
Subject-independent Pain Recognition using Physiological Signals and
Para-linguistic Vocalizations
Nadeen Shoukry, Omar Elkilany, Patrick Thiam, Viktor Kessler and Friedhelm Schwenker
a
Ulm University, Institute of Neural Information Processing, 89081 Ulm, Germany
Keywords:
Machine Learning, Pain Recognition, Affective Computing, Biopotential Signals, Para-linguistic Data.
Abstract:
Pain is the result of a complex interaction among the various parts of the human nervous system. It plays
an important role in the diagnosis and treatment of patients. The standard method for pain recognition is
self-report; however, not all patients can communicate pain effectively. In this work, the task of automated
pain recognition is addressed using para-linguistic and physiological data. Hand-crafted and automatically
generated features are extracted and evaluated independently. Several state-of-the-art machine learning algo-
rithms are applied to perform subject-independent binary classification. The SenseEmotion dataset is used for
evaluation and comparison. Random forests trained on hand-crafted features from the physiological modal-
ities achieved an accuracy of 82.61%, while support vector machines trained on hand-crafted features from
the para-linguistic data achieved an accuracy of 63.86%. Hand-crafted features outperformed automatically
generated features.
1 INTRODUCTION
Pain is the result of a complex interaction among the
various parts of the nervous system and plays an im-
portant role in the diagnosis and treatment of patients.
It varies in duration, intensity, and meaning. For ex-
ample, the sudden pain associated with syndromes,
such as causalgia, can be contrasted with the progres-
sive pain related to some types of cancer (Turk et al.,
1983). Furthermore, there are physical and psycho-
logical causes of pain. While physical pain has con-
crete causes, psychogenic pain occurs without exter-
nal stimuli (Engel, 1959). These factors complicate
the pain assessment task.
The standard method for pain assessment is self-
reporting, which assumes that the subjects experi-
encing the pain are capable of comprehending the
task and communicating their feelings effectively
(Zwakhalen et al., 2006). This assumption, however,
does not hold with elderly patients suffering from de-
mentia or cognitive disabilities. For these patients,
pain may become a barrier to social inclusion (Velana
et al., 2016). Moreover, if it is not managed correctly,
it may induce pathological effects, such as higher
blood pressure and rapid heart rates, that may lead to
further complications (Turk and Gatchel, 2018). With
a
https://orcid.org/0000-0001-5118-0812
the growth in the proportion of the population above
the age of 65 in modern industrial societies, more reli-
able pain recognition and assessment methods are re-
quired, possibly not to replace but to aid self-reports
(Velana et al., 2016).
In this paper, two different approaches for au-
tomatic pain recognition are explored. The first
approach relies on physiological signals, collected
through sensors attached to the bodies of patients,
while the second approach depends on para-linguistic
vocalizations, such as moans and similar sounds,
which do not belong to a standardized language.
State-of-the-art machine learning algorithms are ap-
plied to perform binary, subject-independent classi-
fication, and their accuracies are compared briefly.
Leave-one-subject-out cross-validation is used as the
evaluation approach. Assessment is performed on the
recently recorded SenseEmotion dataset (Velana et al.,
2016).
The rest of this work is organized as follows: In
section 2, an overview of related work is presented.
In section 3, the dataset itself is described with more
focus on the physiological and para-linguistic modali-
ties. The data preprocessing and preparation steps are
described in section 4. The conducted experiments
and their results are provided in section 5. Finally,
this work is concluded in section 6.
142
Shoukry, N., Elkilany, O., Thiam, P., Kessler, V. and Schwenker, F.
Subject-independent Pain Recognition using Physiological Signals and Para-linguistic Vocalizations.
DOI: 10.5220/0008912201420150
In Proceedings of the 9th International Conference on Pattern Recognition Applications and Methods (ICPRAM 2020), pages 142-150
ISBN: 978-989-758-397-1; ISSN: 2184-4313
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 RELATED WORK
This section provides an overview of the research re-
lated to the pain recognition task with more focus on
the two relevant modalities: physiological signals and
para-linguistic vocalizations.
Accurate pain recognition requires complex anal-
ysis and a consideration of the environment where the
pain occurs (Hammal and Cohn, 2014). With this
fact in mind, several databases have been developed
to support research on pain recognition. One of the
first of these databases is the UNBC-McMaster Shoul-
der Pain Expression Archive Database, which com-
prises data from patients suffering from shoulder pain
(Lucey et al., 2011). To collect the data, patients were
asked to perform exercises using their limbs, and their
facial expressions were captured and annotated. The
database was limited to this modality.
The EmoPain Dataset is another pain recognition
database, which focused on chronic pain and included
data from both patients and healthy individuals (Aung
et al., 2016). To collect the data, patients performed
exercises in a rehabilitation setting. This database
contained multi-modal data, including multiple-view
face videos, audio signals, 3D motion capture data,
and electromyographic signals.
The BioVid Heat Pain Database provided vi-
sual and biopotential data to advance the recogni-
tion of acute pain (Walter et al., 2013). Similarly,
the SenseEmotion dataset provided a multi-modal
database on acute pain, which was artificially in-
duced using heat stimulation in healthy patients (Ve-
lana et al., 2016).
2.1 Para-linguistic Data
Most of the previous machine learning projects done
in automatic pain recognition relied on facial expres-
sions, bio-potential signals, and in some cases the fu-
sion of both data modalities (Thiam and Schwenker,
2017; Thiam et al., 2017; Sellner et al., 2018;
Schwenker, 2018). In a single study, para-linguistic
audio signals were used along with other data modal-
ities to train pain recognition models (Thiam et al.,
2019). However, para-linguistic audio signals were
not used on their own to recognize pain before.
