
Low Temperature Plasma Vacuum Sterilization of Medical Devices
by using SterAcidAgent
®
: Description and Distinctive Characteristics
Aleksei E. Zhdanov
1a
, Ilya M. Pahomov
2
, Alexey I. Ulybin
2
and Vasilii I. Borisov
1b
1
Ural Federal University, Institute of Radio Electronics and Information Technology-RTF, Mira Str., Yekaterinburg, Russia
2
Lidkor LLC, Posadskaya Str., Yekaterinburg, Russia
Keywords: Low Temperature Plasma, Atmospheric-pressure Plasmas, Sterilization, RF Discharge, UV Radiation,
Diffusion, Exposition, Peroxide, Organic Acid, Sterilized Agent.
Abstract: This article presents a description and distinctive characteristics of the new method of low-temperature
sterilization. This method based on using a mixture which is consists of peroxide and organic acid as the
sterilized agent (SterAcidAgent
®
). This study shows that SterAcidAgent
®
composition has reduced the
concentration of hydrogen peroxide, it also has increased bactericidal property of the mixture. We conducted
studies of the sterilizing activity of 5-carboxylic low molecular weight acids, investigated the effect of basicity
and hydroxyl group in the alpha position on sterilizing activity, and proposed a potential composition for a
new line of sterilizing agents.
1 INTRODUCTION
The low-temperature plasma sterilization method is
used as an alternative to gas sterilization based on
ethylene oxide or formaldehyde vapor (Zhao and Li,
2018). High toxicity of ethylene oxide is a reason for
its increasing usage limitation as well as the strict
requirement of subsequent prolonged ventilation of
sterilized products (Dianfeng, 2016).
Plasma sterilization is carried out at low
temperatures (up to 60 °C) in a dry atmosphere
(Plewa and Yousfi, 2014). A pair of a 60% hydrogen
peroxide aqueous solution (peroxide) H
2
O
2
and its
low-temperature plasma is used as a sterilizing agent
(Xaplanteris and Filippaki, 2019). This combination
of these factors provides the sterilization process
estimated time to be reduced to 35-45 minutes.
According to manufacturers, a vast scope of
instruments and medical devices are not
recommended or eligible to be sterilized in high
temperature and humidity conditions (Suanpoot and
Sornsakdanuphap, 2016). These tools include
surgical, traumatological, ophthalmic, dental
(excluding burs), microsurgical instruments, optical
fibers, laser and optical fibers, electrical cords and
cables, electrical and electronic devices (Li and Hang,
a
https://orcid.org/0000-0002-8594-7660
b
https://orcid.org/0000-0003-0486-7552
2016), electrophysiological catheters (Esmond and
Winfrey, 2016), pens instruments (Ahn and Chae,
2016), breathing circuits, plastic containers and many
other. Implementation of plasma sterilizer appears to
be a decent option to such tools, especially effective
in sterilization of heat-sensitive materials products
and materials prone to active corrosion. Also, the
plasma sterilization method could be used to sterilize
hard-to-reach and finished surfaces. However, wear
and maintain performance of instruments with thin
and sharp working parts could be reduced through
plasma sterilization in a longer period in comparison
to autoclave sterilization method (Smolyakov and
Romadanov, 2015).
Using this method makes it possible to sterilize
the internal surfaces of the channels of medical
devices, such as endoscopes (diameter up to 1 mm
and length up to 3000 mm). Plasma sterilization cause
no harm to the environment since hydrogen peroxide
leaves only non-toxic components (oxygen and
water) after utilization.
The method of low-temperature plasma
sterilization is embodied in the line of low-
temperature plasma sterilizers represented by
Steriplaz
®
models manufactured by Lidkor LLC.
86
Zhdanov, A., Pahomov, I., Ulybin, A. and Borisov, V.
Low Temperature Plasma Vacuum Sterilization of Medical Devices by using SterAcidAgent
R
: Description and Distinctive Characteristics.
