
Estimation of Muscle Fascicle Orientation in Ultrasonic Images
Regina Pohle-Fr
¨
ohlich
1
, Christoph Dalitz
1 a
, Charlotte Richter
2,3
,
Tobias Hahnen
1
, Benjamin St
¨
audle
2,3
and Kirsten Albracht
2,3 b
1
Institute for Pattern Recognition, Niederrhein University of Applied Sciences, Reinarzstr. 49, Krefeld, Germany
2
Institute of Biomechanics and Orthopaedics, German Sport University Cologne, Cologne, Germany
3
Department of Medical Engineering and Technomathematics, Aachen University of Applied Science, Germany
Keywords:
Texture Direction, Orientation Angle, Gray Level Cooccurrence, Vesselness Filter, Projection Profile, Radon
Transform.
Abstract:
We compare four different algorithms for automatically estimating the muscle fascicle angle from ultrasonic
images: the vesselness filter, the Radon transform, the projection profile method and the gray level cooccurence
matrix (GLCM). The algorithm results are compared to ground truth data generated by three different experts
on 425 image frames from two videos recorded during different types of motion. The best agreement with
the ground truth data was achieved by a combination of pre-processing with a vesselness filter and measuring
the angle with the projection profile method. The robustness of the estimation is increased by applying the
algorithms to subregions with high gradients and performing a LOESS fit through these estimates.
1 INTRODUCTION
Human movement results from a coordinated acti-
vation of the skeletal muscles. The muscle fascicle
length and their change in length is critical for the
force and efficiency of the muscle. It is thus necessary
to measure fascicle length, which is usually done from
ultrasonic images (Fukunaga et al., 1997; Fukunaga
et al., 2001; Zhou and Zheng, 2012). An example
of a B-mode ultrasound image of the muscle gastroc-
nemius medialis recorded with an ALOKA Prosound
α7 can be seen in Fig. 1: the fascicles are spanned
between the two aponeuroses.
As the fascicles are interrupted by noise and rarely
are captured in their full length by the imaging pro-
cess, their length must be computed from three dif-
ferent auxiliary observables: the position of the two
aponeuroses and the fascicle orientation angle (pen-
nation). Throughout the present paper, we make the
simplifying assumption that both aponeuroses can be
approximated by straight lines. The fascicle length
can then be computed from the pennation angle at dif-
ferent positions on these lines. We thus only concen-
trate on the problem of finding the aponeuroses and
estimating the pennation angle.
a
https://orcid.org/0000-0002-7004-5584
b
https://orcid.org/0000-0002-4271-2511
When the imaging is done while the muscle is
in motion, the image quality can deteriorate due
to variation of transducer skin contact and position
(Aggeloussis et al., 2010). If the fascicle length or
orientation estimation is not done manually, but semi-
automatic or even fully automatic, this requires thus
robust image processing methods for possibly noisy
videos.
According to (Yuan et al., 2020), algorithms for
automatically estimating the fascicle orientation can
be divided into three different approaches. In the first
category, the tracking is semi-automatic by follow-
ing a manually marked indicidual fibers in subsequent
superficial aponeurosis
deep aponeurosis
pennation
angle
fascicle length
Figure 1: Annotated example of an ultrasonic image of the
muscle gastrocnemius medialis.
Pohle-Fröhlich, R., Dalitz, C., Richter, C., Hahnen, T., Stäudle, B. and Albracht, K.
Estimation of Muscle Fascicle Orientation in Ultrasonic Images.
DOI: 10.5220/0008933900790086
In Proceedings of the 15th International Joint Conference on Computer Vision, Imaging and Computer Graphics Theory and Applications (VISIGRAPP 2020) - Volume 5: VISAPP, pages
79-86
ISBN: 978-989-758-402-2; ISSN: 2184-4321
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
79

frames. This can be done, for example by calculating
the optical flow, like in the UltraTrack software (Far-
ris and Lichtwark, 2016). A disadvantage of these
methods is the cumulative error, which requires man-
ual correction after several frames. In addition, mis-
alignments may result due to significant changes in
the appearance and intensity of the structures between
successive frames. These problems occur particularly
with large displacement fields due to fast motion and
insufficient sampling rates of most currently available
commercial devices.
Methods based on texture feature detection form
the second category, which includes Hough transform
(Zhou and Zheng, 2008), Radon transform (Zhao and
Zhang, 2011), or vesselness filter (Rana et al., 2009).
The disadvantage of these methods is that the result
of the angle estimation may be distorted by speckle
noise and intramuscular blood vessels, which modify
the characteristics of the muscle fascicles.