Environmental and background sound classifi-
cation projects used hand-crafted features of au-
dio signals, such as Mel Frequency Cepstral Coeffi-
cients (MFCC). Support vector machines and random
forests were trained using the audio features to per-
form the classification task (Saki and Kehtarnavaz,
2014; Wang et al., 2008; Lu et al., 2003).
There were previous attempts to use infant para-
linguistic vocalizations (cries) to recognize pain, non-
pain, fear, and hunger. Instead of manually extract-
ing audio features, deep neural networks were trained
on the audio signals to extract features automati-
cally and perform the classification (Chang and Li,
2016). Other studies used hand-crafted features to
train deep neural networks (Abdulaziz and Ahmad,
2010; Petroni et al., 1995). Another study used raw
audio data to train a convolutional neural network
(CNN) and manually extracted audio features to train
a fuzzy support vector machine (FSVM); in this last
study, the FSVM outperforms the CNN and requires
significantly less amount of data to achieve better re-
sults (Barajas-Montiel and Reyes-Garc
´
ıa, 2006).
2.2 Physiological Signals
Several medical studies have established correlations
between physiological responses produced by the au-
tonomic nervous system and the experience of pain
(Ledowski et al., 2009; Colloca et al., 2006; Log-
gia et al., 2011). Many of these responses have been
measured and used as potential indicators of affec-
tive state. They include heart rate, diastolic and sys-
tolic blood pressure, pupil dilation, pulse, respiration,
skin conductance and color, and temperature (Picard,
2000).
Several studies have used electrocardiography,
electromyography, and electrodermal activity to ad-
dress the pain recognition task (Gruss et al., 2015;
Thiam et al., 2019; K
¨
achele et al., 2016). Sig-
nal processing and extensive feature extraction were
used to produce low-dimensional representations of
the information contained in these signals, and the
extracted features were fed to support vector ma-
chines or random forests for classification. These
three signals were also used to develop real-time pain
recognition systems, which are capable of outputting
personalized, continuous estimations of pain inten-
sity (K
¨
achele et al., 2016). Furthermore, unaffected
by differences among the individuals experiencing
pain, electrodermal activity has been found to be the
best performing single modality for pain recognition
(Thiam et al., 2019).
3 THE DATASET
As described by Velana et al., the SenseEmotion
dataset comprises multi-modal data from 45 healthy
participants. Pain was elicited in these participants
using thermal stimuli ranging from 32 to 50.5 degrees
Celsius. A baseline temperature T
0
of 32 degrees Cel-
sius was used commonly in all of the experiments. At
Subject-independent Pain Recognition using Physiological Signals and Para-linguistic Vocalizations
143

the start of an experimental session, calibration was
conducted to determine the pain threshold T
1
and the
tolerance threshold T
3
of each individual participant,
where the pain threshold marks the point where the
participant perceives pain and the tolerance threshold
marks the point where the participant can no longer
bear the pain. The mean of T
1
and T
3
was used to
define another level labelled T
2
. The resulting dataset
consists of 120 events per participant, 30 for each heat
level (Velana et al., 2016).
In this paper, the no-pain state corresponds to the
baseline temperature T
0
, while the pain state corre-
sponds to the pain tolerance threshold T
3
.
3.1 The Para-linguistic Modality
The audio signals used consist of para-linguistic data,
which are non-verbal signals of speech, including
breathing, moaning, and sighing sounds, beyond the
pure transcriptional contents of spoken speech (Cai
et al., 2017).
The recording of the audio signals relied primar-
ily on a digital wireless headset microphone (Line6
XD-V75HS) combined with a directional microphone
(Rode M3). The wireless headset was crucial to al-
low free head movements and record any sounds pro-
duced by the participants. The directional micro-
phone was used to record the ambient acoustic noises
surrounding the participants. Another audio signal
was recorded from the Microsoft Kinect V2 inte-
grated microphone, which was able to capture ambi-
ent noises as well. All recordings were sampled at a
rate of 48kHz and synchronized with both the video
and bio-physiological streams using the SSI frame-
work (Wagner et al., 2013).
3.2 The Physiological Modalities
The physiological modalities were recorded using
the Social Signal Interpretation (SSI) framework syn-
chronously in real-time (Wagner et al., 2013). The
following subsections provide a brief description of
each signal.
3.2.1 Electrocardiography
Electrocardiography is used to trace the electric cur-
rent that the heart muscle generates during a heart-
beat. It provides information about the condition and
performance of the heart. This modality can be used
to extract features such as heart rate, inter-beat inter-
val, and heart rate variability. Heart rate has been
used to differentiate between positive and negative
emotions, with finer differentiation obtainable using
a measure of finger temperature (Kim and Andr
´
e,
2008).
3.2.2 Electromyography
Electromyography is the graphing of the electrical
characteristics of muscles. Three electrodes were
used to measure the activity of the upper-right trapez-
ius muscle and collect data for the SenseEmotion
dataset. Electrical muscle activity is a general in-
dicator of arousal. Increased muscle activity corre-
sponds to increased sympathetic nervous system ac-
tivity. Furthermore, high muscle tension is expected
to occur in the case of pain (Velana et al., 2016).
3.2.3 Electrodermal Activity
The psychogalvanic reflex, or galvanic skin response,
is an alteration in the electrical properties of the skin
that follows harmful or alerting stimuli. Because
sweat glands, which affect skin conductivity, are con-
trolled by the sympathetic branch of the autonomic
nervous system, the galvanic skin response is more
sensitive as an indicator of emotional arousal than
other physiological responses (
¨
Ohman et al., 1993).
