
Water-sensitive Gelatin Phantoms for Skin Water Content Imaging
Gennadi Saiko
1a
and Alexandre Douplik
2b
1
Swift Medical Inc, 1 Richmond St. W., Toronto, Canada
2
Department of Physics, Ryerson University, Toronto, Canada
Keywords: Multispectral Imaging, Tissue Phantoms, Skin Water Content, Skin Moisture.
Abstract: Oxygen supply to tissues can be seriously impacted during wound healing. Edema (accumulation of fluids in
interstitial space) can increase the distance between capillaries, thus decreasing oxygen supply to cells. There
is no standard clinical tool for quantification of edema, and early edema detection (preferably preclinical) is
of great clinical need. Multispectral imaging can be a helpful clinical tool to characterize water content in the
skin. However, to develop and validate this technology, a reliable water-sensitive preclinical model has to be
developed. The scope of this work is to develop a water-responsive skin model and assess the feasibility of
extracting water content using multispectral imaging. Methods: A phantom fabrication protocol has been
developed. The phantoms are based on the gelatin crosslinked with glutaraldehyde. TiO2 nanoparticles were
added to mimic the optical properties of the skin. To emulate various water content, phantoms were dipped
in the water for various duration. The phantoms were imaged using the Multi-Spectral Imaging Device
(MSID) (Swift Medical Inc, Toronto). MSID is a multispectral imaging system for visualization of tissue
chromophores in surface tissues. It uses 12-bit scientific-grade NIR-enhanced monochrome camera (Basler,
Germany) and ten wavelength light source (600-1000nm range) to visualize the distribution of oxy-,
deoxyhemoglobins, methemoglobin, water, and melanin. The imaging distance is 30cm, the field of view:
7x7cm. Results: Initial results show that the developed model mimics the optical scattering properties of the
skin. MSID was able to extract water content using a full set (ten wavelengths) and a subset (three
wavelengths) of channels. Conclusions: A new water responsive model for skin moisture imaging has been
developed. Initial experiments with multispectral imaging of these phantoms show feasibility of tissue water
content imaging with Si-based cameras.
1 INTRODUCTION
Edema (accumulation of fluids in interstitial space) is
a common clinical sign in a wide variety of
conditions. Being a nonspecific finding it often poses
a challenge for the clinician. While in many cases, it
has a benign origin, in other instances, it can be a sign
of life-threatening conditions. Because the
interstitium can easily accommodate several liters of
fluid, a patient’s weight may increase by nearly 10%
before pitting edema is evident. Thus, early detection
of edema (preferably preclinical) is of great
importance, especially for patients with compromised
health (diabetes, kidney or heart conditions, etc.).
Edema can seriously impact wound healing by
restricting oxygen supply to tissues. For example,
a
https://orcid.org/ 0000-0002-5697-7609
b
https://orcid.org/ 0000-0001-9948-9472
edema can increase the distance between capillaries,
thus decreasing oxygen supply to cells, or it may
compress small vessels to shut off the local blood
supply at all, thus creating necrotic tissue.
There is no standard for an objective measurement
of edema. In particular, for peripheral edema, the
most widely-used technique is a subjective clinical
assessment where an examiner applies pressure with
an index finger to the patient’s ankle (Seidel, 1995) to
capture pit depth and the time needed for the skin to
return to its original state (recovery time). Despite
common use, this method has not been proven to be a
sufficiently objective, reliable, or sensitive
assessment of edema. Several quantitative methods to
measure peripheral edema have been proposed, but
they are mostly used in physical therapy and sports
medicine: patient questionnaire, ankle circumference
130
Saiko, G. and Douplik, A.
Water-sensitive Gelatin Phantoms for Skin Water Content Imaging.
DOI: 10.5220/0008919501300134
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 2: BIOIMAGING, pages 130-134
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

(Mora, 2002), figure-of-eight (Mawdsley, 2000),
edema tester (Cesarone, 1999), indirect leg volume
(by series of ankle/leg circumferences) (Latchford,
1997), and water-displacement volumetry (Kaulesar
Sukul, 1993).