The third category includes deep learning ap-
proaches. Cunningham proposed deep residual (Cun-
ningham et al., 2017) and convolution neural net-
works (Cunningham et al., 2018) to estimate the mus-
cle fascicle orientation. One problem with using deep
learning methods is that they require a large amount
of manually measured image data to achieve good re-
sults. Another difficulty is the dependence of the im-
age acquisition and the image distortions on the ul-
trasound transducer, so that adjusted data sets are re-
quired.
In the present article, we compare two estab-
lished methods from the literature with two new ap-
proaches to determine the orientation of textures. As
established methods, we consider vesselness filtering
(Rana et al., 2009) and Radon transform (Zhao and
Zhang, 2011). We compare these with the very re-
cently proposed gray value cooccurence matrix based
texture orientation estimation (Zheng et al., 2018) and
the calculation of the angle using the projection pro-
file (Dalitz et al., 2008). The latter method has been
used for some time in document image analysis for
estimating the rotation of binary documents. Here we
demonstrate that it can be used for gray level images,
too.
In order to evaluate the quality of the different al-
gorithms, we have compared their results with man-
ual estimations of the pennation angle by different ex-
pert observers. As evaluation criteria, we utilized the
intra-class correlation and the mean absolute percent-
age with respect to the inter-observer average, and the
percentage of results within the inter-observer range.
This article is organized as follows: in section 2 &
3 we describe the implemented algorithms, section 4
describes the evaluation method, section 5 discusses
the results and compares the algorithm performances,
and in section 6 we draw some conclusions and give
recommendations for a practical utilization of the al-
gorithms.
2 REGION OF INTEREST
EXTRACTION
To determine the region of interest (ROI), each video
frame is evaluated separately. Firstly, the two black
areas (see Fig.1) are removed. Then, for a reinforce-
ment of the aponeuroses a vesselness filtering (see
section 3.2) is carried out. Then, Otsu’s threshold-
ing method is used generate a binary image of the
filtered image. In the result, the two largest seg-
ments which correspond to the two aponeuroses are
selected. Straight lines are fitted to the lower segment
border of the superficial aponeurosis and to the up-
per segment border of the deep aponeurosis using the
least squares method. The height of the ROI resulted
from the difference between the smallest y-value of
the lower aponeurosis minus 10 pixels and the largest
y-value of the upper aponeurosis plus 10 pixels. The
width of the ROI is calculated from the width of the
image minus a safety area of 10 pixels to the left and
right borders. This ensures that the ROI is always po-
sitioned within the muscle. As the noise level or the
orientation angle may vary over the entire ROI, we
additionally subdivided the entire region horizontally
into eight overlapping subregions. For a fully auto-
mated process, it would be necessary to automatically
pick the subregion with the “best” image quality. To
characterize this quality, we have computed, for every
subregion, the gray value variance as a measure for
contrast, the mean gradient value and the maximum
value of the histogram of the gradients as measures
for edge sharpness.
3 FASCICLE DIRECTION
ESTIMATION
For the determination of the fiber orientation we used
different methods, which are described in the follow-
ing. These methods were either applied directly to the
ROI or a pre-processing step was used for fascicle en-
hancement. For pre-processing, a Vesselness filter or
Radon transformation was optionally applied for im-
age enhancement. Tbl. 1 shows the investigated com-
binations for pre-processing and fascicle orientation
estimation.
VISAPP 2020 - 15th International Conference on Computer Vision Theory and Applications
80

Table 1: Tested combinations for pre-processing and fasci-
cle orientation estimation. “Frangi” denotes the vesselness
filter.
orientation pre-processing
estimation none Frangi Radon
Frangi - x -
Radon - - x
GLCM x x x
projections x x x
3.1 Radon Transform
The Radon transformation determines the line inte-
gral of the function f (x,y) along all straight lines of
the xy plane. For each of these straight lines one can
consider the Radon transform R as a projection of the
function f (x,y) onto a straight line perpendicular to
it. For this reason it was used by (Zhao and Zhang,
2011), (Yuan et al., 2020) to determine the orientation
of the muscle fibers in ultrasound images. It should
be noted that the radon transformation cannot only be
used for direct angle estimation, but also merely as a
pre-processing operation to reinforce fascicles. Such
a pre-processed image E with an enhancement of the
linear structures in the initial image I is achieved by
applying the following equation:
E = R
−1
(sign(R(I)) · R(I)
2
) (1)
where R is the Radon transform and R
−1
is the inverse
Radon transform. The result of the Radon transform
based enhancement is shown in Fig. 2(c). The angle
of the fascicle orientation resulted from the position of
(a) raw data
(b) vesselnes filter (Frangi)
(c) Radon transform
Figure 2: Effect of filtering with the vesselness filter or
the Radon transform on an image recorded during running
movement.
the maximum of the radon transformed. In our tests,
we calculated the radon transformation only for an an-
gular range of 15 to 70 degrees in which the actual
values vary to exclude errors due to the orientation of
the speckle pattern.