3.2.4 Respiration
The respiration signal was captured using an elastic
belt, which was worn by the participants over their
clothing in the thorax area. It is suggested that respi-
ration patterns reflect relaxation and tension (Boiten
et al., 1994). Acute, cutaneous pain stimuli have been
shown to cause increases in inspiratory flow, which
affects inspiration intensity, even under general anaes-
thesia (Jafari et al., 2017). Therefore, the respiration
signal may provide valuable and consistent data for
the pain recognition task.
4 DATA PREPARATION
From the data of the 45 participants of the SenseEmo-
tion dataset, the data of 40 participants could be used.
The other five participants were excluded because
they had erroneous raw data, which were missing one
or more sensor information in certain time segments.
In this section, an overview of the data preparation
steps is presented.
4.1 Para-linguistic Data
The audio signals that were preprocessed and used
came from the wireless headset microphone alone be-
cause it was the only device able to capture sounds
ICPRAM 2020 - 9th International Conference on Pattern Recognition Applications and Methods
144

produced by the participants at a satisfactory level;
it was placed at the nasolabial area, nearer than the
integrated Kinect V2 microphone and the directional
microphone, which were placed at a distance of about
one meter from the participants and captured ambient
noises only (Thiam et al., 2019).
There are several representations of the audio sig-
nals: the raw audio recordings, the audio spectro-
grams, and the hand-crafted features. The audio spec-
trogram is a visual representation of the spectrum of
frequencies of the audio signals as they vary with time
(Mallik et al., 2019). The spectrogram is able to rep-
resent many useful acoustic features, including fre-
quency, pitch, and dB (Chang and Li, 2016).
Each participant experienced 60 stimuli on each
forearm throughout the duration of the experiment.
During the application of each pain/non-pain stimu-
lus, 10 frames of spectrograms were recorded. Sam-
ples of the data spectrograms are presented in figures
1 and 2.
Figure 1: Sample of the data spectrograms (using the left
forearm recorded data of participant 20160404 14 w during
the application of stimulus 1 (pain stimulus)).
Figure 2: Sample of the data spectrograms (using the left
forearm recorded data of participant 20160404 14 w during
the application of stimulus 3 (non-pain stimulus)).
Obtaining hand-crafted features started with the
processing of the data. Several low-level descriptors
were extracted from the raw audio signal. Low-Level
Descriptors (LLDs) are parameters computed from
short time frames of a whole signal to significantly
reduce the processed amount of data. LLDs were ob-
tained using the openSMILE feature extraction toolkit
(Thiam et al., 2019). The most commonly used LLDs
in speech processing are the Mel Frequency Cepstral
Coefficients (MFCCs).
MFCCs have been shown to work well for struc-
tured sounds, such as speech and music, but their per-
formance tends to drop in the presence of noise; there-
fore, other LLDs are analyzed along with the MFCCs.
Obtaining MFCCs is basically the process of convert-
ing audio in the time domain to the frequency domain
to be easily interpreted, analyzed, and processed. One
can think of it as mimicking the human ear cochlea,
which has few filters at high frequencies and more fil-
ters at low frequencies to allow for the detection of
the most quiet sounds possible (Dave, 2013).
MFCCs are known to provide clear insights about
sound data and outstanding results in speech recog-
nition, emotion recognition, and speaker identifica-
tion tasks (Dave, 2013). For the present work, 13
MFCCs were extracted, each combined with its first
and second order temporal derivatives, resulting in a
total of 39 MFCC-based LLDs. In addition to the
MFCCs, another set of LLDs was computed using the
Relative Spectral Perceptual Linear Predictive Cod-
ing (RASTA-PLP), which is an extension to the per-
ceptual linear predictive analysis that improves the
robustness of the computed coefficients against dis-
tortion. For the present work, 6 RASTA-PLP coef-
ficients were extracted, each in combination with its
first and second order temporal derivatives, resulting
in a total of 18 RASTAPLP-based LLDs. Finally, a
third set of LLDs was computed which involved the
root mean square signal energy and logarithmic signal
energy, in combination with their first and second or-
der temporal derivatives. Additionally, the following
descriptors were extracted: the loudness contour, the
zero-crossing rate, the mean-crossing rate, the maxi-
mum absolute sample value, the minimum and max-
imum sample values, and the arithmetic mean of the
sample values. This last set represents a total of 13
LLDs (Thiam et al., 2019).
Then the resulting signals were further processed
to substantially reduce the noise using band-pass fil-
tering, signal smoothing, and detrending. After these
operations, a set of high-level descriptors (HLDs)
was extracted from the previously processed sig-
nals. HLDs are extracted from segmenting the LLDs
based on a fixed window. In the current work, the
following set of 14 statistical functions is applied
on the segmented LLD signals for the extraction
of HLDs: mean, median, standard deviation, maxi-
mum, minimum, range, skewness, kurtosis, first and
second quartiles, inter-quartile, 1%-percentile, 99%-
percentile, and range from 1%- to 99%-percentile
(Thiam et al., 2019).
The MFCC-based feature vectors have a total di-
mensionality of 14 × 39 = 546. The RASTA-PLP-
based feature vectors have a total dimensionality of
14 × 18 = 252, and the last set of feature vectors
from the temporal domain has a total dimensionality
of 14×13 = 182. In the following step of preprocess-
ing, the HLDs were standardised individually and per
Subject-independent Pain Recognition using Physiological Signals and Para-linguistic Vocalizations
145
participant using the z-score (Thiam et al., 2019).
4.2 Physiological Data
Both hand-crafted and automatic features were used
to perform pain recognition using the physiological
modalities.
