Average ± SD
CHL-Ph-A
- 0 0 0 9,65 ± 0,03
1 20 15 5 7,78 ± 0,11
2 20 15 10 7,36 ± 0,14
3 20 30 5 7,46 ± 0,06
4 20 30 10 7,40 ± 0,04
5 40 15 5 6,11 ± 0,16
6 40 15 10 5,90 ± 0,12
7 40 30 5 4,52 ± 0,02
8 40 30 10 6,48 ± 0,03
9 30 22 7.5 6,48 ± 0,05
3.2 Membrane Rupture after
Chlorin-PDT
The integrity of the bacteria membrane present in the
biofilm was determined by Fluorescence Microscopy
using the Live/Dead kit, which contains two markers,
the fluorescent green SYTO
®
9 (S) and the fluorescent
red propidium iodide (PI). The probe S penetrates
both into intact cells or not because of its low
molecular weight; however, the PI only penetrates
cells with the damaged cytoplasmic membrane
because of its high molecular weight resulting in the
reduction of S intensity when both dyes coexist in the
cell. Figure 4 shows the results.
The CHL-Ph-A
chlorin associated with photodynamic therapy
enabled the cytoplasmic membrane disruption of
MRSA in the biofilm, revealing the red color (Fig.4
PHOTOPTICS 2020 - 8th International Conference on Photonics, Optics and Laser Technology
94
B). On the other hand, the presence of membrane
integrity is represented in green (Fig.4 A).
Figure 4: Fluorescence microscopy of MRSA biofilm: (A)
Control and (B) after photoinactivation using CHL-Ph-A.
3.3 Analysis of MRSA Photoxidized
after Chlorin-PDT
Scanning Electron Microscopy (SEM) was used to
characterize the biofilm structure, bacterial
morphology, as well as to evidence the photodynamic
process. For this, S. aureus biofilm was submitted to
SEM analysis in the best experimental conditions for
CHL-Ph-A photooxidation using FFD 2
3
. The
parameters used were: C = 5 µmol L
-1
; IT = 40 min,
and LD = 30 J cm
-2
.
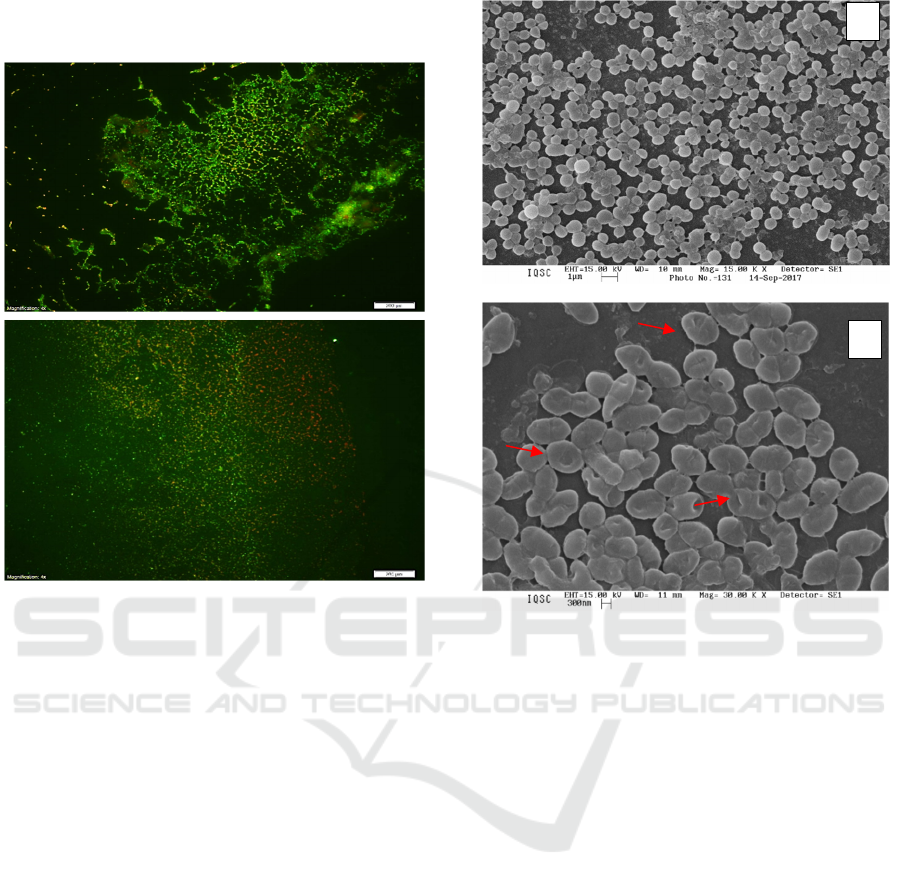
Through Figure 5, it is possible to observe by
Scanning Electron Microscopy the bacterial
arrangements of Staphylococci colonies as well as the
bacterium-bacterium interactions in biofilm
presenting links between them through fimbria
(highlighted in yellow). After A-PDT (Fig. 5B), a
damaged structure was observed (highlighted in red),
denoting photooxidation of cell membrane
components in which cocci do not have a definite
shape but a turgid structure as a deformation.