3.2 Vesselness Filter
Muscle fascicles appear in ultrasound images as
vessel-like tubular structures, so that in (Rana et al.,
2009) the multiscale vesselness filter developed by
Frangi (Frangi et al., 1998) was used to enhance them.
In the first step of this filter, images are convolved
with Gaussian kernels. Then the Hessian matrix of
these convolved images is computed. Their eigen-
values provide information related to the direction of
line-like structures. The eigenvector in the direction
of the smallest eigenvalue yields the orientation angle
at the respective pixel position. For our tests we used
the implementation in libfrangi
1
whereby we only al-
lowed angles within our chosen range of 15 to 70 de-
grees in order to suppress responses from dominating
horizontal or vertical structures. All values outside
this range were set to zero in the result image. To
estimate a total orientation angle from all the local
angles estimated at non-zero pixels, we estimated the
angle distribution with a kernel density estimator with
“Silverman’s rule of thumb” (Sheather, 2004) and de-
termined the angle maximizing this density.
Like the radon transform, the vesselness filter can
alternatively also merely be used as a pre-processing
operation for enhancing fascicle structures. An exam-
ple is shown in Fig. 2(b).
3.3 Projection Profile
The projection profile method (Dalitz et al., 2008) es-
timates the orientation angle α as the angle with the
highest variation of the skewed projection profile
h
α
(y) =
x=∞
∑
x=−∞
f (x cos α − y sinα,x sin α + ycos α)
(2)
where f (x, y) is the gray value of the ultrasound image
at position (round(x),round(y)) and zero outside the
image. The variation of this profile is defined as
V (α) =
y=∞
∑
y=−∞
[h
α
(y + 1) − h
α
(y)]
2
(3)
In our implementation we calculate the variation for
an angle range from 15 to 70 degrees with a step width
of 0.5 degrees, which corresponds to the possible an-
gles occurring for our recording conditions. Then we
1
https://github.com/ntnu-bioopt/libfrangi
Estimation of Muscle Fascicle Orientation in Ultrasonic Images
81

select the angle corresponding to the highest varia-
tion.
3.4 Graylevel Cooccurence
The gray level cooccurence matrix (GLCM) repre-
sents an estimate of the probability that a pixel at
position (x, y) in an image with a graylevel g
1
has a
graylevel g
2
at position (x + dx,y + dx ). The GLCM
has a size of g
max
×g
max
, whereby g
max
−1 is the max-
imum of the gray levels in the image. If arbitrary rela-
tive positions are used to calculate the GLCM, the tex-
ture orientation can be estimated. Zheng (Zheng et al.,
2018) applied this method to evaluate SAR images of
the sea surface. For the calculation of the GLCM, we
utilized in the method that Zheng et al. called “scheme
1”. If the shift vector (dx,dy) = (r · cosα,r · sinα)
corresponds with the texture orientation, the diagonal
elements of the GLCM attain high values. For the es-
timation of the fascicle orientation, we apply the crite-
rion suggested in (Zheng et al., 2018), i.e., the degree
of concentration C of larger elements of the GLCM
with respect to the diagonal line:
C(r, α) =
g
max
−1
∑
m=0
g
max
−1
∑
n=0
(m − n)
2
· GLCM(m,n; r, α)
(4)
The weight (m − n)
2
, which increases with increasing
distance of the matrix element from the diagonal, re-
sults in smaller values for images with a strong line
structure if the angle α corresponds to the orientation
of this structure. In our experiments we used a maxi-
mum r of 40 and an angle range of 15 to 70 degrees.
The used angle corresponded to the angle α with the
lowest concentration value.
3.5 Local Regression
Due to the noisy nature of the images, the angle es-
timate can fluctuate considerably between adjacent
frames and subregions. It is thus natural to seek a
more robust angle estimate by means of local regres-
sion. To this end, we optionally apply Cleveland
& Devin’s LOESS method (Cleveland and Devlin,
1988), which is a distance weighted least squares fit
over the k nearest neighbors with weight
W
h
(z) =
(
1 − (z/h)
3
3
for |z| < h
0 otherwise
(5)
where h is the distance to the k-th nearest neighbor.
In our case, the predictor is the frame number and the
dependent variable is the pennation angle.
A B C all
0 2 4 6 8
expert
spread in degrees
Figure 3: Spread per frame of the pennation angle estimates
of the three experts.