Several quantitative technologies have been used
to assess edema or the hydration of skin non-
invasively. They include skin impedance methods,
ultrasound, and magnetic resonance imaging, and
spectroscopy.
The major part of existing methods of measuring
of edema suffers from various shortcomings; namely,
subjectivity (Brodovicz, 2009), inability to detect on
early stages (Hedlund, 1985), and impossibility to be
applied in certain clinical or field settings (e.g., water
displacement for postoperative patients, MRI, and
ultrasound in field settings).
Thus, it would be useful to have a more widely
accessible way to investigate edema and ideally
visualize it in various clinical and field settings.
Optical spectroscopy and imaging, along with the
skin impedance measurements (Mayrovitz, 2007), are
promising modalities for the non-invasive diagnosis
and monitoring of skin water content. However,
optical imaging has several inherited advantages over
skin impedance measurements: a) it is able to
visualize (image) the large field of view, while skin
impedance techniques are essentially one point
measurements, prone to operator’s errors, b) it is a
remote measurement, while skin impedance requires
contact with the skin and sterilization after each use.
Thus, optical water content imaging would be a
useful clinical tool adjuvant to diagnostics of
peripheral vascular disease and pressure injuries.
However, the development of such imaging
technology is complicated in part due to the lack of
the gold standard and established water responsive
models. For example, its comparison and/or
validation with skin impedance techniques is not a
straightforward task such as various geometries of
skin impedance probes have different sampling
depths, which can be incomparable with an optical
sampling depth at a particular wavelength.
Thus, one of the important steps in developing the
water content imaging modality is to develop a
controllable water-responsive model, which can be
used to validate/calibrate the technology in the
absence of the gold standard.
While multiple experimental (Pogue, 2006;
Ohmae, 2018) and computational (Kainz, 2018)
models have been developed to mimic the optical and
RF properties of the human tissues, still there is a
need in versatile water-responsive models.
Our group works on multispectral imaging moda-
lities adjuvant to tissue oximetry. In our previous
works, we have developed several phantom models
and demonstrated the feasibility of multispectral
visualization of methemoglobin in the tissue (Saiko,
2018).
This scope of this work is to develop a water-
responsive skin model and assess the feasibility of
multispectral imaging of water content in the skin.
2 METHODS
Water content within the stratum corneum gradually
increases from about 10% to about 30% between the
surface and deeper layers, followed by an abrupt
increase to about 70% in the epidermis (Warner,
1988).
The water content of the different skin layers is of
interest in different fields. While the water content of
the epidermis is the primary interest in the skincare
industry, subepidermal water accumulation is of
interest in wound care.
Water content imaging of the different skin layers
can be based on various light absorption bands. Water
absorption at 1440nm is 30 times stronger than at
1190nm, which in turn more than two times stronger
than absorption at 970nm. Given this hierarchy and
corresponding light penetration depths it is possible
to expect that 1440nm and 1920nm wavelengths are
suitable for imaging of water content in uppermost
skin layers (stratum corneum), while 970nm
and1190nm can be used for water content
determination and imaging in deeper skin layers,
including epidermis, dermis (1190nm) and even
subcutaneous tissues (970nm). Illumination of the
skin with these wavelengths will integrate signals
from various depths (up to a few millimeters) and thus
will not be sensitive to skin conditions in surface
layers, which due to their small thickness and low
water content account only for a small fraction of
water in integration volume.
For our purposes, we selected 970nm range,
which a) have the required sampling depth (dermis
and subcutaneous tissues), and b) can be implemented
using inexpensive Si-based sensors.
To explore the feasibility of quantification of
water content in the skin, we have developed a water-
responsive skin model. The model is based on a
mechanical gelatin-based human skin model
(Dabrowska, 2017) with adaptations to mimic the
optical parameters of the skin.
Water-sensitive Gelatin Phantoms for Skin Water Content Imaging
131
2.1 Materials
The phantoms were based on the type A gelatine
derived from porcine skin, with 300 g gel strength
(G2500, Sigma-Aldrich, Canada), which does not
have significant optical absorption and scattering in
the visible range. Each phantom contained 10%
(w/w) of type A gelatin.