The hand-crafted features were engineered using
the same methodology presented in (Thiam et al.,
2019). The work presented in this paper has made
use of 335 hand-crafted features after they were pro-
vided by the authors. Out of these 335 features, 10
features were removed because they had zero variance
among the dataset samples. Furthermore, 14 features
were dropped because more than 25% of their val-
ues were undefined, under the assumption that they
would not be beneficial to the learning task. The
median value was calculated independently for each
feature and used to replace what remained of miss-
ing or undefined values. Finally, before they were fed
to support vector machines and neural networks, the
remaining 311 features were standardized. The stan-
dardization step was skipped for random forests.
Standardization for feature x was done by sub-
tracting the mean µ and dividing by the standard de-
viation σ to produce the standardized feature z as fol-
lows:
z =
x −µ
σ
(1)
For the automatic extraction of features, Butter-
worth filters were used to process the raw signals of
the four modalities before the pain events were iso-
lated. A fifth-order Butterworth bandpass filter was
applied to the electrocardiography signals with a fre-
quency range of [0.4, 35] Hz. A third-order Butter-
worth bandpass filter was applied to the respiration
signals with a frequency range of [0.2,0.8] Hz. A
third-order Butterworth bandpass filter was applied to
the electromyographic signals with a frequency range
of [0.05,25] Hz, and a third-order Butterworth low-
pass filter was applied to the electrodermal activity
signals with a cut-off frequency of 0.2 Hz.
To mitigate inter-subject variance, all the signals
were normalized to fit in the range [−1, 1]. For the
training set signals, the normalization was done as fol-
lows:
z
i
= 2 ·
x
i
−x
min
x
max
−x
min
−1 (2)
where z is the output signal, x is the input signal, x
min
is the minimum value of the input signal, x
max
is the
maximum value of the input signal, and the subscript
i is used to index signal values.
The average maximum and minimum over all the
training samples were calculated for every modality
as follows:
max
avg
=
1
m
m
∑
j=1
x
max, j
(3)
min
avg
=
1
m
m
∑
j=1
x
min, j
(4)
where m is the number of samples in the training set,
x
max, j
is the maximum of the j
th
sample, and x
min, j
is
the minimum of the j
th
sample.
Finally, the test data was normalized as follows:
z
i
= 2 ·
x
i
−min
avg
max
avg
−min
avg
−1 (5)
where z is the output signal, x is the input signal, the
subscript i is used to index signal values, and max
avg
and min
avg
are obtained from equations 3 and 4 re-
spectively.
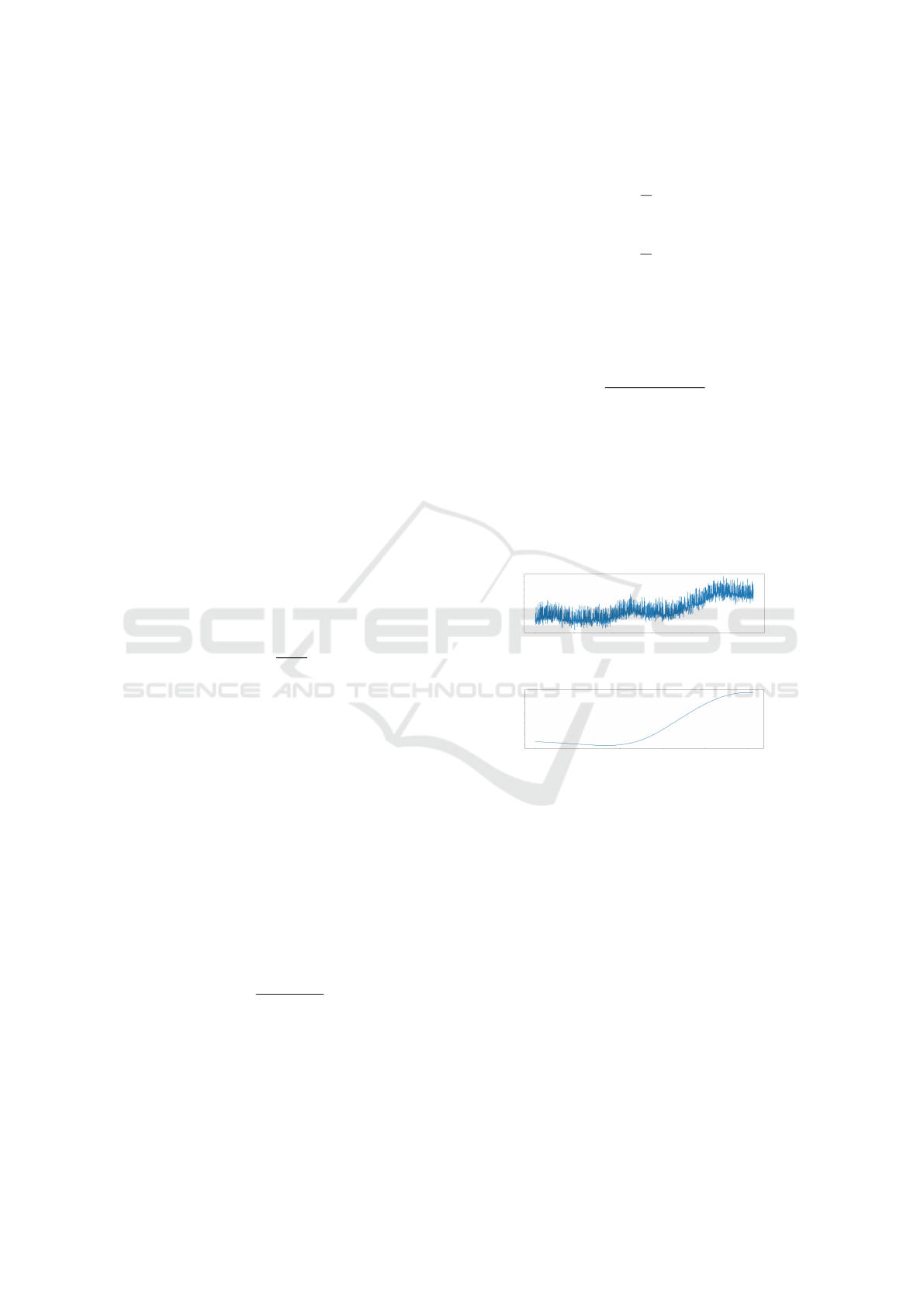
Figure 3 shows a sample of the electrodermal ac-
tivity signal before any preprocessing, while figure 4
shows the same sample after the previously described
preprocessing and normalization methods are applied.