DOI: 10.5220/0008934500860093
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 1: BIODEVICES, pages 86-93
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
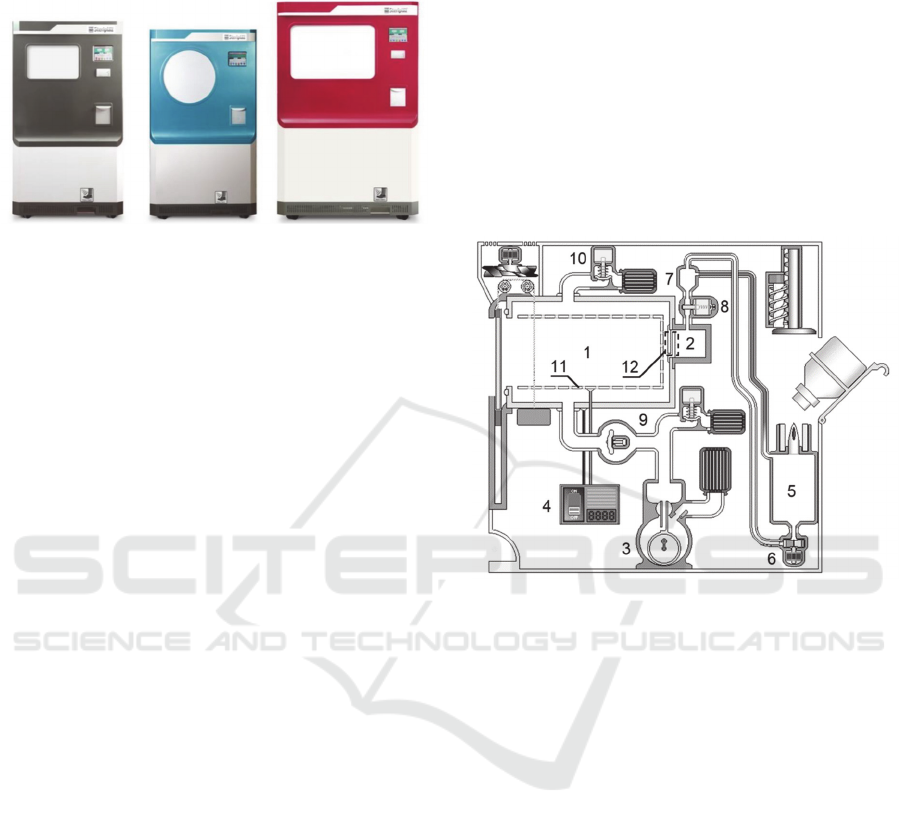
These sterilizers have usable volumes of sterilization
chambers of 50, 80 and 126 litters (Fig.1).
Figure 1: Model Line of Steriplaz
®
.
Limitation of plasma technology is based on the
evaluation of technology itself in numerous system
configurations (Oshita and Kawano, 2015). Thus,
there is an explanation of increased interest in the
plasma used for the microorganism inactivation as a
substitute for other non-thermal sterilization
procedures (Suanpoot and Han, 2015). Publications
(Jeništa, 2016) evidence this fact and highlight
several advantages (Yang and Yan, 2015) and
disadvantages (Gil'man, 2003) (for example, the need
for extensive research to determine the most plasma-
resistant microorganisms (Deilmann and Thei, 2008))
associated with method (Xu and Wang, 2019). This
article summarizes the results of plasma sterilization
using SterAcidAgent
®
. The microbiological
assessment shows the effectiveness of
microorganism’s inactivation technology based on
SterAcidAgent
®
.
2 METHODS
2.1 Technical Implementation of
Developed Sterilization Method
All acids are water-soluble, prepared in non-
explosible concentrations, and stored in accordance
with peroxide storage rules. All reagents were
purchased by Lidkor LLC. The experiments were
performed on a Steriplaz-120 brand sterilizer, and
bactericidal tests were performed on biological and
chemical indicators manufactured by Lidkor LLC.
Fig.2 shows a simplified diagram of a plasma
sterilization device, where:
1 - a sterilization chamber;
2 - a sterilizing agent evaporator;
3 - a vacuum pump;
4 - a high-frequency generator;
5 - a container with a sterilizing agent;
6 - a pump of sterilizing agent;
7 - a sterilizing agent measuring cup;
8 - valve for injection of a sterilizing agent;
9 - valve of a vacuum pump;
10 - atmospheric valve;
11 - chamber walls;
12 - concentration equalization slit.
The designed method may be divided into 7 main
stages, which are described below and referred to the
elements of a simplified diagram of the plasma
sterilization device (Fig. 2) and an influence of
sterilization chamber pressure on time (Fig.3).
Figure 2: Simplified Diagram of a Plasma Sterilization
Device.
2.1.1 Evacuation
The sterilization chamber and evaporator heating
initiate the start of operation of the sterilization
device. Sterilization chamber (Fig. 2, 1) is heated in
the range from 45 to 50 degrees Celsius and of the
sterilizing agent (Fig. 2, 2) is heated to 110 degrees
Celsius. When the device is ready for sterilization, the
operator places the object into the chamber and fills
the container with a sterilizing agent (Fig. 2, 1).
Atmospheric pressure valve (Figs. 2, 10) closing
and vacuum pump starting proceed automatically,
providing the first target pressure (at least 25 Pa)
(Figs. 2, 3).
At this stage, pressure reduces linearly to the
value of at least 200 Pa. After reaching that point, the
pressure inside the chamber decreases exponentially
to the first target pressure value. The evacuation
process shown in curve 3 may be divided into two
parts. The first part is a low vacuum (linear), where
the main influence is caused by the chamber volume.