Figure 5: Scanning Electron Microscopy of MRSA
Biofilm: (A) Control and (B) After photoinactivation using
CHL-Ph-A.
4 CONCLUSIONS
The results suggest that A-PDT of MRSA biofilm
using CHL-Ph-A has excellent potential to combat
MRSA biofilm since reductions up to 5.13 Δlog
10
were reached. The efficiency of photooxidation and
the potential to eradicate biofilm was observed and
proven by SEM and Fluorescence Microscopy. The
results suggest that antimicrobial photodynamic
therapy using this new chlorin may be a good
alternative for the treatment of antibiotic-resistant
bacterial infections.
Overall, A-PDT is expected to be implemented to
ensure a broad bacterial inactivation. It is also
expected that the use of multivariate models, such as
complete factorial design 2
3
be usefull in future
experiments aiming to reduce costs and experimental
time in order to search for better responses that
enable even greater photoinactivation.
B
*
*
*
*
*
A
B
A
Photoinactivation of Methicillin-Resistant S. Aureus Biofilm using a New Chlorin as Photosensitizer
95

ACKNOWLEDGMENTS
This study was financed in part by the Coordenação
de Aperfeiçoamento de Pessoal de Nível Superior -
Brasil (CAPES) - Finance Code 001; Conselho
Nacional de Desenvolvimento Científico e
Tecnológico (CNPq) and Fundação de Amparo à
Pesquisa do Estado de São Paulo/Centro de Pesquisas
de Ótica e Fotônica (FAPESP/CEPOF) 2013/07276-
1.
REFERENCES
Amaral, L.S.; Azevedo. E.B.; Perussi, J.R, 2018. The
Response Surface Methodology speeds up the search
for optimal parameters in the photoinactivation of E.
coli by Photodynamic Therapy. Photodiagnosis and
Photodynamic Therapy, v. 22, p. 26-33.
ASM, 2015. Antimicrobial Agents and Chemotherapy.
Available at: http://aac.asm.org/site/misc/journal-
ita_abb.xhtml#04
De Oliveira, K.; De Souza, J.; Assis, B. Brocksom, T, 2014.
Chlorins: natural sources, synthetic developments, and
main applications. Current Organic Synthesis, v. 11, n.
1, p. 42-58.
Hamblin, M. R.; Mroz, P., 2008. Advances in
Photodynamic Therapy: Basic, Translational, and
Clinical. Norwood, MA: Artech House.
Linares, I. A. P.; Oliveira, K. T., and Perussi, J. R., 2017.
Chlorin derivatives sterically-prevented from self-
aggregation with high antitumor activity for
photodynamic therapy. Dyes and Pigments, v. 145, p.
518-527.
Stanislaw, K.; Kwiatkowski. S.; Knap, B.; Przystupski, D.;
Saczko, J.; Kędzierska, E.; Knap-Czop, K. Kotlińska,
J.; Michel, O.; Kotowski, K.; Kulbacka, J., 2018.
Photodynamic therapy – mechanisms, photosensitizers
and combinations. Biomedicine and Pharmacotherapy,
v. 106, p. 1098-1107.
Sobotta, L.; Paulina S.; Jaroslaw, P.; Jadwiga, M., 2018.
Non-porphyrinoid photosensitizers mediated
photodynamic inactivation against bacteria. Dyes and
Pigments, v. 163, p. 337-355.
Wiesch, P.A.; Kouyos, R.; Engelstadter, J.; Regoes, R.R.;
Bonhoeffer, S., 2011. Population biological principles
of drug-resistance evolution in infectious diseases.
Lancet infects dis., v. 11, n.3, p.236-47.
PHOTOPTICS 2020 - 8th International Conference on Photonics, Optics and Laser Technology
96