Figure 3: A sample from the electrodermal activity signal
of one of the dataset samples before any preprocessing.
Figure 4: The same sample from figure 3 after it went
through the preprocessing pipeline.
5 EXPERIMENTS AND RESULTS
In this section, the different experiments that were
conducted are presented along with their results.
All the experiments were conducted using the com-
bined data from the left and right forearms from the
SenseEmotion dataset.
Several algorithms were applied to the problem.
Support vector machines work by maximizing the
margin between the training instances and the clas-
sification boundary (Boser et al., 1992). A random
forest is an ensemble classification model, where de-
cision tree classifiers are trained on different subsets
of the training data. The predictions of the individ-
ual decision tree classifiers are then combined to-
gether through a voting scheme to produce the final
ICPRAM 2020 - 9th International Conference on Pattern Recognition Applications and Methods
146

output of the random forest (Breiman, 2001). Neu-
ral networks are nonlinear models that are trained us-
ing back-propagation and that output predictions us-
ing the feed-forward operation (Schwenker, 2018).
5.1 Para-linguistic Data
The para-linguistic data were used to train a support
vector machine, a random forest, a naive Bayes clas-
sifier, a voting classifier, and a deep neural network.
Randomized search cross-validation was used to tune
the hyperparameters of the different models. The
data was evaluated using 10-fold cross-validation and
leave-one-subject-out cross-validation.
5.1.1 Support Vector Machines
The three hand-crafted features from the audio sig-
nals were combined and fed to a support vector ma-
chine. Randomized cross-validation search was used
to tune three hyperparameters. The resulting hy-
perparameters from this search were kernel = RBF,
C = 2 and γ = 1/3. These hyperparameters achieved a
mean accuracy of 64.94% over the 10 cross-validation
folds. The same model was evaluated using leave-
one-subject-out cross-validation and achieved a mean
accuracy of 63.86% over the 40 subjects.
5.1.2 Random Forests
The three hand-crafted features from the audio sig-
nals were combined and fed to a random forest. Ran-
domized cross-validation search was used to tune
the hyperparameters. The resulting hyperparameters
from this search were number of estimators (trees) =
200, maximum features = 1/
√
3, maximum depth =
17, minimum samples to split = 40, and minimum
samples per leaf = 2. This combination achieved a
mean accuracy of 63.52% over the 10 cross-validation
folds. This model achieved a mean accuracy of
61.20% with the same combination of hyperparam-
eters in the leave-one-subject-out cross-validation.
5.1.3 Naive Bayes Classifiers
The three hand-crafted features from the audio signals
were combined and fed to a naive Bayes classifier.
It achieved a mean accuracy of 61.97% over the 10
cross-validation folds and a mean accuracy of 61.68%
over the 40 subjects.
5.1.4 Voting Classifier
A voting classifier is a classifier that formulates the
classification decision based on the aggregation of the
votes from several classifiers. The voting classifier
consisted of a support vector machine, a logistic re-
gression classifier, a Gaussian naive Bayes classifier,
and a random forest. The voting classifier achieved a
mean accuracy of 63.87% over the 10 cross-validation
folds and a mean accuracy of 63.28% in the leave-
one-subject-out cross-validation.
5.1.5 Neural Network
A pre-trained model was used to do automatic fea-
ture extraction from the audio spectrograms. The pre-
trained model used was VGG16, a 16-layer model
originally trained on the ImageNet database (Si-
monyan and Zisserman, 2014). The classification
was done using a bidirectional long short-term mem-
ory neural network (LSTM). A bidirectional LSTM
uses previous and upcoming information from the
sequence while learning (Graves and Schmidhuber,
2005). Dropout with a keep probability of 0.5 was
used to reduce overfitting (Srivastava et al., 2014).
The output layer used the sigmoid activation function.
The model was trained using binary cross-entropy
and the Adam optimizer (Kingma and Ba, 2014). It
was first trained for 20 epochs with a batch size of
64. It achieved 49.47% mean accuracy over the 10
cross-validation folds and 49.01% mean accuracy in
the leave-one-subject-out cross validation. After the
same model was trained for 50 epochs, it achieved a
mean accuracy of 50.35% over the 10 cross-validation
folds and 50.12% in the leave-one-subject-out cross-
validation.
5.2 Physiological Data
The physiological data were used to train random
forests, support vector machines, neural networks, au-
toencoders, and convolutional neural networks. 5-
fold cross-validation was used to tune the hyperpa-
rameters of the different models. Then Leave-one-
subject-out cross-validation was used as the final eval-
uation metric. Table 1 provides a summary of the
achieved results.
5.2.1 Random Forests
The hand-crafted features from the four physiologi-
cal modalities were concatenated and fed to random
forests. Two searches of the hyperparameter space
were conducted. In both, entropy was used to grow
the trees. 240 combinations of hyperparameters were
tried in total. The best setting had a maximum depth
of 20 levels per tree. All features were examined at
every node, and a minimum of 10 samples was needed
Subject-independent Pain Recognition using Physiological Signals and Para-linguistic Vocalizations
147

to create child nodes in the trees. A total of 100 deci-
sion trees were trained. This setting achieved a mean
accuracy of 83.36% on the 5 cross-validation folds
and a mean accuracy of 82.61% on the 40 subjects
of the leave-one-subject-out cross-validation.