TiO2 particles (Sigma-Aldrich, Canada) were
used to mimic the scattering property of the skin.
Glutaraldehyde (Sigma Aldrich, Canada) was
used for the cross-linking of gelatin.
2.2 Phantom Fabrication
We have developed the following protocol for
phantom fabrication:
1. Prepare a 10 wt% solution of gelatine (type A,
bloom no 300, Sigma Aldrich) in distilled water
by continuous stirring at 60 °C for 2 h.
2. Mix with a known concentration of a scattering
agent (TiO2 nanoparticles).
3. Place the final solution in an ultrasonic bath at a
temperature of 37 – 38 degrees to degas for 2
minutes to disrupt the air bubbles trapped inside
the phantom,
4. Pour the solution into the Petri dish (with wax
paper layer) to solidify: 2mm layer.
5. Leave the phantom to dry for 24 h at room
temperature.
6. Gelatin crosslinking: Place the phantom in 1 wt%
solution of glutaraldehyde (Sigma Aldrich) in
Dulbecco's PBS buffer (DPBS, GIBCO) for 24 h
at room temperature under continuous stirring
(130 rpm).
7. Rinse phantoms with distilled water.
8. Dry phantoms by wrapping in paper towels and
placing between two boards with the use of
weight, to avoid ripples caused by drying-related
contraction.
9. Change paper towels daily and measure the
weight of each phantom. It is considered to be dry
after mass stabilization (about 6 days).
To emulate various hydration levels in the skin, the
samples were dipped in the water for various time.
Electronic scales measured weight before and after
dipping.
2.3 Imaging
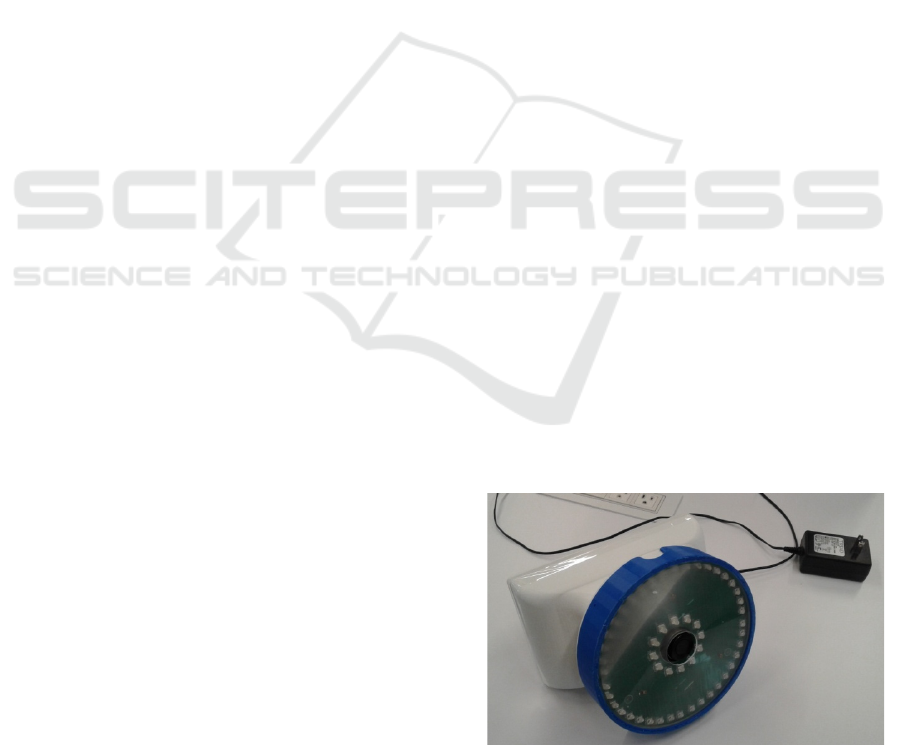
The Multi-Spectral Imaging Device (MSID) (Swift
Medical Inc, Toronto) is a multispectral imaging
system (see Figure 1) for visualization of the
distribution of oxy-, deoxyhemoglobins,
methemoglobin, water, and melanin in the skin. The
MSID consists of a 10-channel illumination unit, a
scientific-grade camera (a 12-bit NIR-enhanced
monochrome camera acA1300-60gmNIR (Basler,
Germany)), and a processing unit, which coordinates
them and collect data. Ten channels of the
illumination unit illuminate the target area with 630,
660, 690, 735, 810, 830, 850, 880, 940, and 970nm,
respectively. Each channel consists of 4 high power
LEDs arranged into a circle. The illumination unit
produces a sequence of light flashes, each flash at a
particular wavelength, while the camera captures a
series of images, each with illumination at particular
wavelength. The acquired images are arranged into a
3D hypercube (, i, j) for further processing. The
imaging distance is 30cm, the field of view: 7x7cm.