4 EVALUATION METHOD
In order to evaluate and compare the different algo-
rithms, we have asked three different experts to manu-
ally draw the aponeurosis and fascicle orientation into
ultrasonic images with the user interface of the Ultra-
Track software (Cronin et al., 2011). The images were
taken from two different videos, which were recorded
each with an ALOKA Prosound α7 for five consec-
utive stance phases (touchdown to toe-off) of the left
foot during walking (video “W”) and running (video
“R”). This resulted in a total of 425 different frames.
The muscle fascicles in the R video were less clearly
visible tan in the W video due to the shakier trans-
ducer skin contact during running.
Each frame was examined three times by every ex-
pert, but on different days. We thus had nine differ-
ent manually estimated angles for each frame. This
was done to estimate the accuracy of the expert opin-
ion. The intra-class correlation ICC3 (Shrout and
Fleiss, 1979) between the experts’ angle estimations
was 0.97, which means that there was good agree-
ment among the experts which angles were higher and
which were lower. On the other hand, the average an-
gle spread per frame was 1.9
◦
for expert A, 1.7
◦
for
expert B, 1.1
◦
for expert C, and 3.2
◦
over all experts.
Box-Plots for the spread distribution can be seen in
Fig. 3. The spread between experts was thus consider-
ably greater than within each expert, and we conclude
that we cannot expect an algorithm to estimate the an-
gle with an accuracy greater than about two degrees.
Part of the inter- and intra-observer variation can
be explained by varying fascicle orientations for dif-
ferent image regions. We therefore split the region
of interest into eight slightly overlapping subregions
and ran the algorithms on each subregion plus on the
VISAPP 2020 - 15th International Conference on Computer Vision Theory and Applications
82
190 200 210 220 230 240
20 25 30 35
frame
angle
projection
GLCM
Frangi
Radon
(a) walking (video W)
270 275 280 285 290 295
20 25 30 35 40 45
frame
angle
projection
GLCM
Frangi
Radon
(b) running (video R)
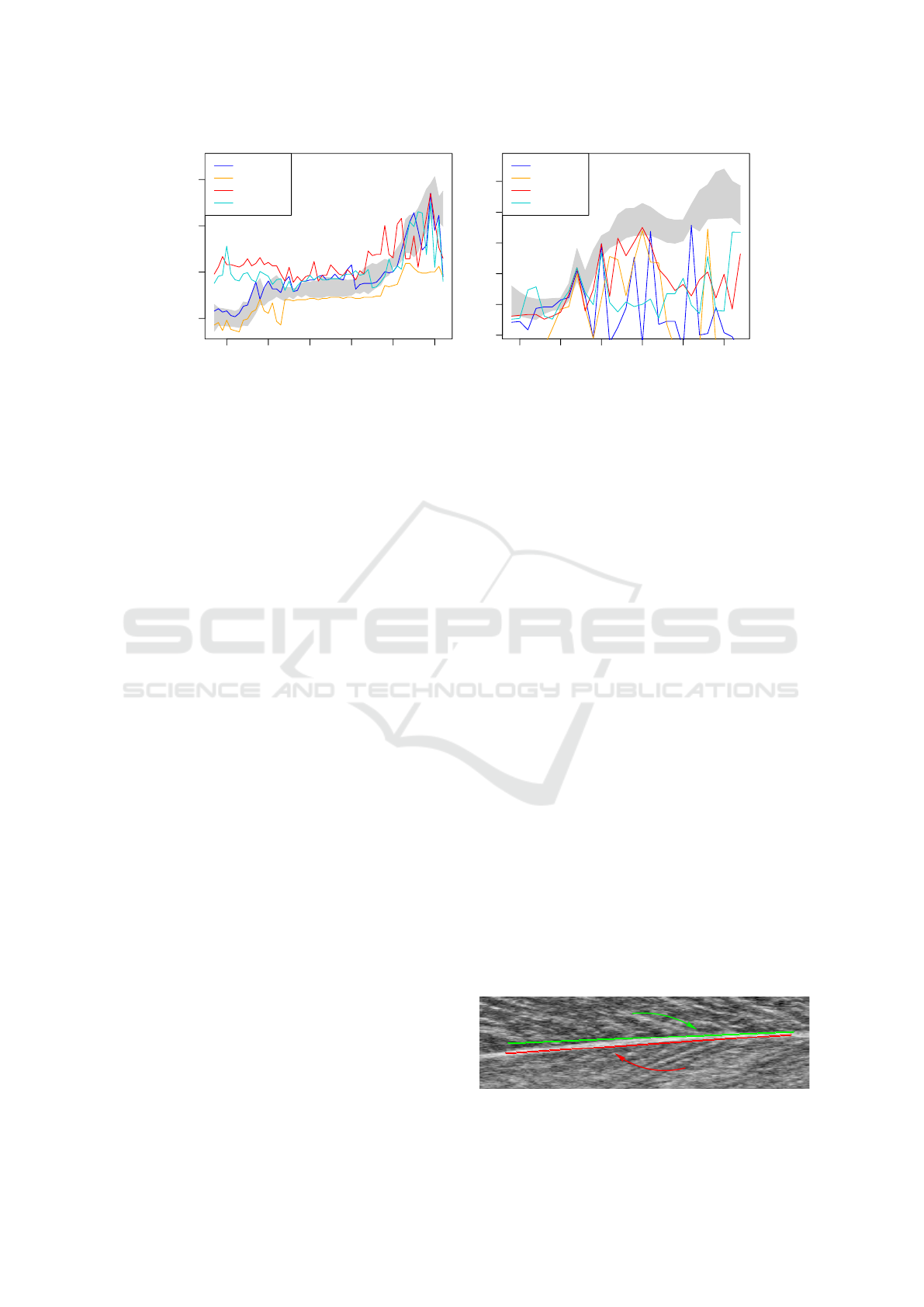
Figure 4: Angle estimations of the different algorithms applied to the entire region of interest for two typical steps of motion.