The second part is high vacuum (nonlinear), where
the vacuum time depends on the inner surface area of
Low Temperature Plasma Vacuum Sterilization of Medical Devices by using SterAcidAgent
R
: Description and Distinctive Characteristics
87
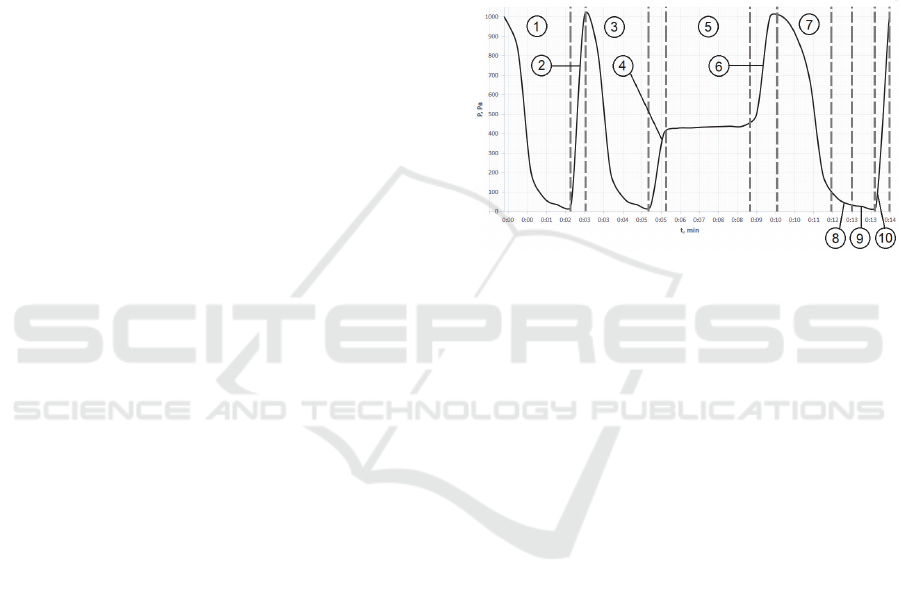
the sterilization chamber. The inside chamber
pressure is represented on curve 3 (Fig.3, 3).
Camera pre-drying cycle is displayed on curves 1
and 2. Curve 1 shows evacuation to 100 Pa, after
which the RF generator is turned on to the maximum
vacuum value. Curve 2 shows the opening of the
atmospheric valve and increasing the pressure to
atmospheric value. If additional camera drying is
required the user can select the camera pre-drying
cycle manually.
2.1.2 Injection of a Sterilizing Agent
Hydraulic pump operation start provides the supply
of the sterilizing agent in the measuring cup (Fig. 2,
6), after which the sterilizing agent is injected into the
evaporator (Fig. 2, 2) by the opening of the measuring
cup valve (Fig. 2, 8).
Curve 4 (Fig.3, 2) shows the change in pressure
inside the chamber (Fig.2, 1) when opening the
atmospheric valve (Fig.2, 10). An immediate increase
in pressure up to no more than 400 Pa occurs as a
result of the injection valve opening (Fig.2, 8) since
the measuring cup (Fig.2, 7) has connection with the
atmosphere.
2.1.3 Diffusion of the Sterilizing Agent
In the evaporator (Fig.2, 2), the sterilizing agent
changes its aqueous state of aggregation to gaseous.
Using the concentration equalization slit (Fig.2, 12) is
essential to reach the sterilizing agent concentrations
alignment between the evaporator (Fig.2, 2) and the
chamber (Fig.2, 1) (or the penetration of the
sterilizing agent into the chamber (Fig. 2, 1)).
Diffusion initiates with the sterilizing agent
subside on the sterilized object. The diffusion process
of an organic acid-based sterilizing agent is presented
in more detail in the second paragraph described
below.
Concentration gradient and sterilizing agent
diffusion inside the chamber (Fig.2, 1) are the reason
for the alignment of concentrations between the
chamber and the evaporator as shown in Curve 5
(Fig.3, 5). Initially, the pressure increases
exponentially due to the pressure gradient inside the
chamber. The linear part of the curve corresponds to
the sterilizing agent evaporation and an increase in
the temperature inside the chamber as a result.
2.1.4 Opening of the Atmospheric Valve and
Subsequent Diffusion of the Sterilizing
Agent
Some of the objects, like endoscopes and tubular
systems, have cavities inaccessible for penetration of
the sterilizing agent. Thus, an increase in pressure to
1000 Pa occurs by opening the atmospheric valve for
penetrating the sterilizing agent into the tubular
systems (Fig.2, 10) and allow the sterilizing agent to
persist action on bacteria on the surface of the
sterilized object.
Figure 3: Graph of the Pressure Versus Time of the Plasma
Sterilization Process.
The change in pressure inside the chamber (Fig.2,
1) when opening the atmospheric valve (Fig.2, 10) is
represented on curve 6 (figure 3).
The engagement of the RF generator (Fig.2, 4)
generates a short-term increase in pressure associated
with the transfer of plasma energy to condensed
water, thereby contributing to the evaporation
process.
The effect of radiofrequency discharge on a
sterilizing agent is presented in more detail in the
third paragraph, described below.
The evacuation process is shown in curve 7
(Fig.3) is similar to the process in curve 3.
The introduction into the plasma chamber
promotes a brief increase in pressure, shown in curve
8 (Fig.3). The plasma inside the chamber can
effectively destroy any pathogen present in the
working area (Qi-Kang and Si-Jing, 2019). As
reported in recent studies (Stulić and Vukušić, 2019),
it is known that atmospheric pressure plasma is highly
destructive towards microorganisms (Li and Zhou,
2019), which makes it an object of potential use for
various biological and medical purposes.