2.4 Image Processing
During each measurement, the MSID device captures
11 images: ten with illumination at a particular
wavelength and one without additional illumination
(ambient light only). The processing consists of the
following consequential steps: a) calculate diff
images (subtract the image without additional
illumination from the image with illumination at a
particular wavelength), b) obtain reflectance images
by dividing the diff image on the diff image of the
reference object, c) extract index of absorption
a
from reflectance using tissue light transport model
(e.g., Beer-Lambert), d) extract tissue chromophore
concentrations using least square fitting.
To emulate a compact device scenario, various
subsets of captured images (10 or 3 of them) were
used to extract water content.
The MSID device uses the 12bit scientific-grade
camera (acA1300-60gmNIR (Basler, Germany)).
False-color maps were used to visualize water
content.
Figure 1: Multi-Spectral Imaging Device (MSID).
BIOIMAGING 2020 - 7th International Conference on Bioimaging
132
3 RESULTS
Rough calculations based on the model developed in
(Saiko, 2019) show that we can expect approximately
-0.4% change in reflectance for every 1% increase in
the absorption coefficient at 970nm. Given that the
water is the primary absorber in this range we can
expect that it will be possible to detect even
preclinical peripheral edema with an expected
increase in water content. In particular, we expect that
a 50% increase in interstitial fluid volume for water
content in the skin, adipose tissue, connective tissue
will translate into a 5-6% increase in total tissue water
content (see Table 1). This increase will translate into
-2.0...-2.4% change in tissue reflectance, which is
possible to detect even using an 8-bit camera.
The developed phantoms were visualized using
MSID. To emulate various water content in the tissue,
phantoms were dipped in the water for the various
duration.
Table 1: Initial and expected water content for different
tissue types.
Tissue
Initial water
content, %
(Braunwald,
1994)
Expected water
content for 50%
increase in IFV, %
Skin 72 79
Skeletal muscle 76 77.4
Adipose tissue 14 18.5
Connective tissue 80 86
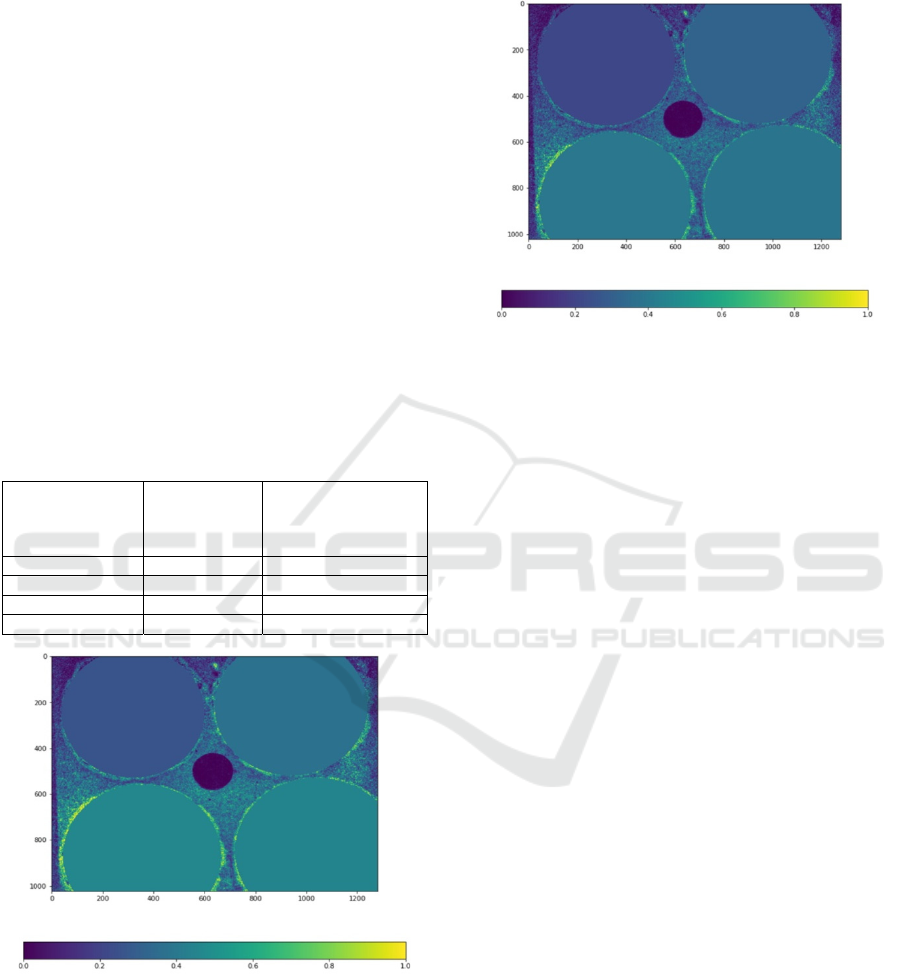
Figure 2: Water content extraction using ten wavelengths.
Dry sample (upper left), sample dipped for several seconds
(top right), 5 min (bottom left), and 20 min (bottom right).
The results of water content extraction using all
ten channels and three channels are presented in