The gray area is the inter-observer range.
entire region. For each algorithm, we then measured
the following performance indicators for each of these
nine regions:
• the intra-class correlation (ICC3) with the inter-
observer average; this measures how well the es-
timated angles follow the curve shape
• the mean absolute error (MAE) with respect to the
inter-observer average; this measures the overall
error in the estimation in degrees
• the percentage of values inside the inter-observer
range (hit)
5 RESULTS
As the pennation angle is defined as the angle between
the deep aponeurosis and the muscle fascicles, there
are two possible sources of error for its estimation:
errors in the estimation of the aponeurosis’ slope, and
in the estimation of the fascicle orientation. We there-
fore first evaluated the aponeurosis estimation, and
then the estimation of the pennation angle. Moreover,
to derive recommendations for pre-processing filter-
ing, we report results for the different combinations of
pre-processing and estimation algorithms listed above
in Tbl. 1.
5.1 Aponeurosis Slope
In video “R”, the deep aponeurosis was very close to a
straight line, and algorithm and expert opinion about
its slope angle was in good agreement: ICC3=0.926,
MAE=0.286
◦
, hit=71%.
In video “W”, the deep aponeurosis was curved
slightly (see Fig. 5) and the experts tended to estimate
the slope at the right end, whilst the algorithm com-
puted an average slope over its entire width. This had
the effect that the automatic estimate of its slope an-
gle was on average one degree greater than the expert
opinion: ICC3=0.764, MAE=1.081
◦
, hit=4%.
As the decision at which position the tangential
angle of the aponeurosis is measured is somewhat ar-
bitrary, we conclude that the aponeurosis slope angle
is estimated by our algorithm within the possible ac-
curacy. The difference in slope estimation has no ef-
fect for video “R”, but for video “W” it leads to a
systematic difference of about one degree for the pen-
nation angle, i.e., the automatically estimated penna-
tion angle in video “W” should be about one degree
greater than the manually estimated angle.
5.2 Pennation Angle
For the pennation angle, we have evaluated two dif-
ferent approaches to its estimation. The first approach
models the angle as a single texture feature over the
entire ROI, whilst the second approach models it as
locally and statistically varying and applies a LOESS
fit over subregions of neighboring frames.
5.2.1 Entire Region
It turned out that the results were very different for
the two videos: for all algorithms, all performance in-
dices were considerably better on the less noisy video
manual
algorithm
Figure 5: The aponeurosis curvature in video “W” leads to
a small difference in the aponeurosis slope estimation.