The optimal pressure range for an effective
breakdown of the spark gap lays between 30 to 100
Pa. This increasing pressure step in sterilization
process is displayed on the curve 8 (Fig.3). However,
BIODEVICES 2020 - 13th International Conference on Biomedical Electronics and Devices
88
the breakdown of the gaseous medium relies on
assorted factors. Pressure, temperature, and
composition of the working mixture and the distance
between the electrodes makes the contribution into
breakdown properties.
2.1.5 Exposition
The RF generator is disabled by the control unit
(Fig.2, 4). The plasma burns out during plasma
exposure occurring in chamber. The mechanism of
the UV-radiation formation is presented in more
detail in the fourth paragraph, described below.
2.1.6 Opening the Atmospheric Valve
This stage returns an atmospheric pressure to the
chamber. Pressure equalization between the
atmosphere and the chamber is realized due to the
concentration gradient. An abrupt increase in pressure
to atmospheric is displayed on curve 10 (Fig. 3).
2.2 Diffusion Process of an Organic
Acid-based Sterilizing Agent
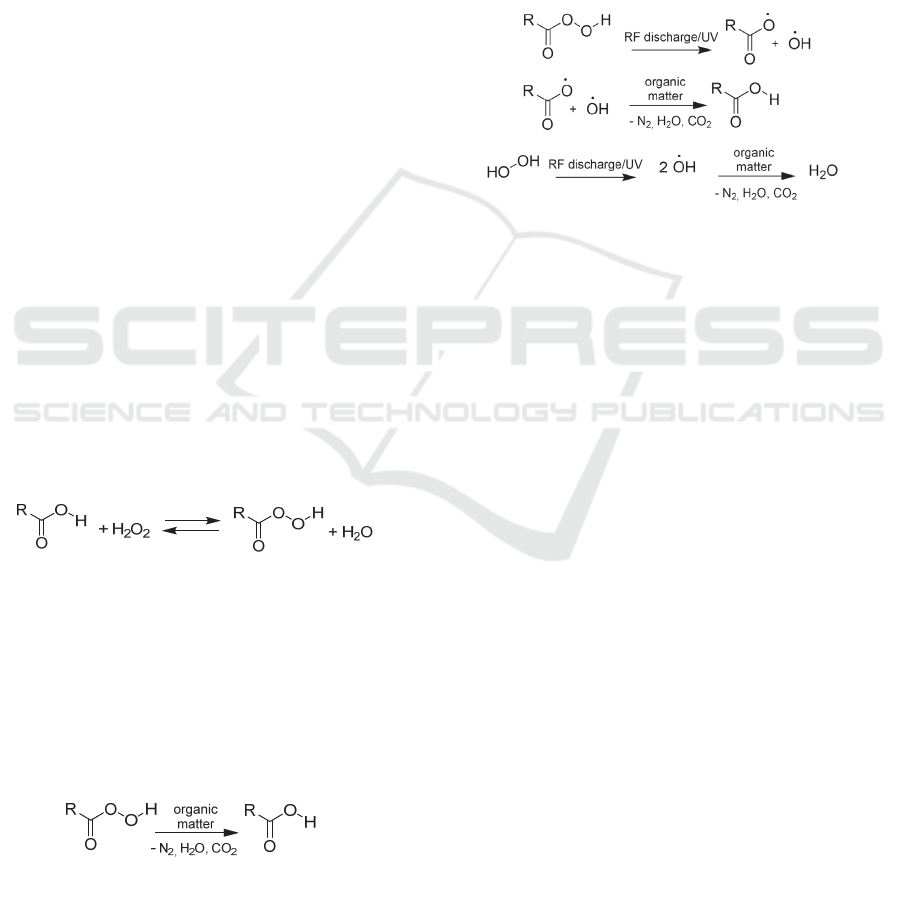
Since the second half of the twentieth century, a wide
scope of oxidation reactions is realized through the
organic peracids action (Ki and Masur, 2019).
Currently, organic peracids are regularly used in
diverse bleaching and cleaning products and
disinfectants (Li and Ma, 2019). Organic peracids
could be obtained by the reversible reaction of
hydrogen peroxide with organic acids (1), but the
yields do not exceed 10% without catalyst.
(1)
Substituent R in α-position to carboxyl group
determines the oxidizing potential of organic
peracids. Higher oxidative activity, in comparison to
hydrogen peroxide, and, as a result, more effective
sterilization, is provided by more powerful electron-
withdrawing substituent R. The peracids affect
organic matter similarly to other strong oxidizing
agents, which means the carbon dioxide, nitrogen,
and water in reaction products. Original carbon acid
is regenerated through the oxidation process (2).
(2
)
2.3 The Effect of RF Discharge on a
Sterilizing Agent
All proceeding reactions undergo the same reaction
centre – peroxyl group, prone to homolytic
decomposition, making effect of RF Discharge on
peracids and hydrogen peroxide similar. The O-O
bond cleavage proceeds when exposed both to RF
discharge as well as UV radiation produced. Obtained
radicals are significantly more active oxidants
comparing to starter compounds, which leads to
increased sterilization activity (3).
(3)
It’s noteworthy, that obtaining radicals are
highly unstable, that provides not only effective
oxidative sterilization but also self-oxidation to
carbon dioxide and water, which has a positive
influence on sterilization camera cleanness.