Figure 2 and Figure 3, respectively. Four samples on
these figures have various water content: dry sample
(upper left), and samples dipped for several seconds
(top right), 5 min (bottom left) and 20 min (bottom
right).
Figure 3: Water content extraction using three wavelengths.
Dry sample (upper left), sample dipped for several seconds
(top right), 5 min (bottom left), and 20 min (bottom right).
4 DISCUSSION
Our initial calculations show that the
multispectral/hyperspectral imaging using a 970nm
band and Si-based sensor can retrieve clinically
relevant changes in the tissue water content.
These calculations were supported by our initial
experiments on phantoms, which demonstrate the
feasibility of water content imaging using Si-based
cameras. The water content was successfully
extracted using the full set of channels (10 channels)
and its subset (3 channels, including 970nm). Results
are almost identical, so we can conclude that three
illumination wavelengths may be sufficient for water
content visualization. These results are in line with
preliminary experiments on volunteers reported
separately (Saiko, 2020).
Initial experiments also show that the developed
gelatine-based phantoms a) demonstrate optical
properties similar to the skin, and b) demonstrate
required water-responsive properties. We will report
phantom validation results separately. The current
study with dipping phantoms in the water was a
proof-of-concept. In the future, we plan to vary
phantom water content in a more quantifiable way by
spreading a certain amount of water over its surface
and comparing water content results with those
obtained by bioimpedance measurements.
It should be noted that the cross-linked phantoms
changed their color from clear to pink during
crosslinking. In future work, we plan to investigate
Water-sensitive Gelatin Phantoms for Skin Water Content Imaging
133

these color changes and find conditions that minimize
them or investigate other cross-linking agents.
5 CONCLUSIONS
A new water responsive model for skin water content
imaging has been developed. Initial experiments with
multispectral imaging of these phantoms show the
feasibility of tissue water content imaging with Si-
based cameras using a 970nm band.
ACKNOWLEDGEMENTS
Authors are thankful to Burhan Hussein and Andrei
Betlen for help with phantom fabrication and image
processing.
REFERENCES
Seidel H.M, Ball J.W, Dains J.E, et al., 1995. Heart and
blood vessels. In: Schrefer S, ed. Mosby’s Guide to
Physical Examination, St. Louis, MO: Mosby. 3rd ed.