Estimation of Muscle Fascicle Orientation in Ultrasonic Images
83

190 200 210 220 230 240
20 25 30 35
frame
angle
projection
GLCM
Frangi
Radon
(a) walking (video W)
270 275 280 285 290 295
20 25 30 35 40 45
frame
angle
projection
GLCM
Frangi
Radon
(b) running (video R)
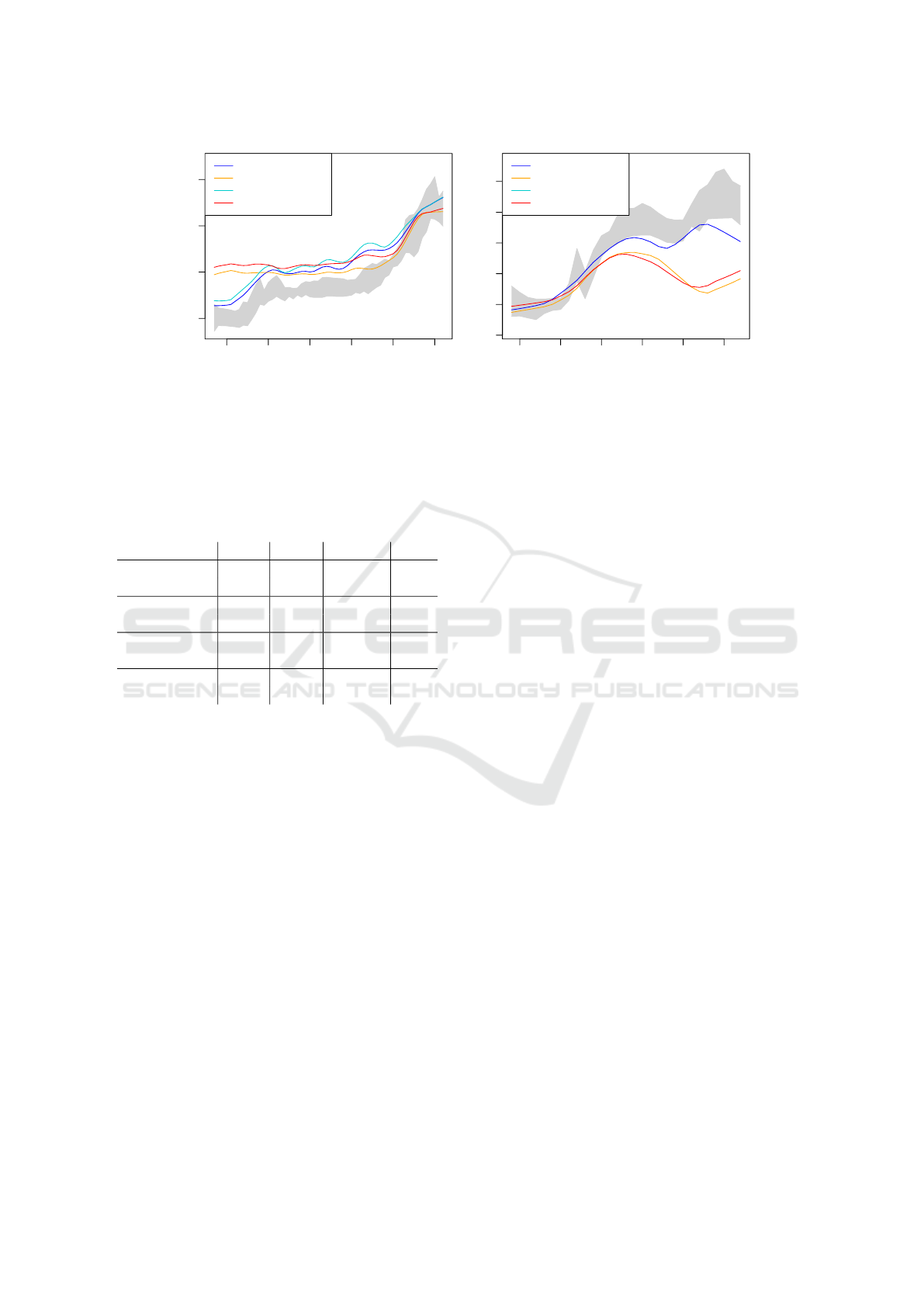
Figure 6: Angle estimations of the different algorithms applied to the three regions with the highest mean gradient and with
LOESS fitting for two typical steps of motion. For video R, the projection method was so far off that its values do not fall into
the displayed angle range. The gray area is the inter-observer range.
Table 2: Angle estimation performance indices of the dif-
ferent algorithms applied to the entire region of interest.
algorithm video ICC3 MAE hit
projection W 0.871 1.231
◦
58%
R -0.003 9.335
◦
35%
GLCM W 0.784 2.221
◦
33%
R 0.180 10.437
◦
10%
Frangi W 0.552 2.998
◦
17%
R 0.524 5.493
◦
33%
Radon W 0.540 2.309
◦
42%
R 0.430 6.506
◦
32%
“W” (see Tbl. 2). The best performing algorithm
was the projection profile method, followed by the
GLCM. As can be seen in Fig. 4(a), the angles es-
timated by the other two algorithm follow the curve
shape with lesser agreement, which corresponds to
poorer ICC3 values in Tbl. 2.
For video “R”, however, neither of the algorithms
yielded satisfying results, as can be concluded from
the poor performance indices in Tbl. 2 and the random
fluctuations of the estimated angles inf Fig. 4(b).