2.4 The Mechanism of UV Radiation
Formation
Inactivation or removal of biological contaminants
(pathogens) under influence of non-thermal plasma
has been reported in papers (McEvoy and Rowan,
2019). Thus, moderate processes for gentle biological
deactivation could be realized through plasma action
as reported for sensitive products (Homola and
Pongrac, 2019), medical instruments (Chumakov and
Taranchuk, 2018) and implants (Souza and Ferreira,
2012). UV radiation has a lower antimicrobial effect
than direct plasma treatment (George and Barrett,
2019). Moreover, biological deactivation using non-
thermal plasma can include processes involving
active radicals or ions (Vasilets and Gutsol, 2009).
3 RESULTS AND DISCUSSION
Chemical indicators CI PCD and biological indicators
BI PCD with a class G culture (Stearothermophilus
culture of Lidkor LLC) were used for effectiveness
evaluation of the sterilizing agent. For each
sterilization iteration, two chemical and two
Low Temperature Plasma Vacuum Sterilization of Medical Devices by using SterAcidAgent
R
: Description and Distinctive Characteristics
89
biological indicators were used. Result was
considered as a successful in the case of positive
results of both biological indicators.
A portion of acid and the required amount of
water was added to 60% hydrogen peroxide to
prepare solutions with given mass concentrations.
The resulting solutions were kept in the dark for 10
days to form a sufficient amount of peracids.
As part of the study, a set of low molecular weight
carboxylic acids including acetic, propionic, lactic,
oxalic and citric acid were selected for the
experiments. During the study, the concentration of
acids ranged from 5 to 15% in increments of 2.5%,
the concentration of hydrogen peroxide changed from
50 to 20% in increments of 5% and from 20 to 10%
in increments of 2%.
The critical concentrations at which sterilization
was possible (the minimum acid concentration at the
minimum peroxide concentration) are shown in Table
1.
Table 1: Critical Concentrations.
Acid
Acetic
Propionic
Lactic
Oxalic
Citric
Acid/
Peroxid,
%
12.5/20
15/20
15/16
15/20
15/10
Figure 4: Selection of the Optimal Composition.
Obtained results for chosen original carbon acids are
provided in Tables I-VI. Each table represents the
sterilization activity of carbon acid and hydrogen
peroxide mixture (Fig.4). “Plus” and “minus” signs in
the table cells mean effective and ineffective
sterilization respectively based on indicator response.
Sterilization is considered complete if both results
are “plus”. It is noteworthy that hydrogen peroxide
aqueous solution without carbon acids persist
effective sterilization only in concentrations above
52,5%.
The results of the experiments on the selection of
the optimal composition and a comparative table are
shown in Table 2-7.
Sterilization activity should be associated with
peracid oxidative potential; hence it should be
determined by the carboxyl group substituent.
Actually, an increase in sterilization activity
correlates with an increase of the electron-
withdrawing group influence for the series of
propionic, acetic and lactic acids.
Table 2: Propionic Acid Sterilization Potential.
Acid
Peroxi
d
5,0 7,5 10,0 12,5 15,0
45 +/+ +/+ +/+ +/+ +/+
40 +/+ +/+ +/+ +/+ +/+
35 +/- +/+ +/+ +/+ +/+
30 -/- +/+ +/+ +/+ +/+
25 -/- -/- +/+ +/+ +/+
20 -/- -/- -/- +/- +/+
Table 3: Oxalic Acid Sterilization Potential.
Acid
Peroxi
d
5,0 7,5 10,0 12,5 15,0
45 +/+ +/+ +/+ +/+ +/+
40 +/+ +/+ +/+ +/+ +/+
35 +/- +/+ +/+ +/+ +/+
30 -/- +/+ +/+ +/+ +/+
25 -/- -/- +/+ +/+ +/+
20 -/- -/- -/- +/- +/+
Table 4: Acetic Acid Sterilization Potential.