5.2.2 Support Vector Machines
The hand-crafted features from the four physiological
modalities were concatenated and fed to support vec-
tor machines with both polynomial and Radial-Basis
Function (RBF) kernels.
With the RBF kernel, 195 hyperparameter combi-
nations were evaluated in total. The best combination
was C = 2000 and γ = 2 ×10
−5
. This combination
achieved a mean accuracy of 81.94% over the 5 cross-
validation folds and a mean accuracy of 81.34% over
the 40 subjects.
With the polynomial kernel, 297 hyperparameter
combinations were evaluated in total. The best com-
bination of hyperparameters was degree = 3, C = 20,
and γ = 2 ×10
−3
. This combination achieved a mean
accuracy of 78.78% over the 5 cross-validation folds
and a mean accuracy of 78.04% over the 40 subjects.
5.2.3 Neural Networks
Several architectures of neural networks with fully
connected layers were tested. The number of hidden
layers ranged from 2 to 4. The Exponential Linear
Unit (ELU) function was used as the activation func-
tion for the hidden layers. The sigmoid function was
used as the activation function for the output layer.
Batch normalization and He initialization were used
(Ioffe and Szegedy, 2015; He et al., 2015). Dropout
with a keep probability of 0.5 was applied to the out-
puts of all the hidden layers in the networks (Srivas-
tava et al., 2014). Binary cross-entropy was mini-
mized using the Adam optimizer to train the networks
(Kingma and Ba, 2014).
The best performing architecture had 3 hidden
layers with 32, 16, and 4 units respectively. It
achieved a mean accuracy of 81.96% on the 5 cross-
validation folds and a mean accuracy of 81.57% on
the 40 subjects.
5.2.4 Autoencoders
A single autoencoder was trained on all four modal-
ities at once to perform automatic feature extraction.
The input to this autoencoder consisted of 10240 nor-
malized signal values. The autoencoder consisted of
three hidden layers with 128, 64, and 128 units re-
spectively. The 64-unit layer was used as the encod-
ing layer. Batch normalization and He initialization
were used. The ELU function was used as the activa-
tion for the hidden layers of the autoencoder. A small
neural network consisting of two layers with 32 and
1 units respectively was used on top of the encoding
layer to perform the classification. This architecture
achieved a mean accuracy of 70.00% on the 40 sub-
jects in leave-one-subject-out cross-validation.
5.2.5 Convolutional Neural Networks
A 1D convolutional neural network with 3 convolu-
tional layers was trained on the normalized signals
of the four modalities to perform automatic feature
extraction and classification (Kiranyaz et al., 2019).
Each layer had 96 filters, and each filter was of size
3. The three layers were followed by a global averag-
ing layer, which computes the average over each fil-
ter output independently. Finally, a dense layer with
one unit was used to output the classification results.
The binary cross-entropy function was used as the
cost function to train the network. The Rectified Lin-
ear Unit (ReLU) function was used as the activation
function for the convolutional layers. The sigmoid
function was used as the activation function for the
output layer. The model was trained using stochastic
gradient descent. This architecture achieved a mean
accuracy of 76.90% on the 40 subjects in leave-one-
subject-out cross-validation.
Table 1: A summary of the results of the six algorithms
that were applied to the physiological modalities to per-
form pain recognition. The mean accuracy of leave-one-
subject-out cross-validation is presented. Random forests,
support vector machines (SVM), and neural networds used
hand-crafted features, while autoencoders and 1D convolu-
tional neural networks (CNN) performed automatic feature
extraction. For the autoencoders approach, a 2-layer neural
network was used as the classifier.
Algorithm Accuracy
Random Forests 82.61 ±10.74%
Neural Network 81.57 ±10.58%
RBF Kernel SVM 81.34 ±10.62%
Polynomial Kernel SVM 78.04 ±10.57%
1D CNN 76.90 ±12.60%
Autoencoders 70.00 ±12.00%
6 CONCLUSION
In this paper, several machine learning algorithms
have been evaluated and compared on the pain recog-
nition task using data from the SenseEmotion dataset.
On para-linguistic data, support vector machines
were the best performing models in the leave-one-
subject-out cross-validation. The naive Bayes clas-
ICPRAM 2020 - 9th International Conference on Pattern Recognition Applications and Methods
148

sifer was also able to achieve comparable results with
less processing time. Analysis of the trained models
shows that the MFCC values were the most important
features for the classifiers.
The physiological signals achieved higher accu-
racies. This high performance can be attributed to
the use of the electrodermal activity signal, which has
been confirmed as an effective indicator of pain. The
random forests achieved the highest performance on
the hand-crafted features. Support vector machines
and neural networks followed closely. Error analy-
sis has shown that the four models that used hand-
crafted features made similar mistakes in their pre-
dictions. Automatic feature extraction achieved lower
accuracies but produced different classification errors.
Therefore, a combination of both hand-crafted and
automatic features can be tested in the future and may
lead to improvements in accuracy.
In both approaches, the use of hand-crafted fea-
tures outperformed automatic feature extraction. The
difference is especially clear in the case of the
para-linguistic data, where the VGG-16 bidirectional
model performed like a random classifier. This dis-
parity can be attributed to the data size; the dataset
does not contain enough data samples to train auto-
matic feature extractors that can generalize well. Fur-
thermore, the inter-subject variance in the values of
the signals further complicates automatic extraction.