5.2.2 LOESS Fit Over Subregions
To obtain a more robust angle estimator, we calcu-
lated the estimates for eight subregions, selected the
“best” three subregions per frame and made a LOESS
fit over these subregions including the eight neighbor-
ing frames. As our predictor was the frame number,
the distance z in Eq. (5) was measured in frame num-
bers and the number of neighbors was k = 27.
This raises the question, how the “best” subre-
gions are selected for each frame. A human expert
would focus on a region in which the fascicles are
clearly visible, i.e. a region with high contrast or sharp
edges. The three criteria listed in section 2 try to mea-
Table 3: Angle estimations of the different algorithms ap-
plied to the three regions with the highest mean gradient
and with LOESS fitting.
algorithm
video ICC3 MAE hit
projection W 0.975 0.548
◦
86%
R 0.096 12.895
◦
1%
GLCM W 0.926 1.567
◦
27%
R 0.848 4.122
◦
29%
Frangi W 0.733 1.704
◦
49%
R 0.635 6.688
◦
14%
Radon W 0.804 2.007
◦
41%
R 0.868 3.357
◦
36%
sure this property. It turned out that the actual crite-
rion has a smaller effect than the choice of algorithm.
We thus present the results that use the highest mean
gradient as a criterion for the “best” subregions; the
results for the other criteria are similar.
As can be seen from Tbl. 3, the LOESS fit
improves the performance indices in almost all
cases. One notable exception is the projection profile
method for video “R”: in this case the angle estimates
were so far off that they even fell outside the range
of Fig. 6(b), although this algorithm performed best
on video “W”. We thus conclude that the projection
profile method should be used in combination with a
pre-processing filter because it is not robust with re-
spect to high levels of noise.
5.3 Effect of Pre-processing
To see whether using the Radon transform or the ves-
selness filter (“Frangi”) as a pre-processing operation
improves the performance of the other algorithms, we
have first applied these filters and then utilized the
same LOESS approach as in the preceding subsec-
tion. As can be seen from Tbl. 4, this did not improve
VISAPP 2020 - 15th International Conference on Computer Vision Theory and Applications
84
190 200 210 220 230 240
20 25 30 35
frame
angle
projection (Frangi)
projection (Radon)
GLCM (Frangi)
GLCM (Radon)
(a) walking (video W)
270 275 280 285 290 295
20 25 30 35 40 45
frame
angle
projection (Frangi)
projection (Radon)
GLCM (Frangi)
GLCM (Radon)
(b) running (video R)
Figure 7: Effect of the pre-processing filters (in parentheses) on angle estimations applied to the three regions with the
highest mean gradient and with LOESS fitting for two typical steps of motion. For video R, the GLCM method with Frangi
(vesselness filter) pre-processing was so far off that its values do not fall into the displayed angle range. The gray area is the
inter-observer range.
Table 4: Angle estimations after pre-processing applied to
the three regions with the highest mean gradient and with
LOESS fitting.
algorithm video ICC3 MAE hit
projection W 0.946 1.962
◦
25%
(with Frangi) R 0.946 1.871
◦
62%
projection W 0.755 2.231
◦
31%
(with Radon) R 0.665 4.500
◦
29%
GLCM W 0.914 2.408
◦
21%
(with Frangi) R 0.058 39.699
◦
0%
GLCM W 0.718 2.912
◦
20%
(with Radon) R 0.695 4.316
◦
23%
the performance of the GLCM with respect to Tbl. 3,
but for the projection profile method, pre-processing
with a vesselness filter seriously improved the results
for video “R”. Overall, the combination “vesselness
filter and projection profile method” was the best per-
forming algorithm, followed secondly by the GLCM
without pre-processing.
6 CONCLUSIONS
Based upon our experimental evaluation, we recom-
mend two possible algorithms for estimating the pen-
nation angle in ultrasonic images of muscles. The best
performing algorithm was a combination of the ves-
selness filter as a pre-processing operation with the
projection profile method for angle estimation. This
algorithm achieved an intra-class correlation close to
one and had a mean average error less than two de-
grees. The second best algorithm was based on the
gray level cooccurance matrix (GLCM).
Both the robustness and accuracy of the angle esti-
mates are considerably improved by a LOESS fit over
neighboring frames and the subregions with the best
visible edges. In our study, we have selected these
regions automatically on basis of the mean absolute
value of the gradient within the subregion.
In practice, if a semi-automatic processing is pos-
sible, the region selection process could alternatively
done by an expert user. This would also have the ben-
efit that the fascicle length computation can be based
on the selected region. This is of relevance, because
the fascicle length is not well defined if the superficial
and the deep aponeuroses are not parallel. In this case,
a hint by an expert user is necessary in any case where
to set an anchor point of the line used for computing
the fascicle length, which could be chosen, e.g., as the
mid point of the user selected region.