Acid
Peroxi
d
5,0 7,5 10,0 12,5 15,0
45 +/+ +/+ +/+ +/+ +/+
40 +/+ +/+ +/+ +/+ +/+
35 +/+ +/+ +/+ +/+ +/+
30 +/+ +/+ +/+ +/+ +/+
25 -/- +/- +/+ +/+ +/+
20 -/- -/- -/- +/+ +/+
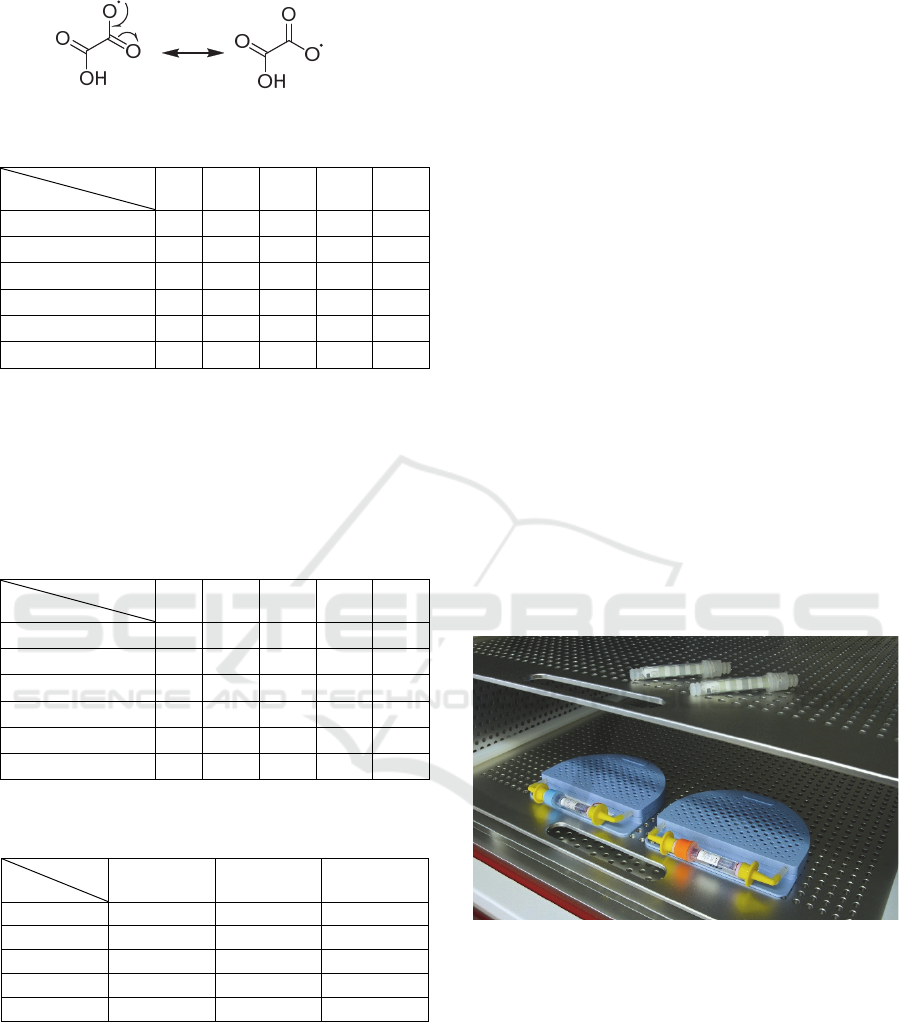
Almost similar results for oxalic and acetic acids
could be explained with oxalic radical increased
stability due to resonance structures set (4). The
highest efficiency was performed by lactic and citric
acids in accordance with the assumption.
BIODEVICES 2020 - 13th International Conference on Biomedical Electronics and Devices
90
(4
)
Table 5: Lactic Acid Sterilization Potential.
Acid
Peroxi
d
5,0 7,5 10,0 12,5 15,0
45 +/+ +/+ +/+ +/+ +/+
40 +/+ +/+ +/+ +/+ +/+
35 +/+ +/+ +/+ +/+ +/+
30 +/+ +/+ +/+ +/+ +/+
25 -/- +/- +/+ +/+ +/+
20 -/- -/- -/- +/+ +/+
Thus, we divide the acid set into two groups,
applicable and non-applicable for further
investigations as a sterilization agent. Propionic and
oxalic acids are considered to be non-applicable due
to propionic acid poor efficiency and oxalic acid
higher toxicity in comparison with acetic acid.
Table 6: Citric Acid Sterilization Potential.
Acid
Peroxi
d
5,0 7,5 10,0 12,5 15,0
45 +/+ +/+ +/+ +/+ +/+
40 +/+ +/+ +/+ +/+ +/+
35 +/+ +/+ +/+ +/+ +/+
30 +/+ +/+ +/+ +/+ +/+
25 +/+ -/- +/- +/+ +/+
20 -/- -/- -/- +/- +/+
Table 7: Lactic, Citric and Acetic Acid Sterilization
Potential in Conditions of Low Peroxide Concentration.
Acid
Peroxi
d
Lactic, 15% Citric, 15% Acetic, 15%
18 +/+ +/+ -/-
16 +/+ -/- -/-
14 -/- -/- -/-
12 -/- -/- -/-
10 -/- +/+ -/-
Most substantial results are recorded for citric
acid, even with unexplainable at this moment's gaps
in sterilization facilities. Also, a high activity in low
peroxide concentration was performed by acetic and
lactic acids which could lead to the decreased
corrosive effect on glass parts of medical equipment.
That makes acetic, lactic and citric acid regarded as
possible compounds for an agent of commercial use.
Moreover, no papers or patents content citric acid as
a possible sterilization agent, which makes it a very
interesting object of further investigations.
A method for experimental mixtures effectiveness
evaluation was developed and consist in usage of pair
of chemical and biological indicators (Fig.5). The
chemical indicator is a test strip with five applied
layers of the active colored substance. Upon
successful sterilization, all colored strips change their
color to pale yellow, a special table for color
comparison is provided. Evaluation of the
sterilization effectiveness using chemical sterilizers is
approximate, but express. Thus, it has the role of a
rapid detector of errors that could possibly occur
during the sterilization process. The biological
indicator represents a combination of a plastic capsule
containing a G. Stearothermophilus bacterial culture
(ATCC 7953) and incubator. This indicator is more
accurate and is used for the final assessment of
sterilization efficiency, but incubation time requires
24 hours to complete.
