
Minimal Complexity Requirements for Proteins and Other
Combinatorial Recognition Systems
George D. Monta
˜
nez
a
, Laina Sanders
b
and Howard Deshong
c
AMISTAD Lab, Department of Computer Science, Harvey Mudd College, Claremont, CA, U.S.A.
Keywords:
Minimum Complexity, Proteins, Information Theory, Recognition Tasks, Classification.
Abstract:
How complex do proteins (and other multi-part recognition systems) need to be? Using an information-
theoretic framework, we characterize the information costs of recognition tasks and the information capacity
of combinatorial recognition systems, to determine minimum complexity requirements for systems performing
such tasks. Reducing the recognition task to a finite set of binary constraints, we determine the sizes of minimal
equivalent constraint sets using a form of distinguishability, and show how the representation of constraint sets
as binary circuits or decision trees also results in minimum constraint set size requirements. We upper-bound
the number of configurations a recognition system can distinguish between as a function of the number of parts
it contains, which we use to determine the minimum number of parts needed to accomplish a given recognition
task. Lastly, we apply our framework to DNA-binding proteins and derive estimates for the minimum number
of amino acids needed to accomplish binding tasks of a given complexity.
1 INTRODUCTION
Complexity is costly (Ho and Pepyne, 2002; Ho et al.,
2003). Ho and Pepyne, in discussing the trade-off be-
tween complexity and fragility, describe the situation
this way (Ho and Pepyne, 2002):
Consequently, as design complexity continues
to increase, the probability of catastrophic bad
outcomes increases. There is no avoiding this;
systems become increasingly fragile as their
complexity increases.
Thus, unless the extra capacity is actually needed, op-
timization towards robustness seems to recommend
eschewing complexity in favor of simplicity. Yet, in
the biological realm complexity abounds (Mitchell,
2009). In particular, molecular machines consisting
of amino acid chains regularly have lengths stretch-
ing to hundreds of residues, offering an exponentially
large space of possible protein structures (Branden
and Tooze, 1999; Milo and Phillips, 2015). For pro-
teins, we are moved to ask an obvious question: is all
this complexity really necessary?
We propose a conditionally affirmative answer to
this question, using tools from information theory and
a
https://orcid.org/0000-0002-1333-4611
b
https://orcid.org/0000-0003-0586-4556
c
https://orcid.org/0000-0002-3473-9239
machine learning. By casting protein substrate match-
ing as an abstract recognition task similar to binary
classification (Bishop, 2006), we demonstrate that
the functional capacity of proteins as information-
processing recognition systems scales linearly with
their size, as measured by their number of amino
acids. To perform complex binding tasks, complex
structures are therefore needed.
We explore the information capacity and informa-
tion cost (referred to as an information burden) of
general recognition tasks. We first define the struc-
ture of recognition tasks, including a discussion of
distinguishability requirements for recognition sys-
tems relative to tasks. Representing tasks as deci-
sion trees and circuits, we provide a concrete logi-
cal structure for these abstract tasks. From this we
apply a search framework to find a lower bound for
the information burdens for tasks. We derive an up-
per bound of the recognizer’s information capacity,
relative to its complexity, using Abu-Mostafa and St.
Jacques’ method for determining the maximum infor-
mation capacity of general forms of memory (Abu-
Mostafa and St. Jacques, 1985). Chaining our bounds,
we apply our findings to protein-DNA binding as a
recognition task. We find that the minimum number
of sector (functional) amino acids needed for any pro-
tein capable of performing a recognition task with in-
formation burden of b bits is linear in b, within a small
constant factor (< 5).
Montañez, G., Sanders, L. and Deshong, H.
Minimal Complexity Requirements for Proteins and Other Combinatorial Recognition Systems.
DOI: 10.5220/0008856800710078
In Proceedings of the 13th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2020) - Volume 3: BIOINFORMATICS, pages 71-78
ISBN: 978-989-758-398-8; ISSN: 2184-4305
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
71

2 STRUCTURE IN
RECOGNITION TASKS
2.1 Defining Recognition Tasks
A recognizer processes a configuration (an object or
collection of data, such as a string) and either accepts
or rejects that configuration based on its features. We
use the term constraint to signify an aspect the rec-
ognizer can test for, that must meet a certain require-
ment. For example, “the configuration starts with the
symbol q” is one possible constraint.
Each constraint may be thought of as a logical
predicate that acts on the configuration and outputs
either true or false. A recognizer’s behavior is ex-
pressed as a logical statement involving its constraints
and the logical connectives ∧ (AND), ∨ (OR), and ¬
(NOT). We require our constraints to be atomic, such
that a constraint cannot be broken down into multiple
simpler constraints joined with connectives. For ex-
ample, “the configuration is 5 or 6 symbols long” is
not one constraint; since it can be broken down at the
“or” connective, it is really two separate constraints.
We express each set of constraints in its minimum
form (i.e., using the fewest number of connectives).
2.2 Distinguishability
Intuitively, if a recognizer treats different configura-
tions differently, then the recognizer must be complex
enough to distinguish between those configurations.
Therefore, some notion of distinguishability can con-
ceivably be used to find a lower bound on the number
of constraints (and by extension, the amount of infor-
mation) needed to perform a recognition task.
2.2.1 Features, Constraints, and Separability
The features of an object are aspects which can be
in one of several possible states, or equivalently, can
take values from within some set of possible values.