ACKNOWLEDGMENTS
Parts of this study were financially supported by the
German Federal Ministry of Economic Affairs and
Energy under grant no. 50WB1728.
REFERENCES
Aggeloussis, N., Giannakou, E., Albracht, K., and Aram-
patzis, A. (2010). Reproducibility of fascicle length
and pennation angle of gastrocnemius medialis in hu-
man gait in vivo. Gait & posture, 31(1):73–77.
Cleveland, W. S. and Devlin, S. J. (1988). Locally weighted
regression: an approach to regression analysis by local
fitting. Journal of the American statistical association,
83(403):596–610.
Estimation of Muscle Fascicle Orientation in Ultrasonic Images
85

Cronin, N. J., Carty, C. P., Barrett, R. S., and Lichtwark, G.
(2011). Automatic tracking of medial gastrocnemius
fascicle length during human locomotion. Journal of
Applied Physiology, 111(5):1491–1496.
Cunningham, R., Harding, P., and Loram, I. (2017). Deep
residual networks for quantification of muscle fiber
orientation and curvature from ultrasound images. In
Annual Conference on Medical Image Understanding
and Analysis, pages 63–73. Springer.
Cunningham, R., S
´
anchez, M., May, G., and Loram, I.
(2018). Estimating full regional skeletal muscle fi-
bre orientation from B-mode ultrasound images us-
ing convolutional, residual, and deconvolutional neu-
ral networks. Journal of Imaging, 4(2):29.
Dalitz, C., Michalakis, G. K., and Pranzas, C. (2008). Opti-
cal recognition of psaltic byzantine chant notation. In-
ternational Journal of Document Analysis and Recog-
nition (IJDAR), 11(3):143–158.
Farris, D. J. and Lichtwark, G. A. (2016). UltraTrack: Soft-
ware for semi-automated tracking of muscle fascicles
in sequences of B-mode ultrasound images. Computer
Methods and Programs in Biomedicine, 128:111–118.
Frangi, A. F., Niessen, W. J., Vincken, K. L., and Viergever,
M. A. (1998). Multiscale vessel enhancement filter-
ing. In International conference on medical image
computing and computer-assisted intervention, pages
130–137.
Fukunaga, T., Ichinose, Y., Ito, M., Kawakami, Y., and
Fukashiro, S. (1997). Determination of fascicle length
and pennation in a contracting human muscle in vivo.
Journal of Applied Physiology, 82(1):354–358.
Fukunaga, T., Kubo, K., Kawakami, Y., Fukashiro, S.,
Kanehisa, H., and Maganaris, C. N. (2001). In vivo
behaviour of human muscle tendon during walking.
Proceedings of the Royal Society of London. Series B:
Biological Sciences, 268(1464):229–233.
Rana, M., Hamarneh, G., and Wakeling, J. M. (2009). Au-
tomated tracking of muscle fascicle orientation in B-
mode ultrasound images. Journal of biomechanics,
42(13):2068–2073.
Sheather, S. J. (2004). Density estimation. Statistical sci-
ence, 19(4):588–597.
Shrout, P. E. and Fleiss, J. L. (1979). Intraclass correla-
tions: uses in assessing rater reliability. Psychological
bulletin, 86(2):420.
Yuan, C., Chen, Z., Wang, M., Zhang, J., Sun, K., and Zhou,
Y. (2020). Dynamic measurement of pennation angle
of gastrocnemius muscles obtained from ultrasound
images based on gradient radon transform. Biomed-
ical Signal Processing and Control, 55:101604.
Zhao, H. and Zhang, L. (2011). Automatic tracking of
muscle fascicles in ultrasound images using localized
radon transform. IEEE Transactions on Biomedical
Engineering, 58(7):2094–2101.
Zheng, G., Li, X., Zhou, L., Yang, J., Ren, L., Chen, P.,
Zhang, H., and Lou, X. (2018). Development of a
gray-level co-occurrence matrix-based texture orien-
tation estimation method and its application in sea
surface wind direction retrieval from SAR imagery.
IEEE Transactions on Geoscience and Remote Sens-
ing, 56(9):5244–5260.
Zhou, G. and Zheng, Y. (2012). Human motion analysis
with ultrasound and sonomyography. In 2012 Annual
International Conference of the IEEE Engineering in
Medicine and Biology Society, pages 6479–6482.
Zhou, Y. and Zheng, Y.-P. (2008). Estimation of muscle
fiber orientation in ultrasound images using revoting
Hough transform (RVHT). Ultrasound in medicine &
biology, 34(9):1474–1481.
VISAPP 2020 - 15th International Conference on Computer Vision Theory and Applications
86