A pair of biological and chemical indicators are
placed in both sterilizer compartments for
experimental sterilizing agent testing. After leak-
proof covers are removed, the capsule with a
biological indicator is placed into a Teflon lumen load
with a silicone hose 1000 mm long and 1 mm in
diameter, simulating endoscopes.
Figure 5: Pairs of Chemical and Biological Indicators.
After the sterilization cycle is complete, the glass
ampoule is crushed, providing a nutrient medium
access to oxygen. A broken ampoule, along with a
control broken ampoule are instantly placed in an
incubator for 24 hours at a temperature of 55 degrees
Celsius (Fig.6). Successful sterilization provides the
growth of a bacteriological culture causing a chemical
indicator to turn pale yellow under the influence of
the bacteria waste products. Sterilization is
considered successful only if both biological
indicators persist in their color.
Low Temperature Plasma Vacuum Sterilization of Medical Devices by using SterAcidAgent
R
: Description and Distinctive Characteristics
91

4 CONCLUSIONS
This article presents a new method of low-
temperature plasma sterilization using a mixture of
hydrogen peroxide and low molecular weight
carboxylic acids as a sterilizing agent as well as a
method for the bactericidal activity evaluation based
on combination of chemical and biological indicators.
The sterilization process includes both chemical
oxidative and physical effects due to low-temperature
plasma and ultraviolet radiation, determining the
complex nature of the method. The carboxylic acid
usage in the composition of sterilizing agents allowed
a 5-6-fold reduction in the working concentration of
a hydrogen peroxide solution, thereby reducing the
corrosive effect on the glass components of expensive
medical equipment.
Figure 6: Biological Indicator Incubator.
Bactericidal activity of hydrogen peroxide and
carbon acid mixtures was investigated, results are
provided in Table 2-7. Final monitoring of sterilizer
effectiveness was carried out by the bacteriological
method, consisting in biotests based on the
deactivation of test culture spores. This paper
represents the results based on the germination of
crops in an incubator. However, additional
bacteriological tests required as a part of the
validation of a new type of Steriplaz
®
sterilizer.
ACKNOWLEDGEMENTS
We are grateful Lidkor LLC represented by Alexey I.
Ulybin for the providing of the equipment and
chemical reagents for the experiments. We commend
for the support provided by the employees of Lidkor
LLC Gleb A. Sudakov and Alexey N. Roznin. The
MathLab software used for data analysis was
provided by Ural Federal University.
REFERENCES
J. Zhao, W. Li, L. Zou, H. Fu, "Analysis of Oilfield
Wastewater Treatment Effect Based on Low
Temperature Plasma Treatment Process", IOP Conf.
Series: Earth and Environmental Science, vol. 170,
№032099, 2018.
Z. Dianfeng, "The Feasibility of Applying AC Driven Low-
Temperature Plasma for Multi-Cycle Detonation
Initiation", Plasma Science and Technology, vol. 18
(11), pp. 1110-1114, 2016.
J. Plewa, M. Yousfi, C. Frongia, O. Eichwald, B.
Ducommun, N. Merbahi, V. Lobjois, "Low-
temperature plasma-induced antiproliferative effects on
multi-cellular tumor spheroids", New Journal of
Physics, vol. 16, №043027, 2014.
C.L. Xaplanteris, E.D. Filippaki, J.K. Christodoulakis,
M.A. Kazantzaki, E.P. Tsakalos, L.C. Xaplanteris,
"Low temperature atmospheric plasma applications and
codification of its' influence on micro-organisms",
CHAOS 2014 - Proceedings: 7th Chaotic Modeling and
Simulation International Conference, pp. 533-549,
2019.
P. Suanpoot, J. Sornsakdanuphap, H. S. Uhm, G. Cho and
E. H. Choi, "Using plasma propagation speed model for
investigation of electron temperature of AR/N2 in non-
thermal atmospheric pressure indirect-plasma jet,"
2016 IEEE International Conference on Plasma Science
(ICOPS), Banff, AB, 2016, pp. 1-3.
X. Li, Y. Hang, J. Wu, S. Jia and A. B. Murphy, "Roles of
metalions and plasma radiation in the interactions
between a capillary discharge plasma and propellants,"
2016 IEEE International Conference on Plasma Science
(ICOPS), Banff, AB, 2016, pp. 1-2.
M. J. Esmond and A. L. Winfrey, "Review of past, present,
and future plasma models for electrothermal plasma
discharge simulation," 2016 IEEE International
Conference on Plasma Science (ICOPS), Banff, AB,
2016, pp. 1-3.
S. Ahn, J. Chae, H. Kim, K. H. Kim and S. Y. Jung,
"Modeling of reduced air plasma reactions for
nanosecond-pulse dielectric barrier discharge," 2016
IEEE International Conference on Plasma Science
(ICOPS), Banff, AB, 2016, pp. 1-4.
A. Smolyakov, I. Romadanov, W. Frias, A. Koshkarov, A.
Y. Raitses and I. Kaganovich, "Instabilities and
transport in plasmas with EXB drift," 2015 IEEE
BIODEVICES 2020 - 13th International Conference on Biomedical Electronics and Devices
92

International Conference on Plasma Sciences (ICOPS),
Antalya, 2015, pp. 1-4.