Data augmentation can be tested in the future as one
method to deal with these problems.
Pain recognition remains a difficult task. The fu-
ture collection of more data, especially from a re-
alistic clinical setting, can help improve the perfor-
mance of the state-of-the-art machine learning algo-
rithms and can allow for the use of deep learning for
feature extraction. Data collection should be coupled
with the development of new methods to deal with
inter-subject variance, which stands in the way of gen-
eralizable results.
REFERENCES
Abdulaziz, Y. and Ahmad, S. M. S. (2010). Infant cry
recognition system: A comparison of system perfor-
mance based on mel frequency and linear prediction
cepstral coefficients. In 2010 International Confer-
ence on Information Retrieval & Knowledge Manage-
ment (CAMP), pages 260–263. IEEE.
Aung, M. S. H., Kaltwang, S., Romera-Paredes, B., Mar-
tinez, B., Singh, A., Cella, M., Valstar, M., Meng, H.,
Kemp, A., Shafizadeh, M., Elkins, A. C., Kanakam,
N., de Rothschild, A., Tyler, N., Watson, P. J.,
d. C. Williams, A. C., Pantic, M., and Bianchi-
Berthouze, N. (2016). The automatic detection of
chronic pain-related expression: Requirements, chal-
lenges and the multimodal emopain dataset. IEEE
Transactions on Affective Computing, 7(4):435–451.
Barajas-Montiel, S. E. and Reyes-Garc
´
ıa, C. A. (2006).
Fuzzy support vector machines for automatic infant
cry recognition. In Intelligent Computing in Signal
Processing and Pattern Recognition, pages 876–881.
Springer.
Boiten, F. A., Frijda, N. H., and Wientjes, C. J. E. (1994).
Emotions and respiratory patterns: review and critical
analysis. International journal of psychophysiology,
17(2):103–128.
Boser, B. E., Guyon, I. M., and Vapnik, V. N. (1992). A
training algorithm for optimal margin classifiers. In
Proceedings of the fifth annual workshop on Compu-
tational learning theory, pages 144–152. ACM.
Breiman, L. (2001). Random forests. Machine learning,
45(1):5–32.
Cai, D., Ni, Z., Liu, W., Cai, W., Li, G., Li, M., Cai, D.,
Ni, Z., Liu, W., and Cai, W. (2017). End-to-end deep
learning framework for speech paralinguistics detec-
tion based on perception aware spectrum. In INTER-
SPEECH, pages 3452–3456.
Chang, C.-Y. and Li, J.-J. (2016). Application of deep learn-
ing for recognizing infant cries. In 2016 IEEE Interna-
tional Conference on Consumer Electronics-Taiwan
(ICCE-TW), pages 1–2. IEEE.
Colloca, L., Benedetti, F., and Pollo, A. (2006). Repeata-
bility of autonomic responses to pain anticipation and
pain stimulation. European Journal of Pain, 10(7):659
– 665.
Dave, N. (2013). Feature extraction methods LPC, PLP and
MFCC in speech recognition. International journal
for advance research in engineering and technology,
1(6):1–4.
Engel, G. L. (1959). psychogenic pain and the pain-
prone patient. The American journal of medicine,
26(6):899–918.
Graves, A. and Schmidhuber, J. (2005). Framewise
phoneme classification with bidirectional lstm and
other neural network architectures. Neural networks,
18(5-6):602–610.
Gruss, S., Treister, R., Werner, P., Traue, H. C., Crawcour,
S., Andrade, A., and Walter, S. (2015). Pain intensity
recognition rates via biopotential feature patterns with
support vector machines. PloS one, 10(10):e0140330.
Hammal, Z. and Cohn, J. F. (2014). Towards multimodal
pain assessment for research and clinical use. In Pro-
ceedings of the 2014 Workshop on Roadmapping the
Future of Multimodal Interaction Research including
Business Opportunities and Challenges, pages 13–17.
ACM.
He, K., Zhang, X., Ren, S., and Sun, J. (2015). Delving
deep into rectifiers: Surpassing human-level perfor-
mance on imagenet classification. In 2015 IEEE In-
ternational Conference on Computer Vision (ICCV),
pages 1026–1034.
Ioffe, S. and Szegedy, C. (2015). Batch normalization: Ac-
celerating deep network training by reducing internal
covariate shift. arXiv preprint arXiv:1502.03167.
Subject-independent Pain Recognition using Physiological Signals and Para-linguistic Vocalizations
149

Jafari, H., Courtois, I., Van den Bergh, O., Vlaeyen, J. W.,
and Van Diest, I. (2017). Pain and respiration: a sys-
tematic review. Pain, 158(6):995–1006.
K
¨
achele, M., Thiam, P., Amirian, M., Schwenker, F., and
Palm, G. (2016). Methods for person-centered contin-
uous pain intensity assessment from bio-physiological
channels. IEEE Journal of Selected Topics in Signal
Processing, 10(5):854–864.
Kim, J. and Andr
´
e, E. (2008). Emotion recognition based on
physiological changes in music listening. IEEE Trans-
actions on Pattern Analysis and Machine Intelligence,
30(12):2067–2083.
Kingma, D. and Ba, J. (2014). Adam: A method for
stochastic optimization. International Conference on
Learning Representations.
Kiranyaz, S., Avci, O., Abdeljaber, O., Ince, T., Gabbouj,
M., and Inman, D. J. (2019). 1d convolutional neural
networks and applications: A survey.
Ledowski, T., Ang, B., Schmarbeck, T., and Rhodes, J.
(2009). Monitoring of sympathetic tone to assess post-
operative pain: skin conductance vs surgical stress in-
dex. Anaesthesia, 64(7):727–731.