Mora S, Zalavras C.G, Wang L, Thordarson DB, 2002. The
role of pulsatile cold compression in edema resolution
following ankle fractures: a randomized clinical trial.
Foot Ankle Int, 23:999-1002.
Mawdsley R.H, Hoy D.K, Erwin P.M, 2000. Criterion-
related validity of the figure-of-eight method of
measuring ankle edema. J Orthop Sports Phys Ther,
30:149-153.
Cesarone M.R, Belcaro G, Nicolaides A.N, Arkans E,
Laurora G, De Sanctis MT, Incandela L, 1999. The
edema tester in the evaluation of swollen limbs in
venous and lymphatic disease. Panminerva Med;41:10-
14.
Latchford S, Casley-Smith J.R, 1997. Estimating limb
volumes and alterations in peripheral edema from
circumferences measured at different intervals.
Lymphology; 30:161-164.
Kaulesar Sukul D.M, den Hoed P.T, Johannes E.J, van
Dolder R, Benda E., 1993. Direct and indirect methods
for the quantification of leg volume: comparison
between water displacement volumetry, the disk model
method and the frustum sign model method, using the
correlation coefficient and the limits of agreement. J
Biomed Eng; 15:477-480.
Brodovicz K.G, McNaughton K, Uemura N, Meininger G,
Girman CJ, Yale SH., 2009. Reliability and Feasibility
of Methods to Quantitatively Assess Peripheral Edema.
Clin Med & Res ; 7(1-2):21-31.
Hedlund LW, Putman C.E, 1985. Methods for detecting
pulmonary edema, Toxicol Ind Health. 1(2):59-68.
Mayrovitz H.N, 2007. Assessing local tissue edema in
postmastectomy lymphedema, Lymphology 40: 87-94.
Pogue B.W. and Patterson M. S, 2006. Review of tissue
simulating phantom for optical spectroscopy, imaging
and dosimetry, JBO. 11(4), 041102.
Ohmae E., Yoshizawa N., Yoshimoto K., Hayashi M.,
Wada H., Mimura T., Suzuki H., Homma S., Suzuki N.,
Ogura H., Nasu H., Sakahara H., Yamashita Y., and
Ueda Y., 2018. Stable tissue-simulating phantoms with
various water and lipid contents for diffuse optical
spectroscopy, BOE 9, 5792-5808.
Kainz, W., Neufeld E., Bolch W., Graff C., Kim C. H.,
Kuster N., Lloyd B., Morrison T., Segars P., Yeom Y.,
Zankl M., Xu G., Tsui B.,. 2018, Advances in
Computational Human Phantoms and Their
Applications in Biomedical Engineering -A Topical
Review. IEEE Trans Rad and Plasma Med Scie,. 3(1):
1-23.
Saiko G, Zheng X, Betlen A, Douplik A, 2018.
Visualization of Methemoglobin Distribution in
Tissues: Phantom Validation, Adv Exp Med Biol 1072,
387-390
Braunwald E., 1994. Edema. In. Harrison’s Principles of
Internal Medicine, Isselbacher KJ, Braunwald E,
Wilson JD, et al., eds.
New York: McGraw-Hill; 13th
ed.
Bhave G, Neilson E.G , 2011, Body Fluid Dynamics: Back
to the Future, JASN, 22(12): 2166-2181.
Saiko G, Betlen A, 2019, Optimization of Band Selection
in Multispectral and Narrow-Band Imaging: an
Analytical Approach, Adv Exp Med Biol. (in press)
Dąbrowska A., Rotaru G.M., Spano F., Affolter Ch.,
Fortunato G., Lehmann S., Derler S., Spencer N.D.,
Rossi R.M., 2017. A water-responsive, gelatine-based
human skin model, Tribology Int, 113, 316-322.
Warner R., Myers M. C., and Taylor D. A., 1988. Electron
probe analysis of human skin: Determination of the
water concentration profile, J. Invest. Dermatol. 90,
218-224.
Saiko G., 2020. On the feasibility of skin water content
imaging adjuvant to tissue oximetry, Adv Exp Med Biol
(accepted).
BIOIMAGING 2020 - 7th International Conference on Bioimaging
134