T. Oshita, H. Kawano, T. Takamatsu, H. Miyahara and A.
Okino, "Temperature Controllable Atmospheric
Plasma Source," in IEEE Transactions on Plasma
Science, vol. 43, no. 6, pp. 1987-1992, June 2015.
P. Suanpoot, G. Han, J. Sornsakdanuphap, H. S. Uhm, G.
Cho and E. H. Choi, "Plasma Propagation Speed and
Electron Temperature in Slow Electron Energy Non-
thermal Atmospheric Pressure Indirect-Plasma Jet," in
IEEE Transactions on Plasma Science, vol. 43, no. 7,
pp. 2207-2211, July 2015.
J. Jeništa et al., "Investigation of mixing of plasma species
in argon-water arc discharge," 2016 IEEE International
Conference on Plasma Science (ICOPS), Banff, AB,
2016, pp. 1-1.
L. Yang, H. Yan, X. Qi, S. Zhao and C. Ren, "Geometry
Effects of SDBD Actuator on Atmospheric-Pressure
Discharge Plasma Airflow Acceleration," in IEEE
Transactions on Plasma Science, vol. 43, no. 10, pp.
3653-3661, Oct. 2015.
A. B. Gil'man, "Low-temperature plasma treatment as an
effective method for surface modification of polymeric
materials", High Energy Chem., vol. 37, no. 1, pp. 17-
23, Jan. 2003.
M. Deilmann, S. Thei, P. Awakowicz, "Pulsed microwave
plasma polymerization of silicon oxide films:
Application of efficient permeation barriers on
polyethylene terephthalate", Surf. Coat. Technol., vol.
202, no. 10, pp. 1911-1917, Feb. 2008.
J. Xu, Z. Wang, C. Chang, C. Song, J. Wu, W. Shang, P.
Tao, T. Deng, "Electrically Driven Interfacial
Evaporation for High-Efficiency Steam Generation and
Sterilization", ACS Omega 2019, Sep. 2019.
P. Qi-Kang, L. Si-Jing, H. Huan, X. Jing-Fei, Z. Li, F. Zhi,
W. Chuan, "Sterilization effect of an atmospheric low
temperature plasma jet on Candida albicans biofilm",
V. Stulić, T. Vukušić, A.R. Jambrak, V. Bačun-Družina, D.
Popović, J. Mrvčić, Z. Herceg, "Quantitative microbial
assessment for Escherichia coli after treatment by high
voltage gas phase plasma", Innovative Food Science
and Emerging Technologies, vol. 53, pp. 26-35, 2019.
X. Li, R. Zhou, B. Zhang, R. Zhou, K. Ostrikov, Z. Fang,
"Design and characteristics investigation of a miniature
low-temperature plasma spark discharge device",
Plasma Science and Technology, vol. 21 (5), 054005,
2019.
S.H. Ki, K. Masur, K.Y. Baik, E. Ha Choi, "Effects of
humidity on room disinfection by dielectric barrier
discharge plasma", Journal of Physics D: Applied
Physics, vol. 52 (42), 425204, 2019.
J. Li, C. Ma, S. Zhu, F. Yu, B. Dai, D. Yang, "A Review of
Recent Advances of Dielectric Barrier Discharge
Plasma in Catalysis", Nanomaterials (Basel), vol. 9
(10), 1428, 2019.
B. McEvoy, N.J. Rowan, "Terminal sterilization of medical
devices using vaporized hydrogen peroxide: a review of
current methods and emerging opportunities", Journal
of Applied Microbiology, vol. 127 (5), pp. 1403-1420,
2019.
T. Homola, B. Pongrac, M. Zemanek, M. Simek,
"Efficiency of Ozone Production in Coplanar Dielectric
Barrier Discharge", Plasma Chemistry and Plasma
Processing, vol. 39 (5), pp. 1227–1242, 2019.
V. Chumakov, A. Taranchuk, V. Stetsiuk and V. Michan,
"A New Technology of Bactericidal Processing of
Koch's Bacillus on the Basis of Pulsed Electromagnetic
Radiation," 2018 IEEE 38th International Conference
on Electronics and Nanotechnology (ELNANO), Kiev,
2018, pp. 271-276.
J. H. C. d. Souza and J. L. Ferreira, "G. stearothermophilus
Spores' Inactivation by a Single Dielectric Barrier
Discharge in Air at Atmospheric Pressure," in IEEE
Transactions on Plasma Science, vol. 40, no. 12, pp.
3482-3484, December 2012.
J. George and J. Barrett, "A Steam Sterilizable Plastic-
Encapsulated Wireless Sensor Module," in IEEE
Transactions on Components, Packaging and
Manufacturing Technology, vol. 9, no. 4, pp. 770-778,
April 2019.
V. N. Vasilets, A. Gutsol, A. B. Shekther, A. Fridman,
"Plasma medicine", High Energy Chem., vol. 43, no. 3,
pp. 229-233, May 2009.
Low Temperature Plasma Vacuum Sterilization of Medical Devices by using SterAcidAgent
R
: Description and Distinctive Characteristics
93