Loggia, M. L., Juneau, M., and Bushnell, M. C. (2011).
Autonomic responses to heat pain: Heart rate, skin
conductance, and their relation to verbal ratings and
stimulus intensity. PAIN, 152(3):592 – 598.
Lu, L., Zhang, H.-J., and Li, S. Z. (2003). Content-based au-
dio classification and segmentation by using support
vector machines. Multimedia systems, 8(6):482–492.
Lucey, P., Cohn, J. F., Prkachin, K. M., Solomon, P. E., and
Matthews, I. (2011). Painful data: The unbc-mcmaster
shoulder pain expression archive database. In Face
and Gesture 2011, pages 57–64.
Mallik, S., Chowdhury, D., and Chttopadhyay, M. (2019).
Development and performance analysis of a low-cost
mems microphone-based hearing aid with three dif-
ferent audio amplifiers. Innovations in Systems and
Software Engineering, 15(1):17–25.
¨
Ohman, A., Esteves, F., Flykt, A., and Soares, J. J. F. (1993).
Gateways to Consciousness: Emotion, Attention, and
Electrodermal Activity, pages 137–157. Springer US,
Boston, MA.
Petroni, M., Malowany, A. S., Johnston, C. C., and Stevens,
B. J. (1995). Identification of pain from infant cry
vocalizations using artificial neural networks (anns).
In Applications and Science of Artificial Neural Net-
works, volume 2492, pages 729–739. International
Society for Optics and Photonics.
Picard, R. W. (2000). Emotions Are Physical and Cognitive,
pages 21–45. MIT press, Cambridge, MA.
Saki, F. and Kehtarnavaz, N. (2014). Background noise
classification using random forest tree classifier for
cochlear implant applications. In 2014 IEEE Inter-
national Conference on Acoustics, Speech and Signal
Processing (ICASSP), pages 3591–3595. IEEE.
Schwenker, F. (2018). Multimodal affect classifica-
tion using deep neural networks. Studientexte
zur Sprachkommunikation: Elektronische Sprachsig-
nalverarbeitung 2018, pages 240–246.
Sellner, J., Thiam, P., and Schwenker, F. (2018). Visualizing
facial expression features of pain and emotion data. In
IAPR Workshop on Multimodal Pattern Recognition of
Social Signals in Human-Computer Interaction, pages
101–115. Springer.
Simonyan, K. and Zisserman, A. (2014). Very deep con-
volutional networks for large-scale image recognition.
arXiv preprint arXiv:1409.1556.
Srivastava, N., Hinton, G., Krizhevsky, A., Sutskever, I.,
and Salakhutdinov, R. (2014). Dropout: a simple way
to prevent neural networks from overfitting. The Jour-
nal of Machine Learning Research, 15(1):1929–1958.
Thiam, P., Kessler, V., Amirian, M., Bellmann, P., Layher,
G., Zhang, Y., Velana, M., Gruss, S., Walter, S., Traue,
H. C., Kim, J., Schork, D., Andre, E., Neumann, H.,
and Schwenker, F. (2019). Multi-modal pain inten-
sity recognition based on the senseemotion database.
IEEE Transactions on Affective Computing.
Thiam, P., Kessler, V., and Schwenker, F. (2017). Hierar-
chical combination of video features for personalised
pain level recognition. In ESANN.
Thiam, P. and Schwenker, F. (2017). Multi-modal data fu-
sion for pain intensity assessment and classification.
In 2017 Seventh International Conference on Image
Processing Theory, Tools and Applications (IPTA),
pages 1–6. IEEE.
Turk, D. C. and Gatchel, R. J. (2018). Psychological ap-
proaches to pain management: A practitioner’s hand-
book. Guilford publications.
Turk, D. C., Meichenbaum, D., and Genest, M. (1983). Pain
and behavioral medicine: A cognitive-behavioral per-
spective, volume 1. Guilford Press.
Velana, M., Gruss, S., Layher, G., Thiam, P., Zhang, Y.,
Schork, D., Kessler, V., Meudt, S., Neumann, H.,
Kim, J., Schwenker, F., Andr
´
e, E., Traue, H. C., and
Walter, S. (2016). The senseemotion database: A
multimodal database for the development and system-
atic validation of an automatic pain- and emotion-
recognition system. In MPRSS 2016, pages 127–139.
Wagner, J., Lingenfelser, F., Baur, T., Damian, I., Kistler,
F., and Andr
´
e, E. (2013). The social signal interpre-
tation (ssi) framework: Multimodal signal processing
and recognition in real-time. In Proceedings of the
21st ACM International Conference on Multimedia,
MM ’13, pages 831–834, New York, NY, USA. ACM.
Walter, S., Gruss, S., Ehleiter, H., Junwen Tan, Traue, H. C.,
Werner, P., Al-Hamadi, A., Crawcour, S., Andrade,
A. O., and Moreira da Silva, G. (2013). The biovid
heat pain database data for the advancement and sys-
tematic validation of an automated pain recognition
system. In 2013 IEEE International Conference on
Cybernetics (CYBCO), pages 128–131.
Wang, J.-C., Lee, H.-P., Wang, J.-F., and Lin, C.-B. (2008).
Robust environmental sound recognition for home au-
tomation. IEEE Transactions on Automation Science
and Engineering, 5(1):25–31.
Zwakhalen, S. M., Hamers, J. P., Abu-Saad, H. H., and
Berger, M. P. (2006). Pain in elderly people with
severe dementia: a systematic review of behavioural
pain assessment tools. BMC geriatrics, 6(1):3.
ICPRAM 2020 - 9th International Conference on Pattern Recognition Applications and Methods
150