For any given object, there are myriad ways to “fea-
turize” it so as to arrive at some finite number of fea-
tures with which to capture the important aspects of
the object. As Socrates told Phaedrus, there are many
ways in which to carve Nature (and we typically seek
to do so at her “joints”). A common way to represent
an object as a collection of features is as a vector in
a high-dimensional Euclidean space, where each in-
dex of the vector corresponds to a different feature.
The purpose of features in recognition tasks is to dis-
tinguish between objects based on their feature signa-
tures, where a feature signature corresponds to the
specific feature values for an object, or equivalently,
refers to the specific vector in the high-dimensional
feature space that represents the object.
Given a featurization of an object (i.e., represent-
ing an object as a feature signature), any two objects
with the same feature signature will be indistinguish-
able in that feature space. This suggests that to distin-
guish between all objects in some set we are consid-
ering, we require a minimum collection of features,
based on the number of objects we are trying to dis-
tinguish and on which distinctions matter. By the pi-
geonhole principle, if we have fewer possible feature
signatures than we have objects, then at least two ob-
jects will share a signature and the collection of ob-
jects will not be fully distinguishable within that fea-
ture space. This will require us to add more features
(or values they can take) to separate the overlapping
objects.
Therefore, we say that a pair of objects is distin-
guishable with respect to a feature space if and only
if they have different feature signatures in that space.
As in the previous section, we can draw an equiva-
lence between constraint sets and feature sets, con-
sidering each constraint as a binary feature. Thus, we
may also speak of the distinguishability of a pair of
configurations with respect to a constraint set.
For recognition tasks, we must distinguish be-
tween configurations that are accepted by the recog-
nizer, and those that are not. This is the only distinc-
tion that matters for the task, and thus, we only require
distinguishability for configurations with different la-
bels (accepted or rejected). We say a set of configu-
rations is separable with respect to a feature space if
every pair of configurations in that set having differ-
ent labels is distinguishable. Given a set of possible
constraints viewed as binary features, we define the
minimum set of constraints for the recognition task
as the smallest subset of those features under which
the set of objects under question remains separable
within the smaller space.
3 REPRESENTING
RECOGNIZERS
As we have seen, recognizers’ behavior can be de-
scribed using logical statements. We may find it
convenient to re-express these statements in terms of
other structures, such as decision trees or circuits, for
easier analysis. We show how to do so in the next sec-
tion, proving that such corresponding structures exist
for all finite constraint sets.
BIOINFORMATICS 2020 - 11th International Conference on Bioinformatics Models, Methods and Algorithms
72

3.1 Decision Trees
Decision trees (Quinlan, 1986) are a simple way to
represent recognizers. Every non-leaf represents a
constraint and has two branches off of it: one branch
represents the satisfying of the constraint, and the
other represents not satisfying it. Leaves represent
the recognizer either accepting or rejecting a configu-
ration. We begin reading in a configuration from the
tree’s root and traverse it until we reach a leaf. The
process of constructing these trees from a set of con-
straints is outlined below, omitting details concerning
the order in which you choose features via maximized
information gain (as in (Quinlan, 1986)).
Theorem 1. There exists a decision tree for every fi-
nite set of constraints.
Proof. Recall that every set of constraints may be ex-
pressed as a logical statement. Our proof is by strong
induction on the number n of logical connectives in
the constraints’ logical statement.
For the base case, consider n = 0. Note that a log-
ical statement with 0 logical connectives represents
just one constraint, and the statement evaluates true if
and only if that constraint is met. We may construct
a trivial decision tree for the set of constraints as fol-
lows. One node represents the constraint; if we meet
the constraint, we reach an accepting leaf; otherwise
we reach a rejecting leaf.
For the inductive hypothesis, we suppose that for
some k ≥ 0, every logical statement with k or fewer
connectives has a corresponding decision tree.
For the inductive step, we consider the case where
n = k + 1. Every logical statement N with k + 1 con-
nectives falls into one of the cases below:
1. N is of the form ¬A. Since A contains k connec-
tives, by the inductive hypothesis a decision tree
exists for A. We construct a decision tree for N
using the tree for A by flipping the tree’s leaves:
accepting leaves are now rejecting and vice-versa.
2. N consists of two logical statements A and B (re-
spectively containing a and b connectives each,
where a+b = k) joined by a connective. Note that
A, B each have ≤ k connectives, so by the induc-
tive hypothesis they have corresponding decision
trees. Then N falls into a subcase below.
(a) N = A ∧ B. To construct a decision tree for N,
we take the decision tree for A and replace ev-
ery accepting state with a copy of the decision
tree for B.
(b) N = A ∨ B. To construct a decision tree for N,
we take the decision tree for A and replace ev-
ery rejecting state with a copy of the decision
tree for B.
Thus, if every logical statement with k or fewer
connectives has a corresponding decision tree, so does
every logical statement with k + 1 connectives. By
strong induction, we can see that every logical state-
ment with n ≥ 0 connectives has a corresponding de-
cision tree. Thus, since every set of constraints is
represented by a logical statement, every set of con-
straints has a corresponding decision tree.
Notice that there could be many different deci-
sion trees for the same task. We would like to fo-
cus on minimal decision trees: decision trees with
the fewest nodes needed to perform their recognition
task. The size of this tree is one way to measure the
minimum complexity of the recognizer.
Corollary 1.1. There exists a minimal decision tree
for every finite set of constraints.
Proof. The proof follows directly by establishing that
a decision tree exists for every constraint set by The-
orem 1, then invoking the well-ordering principle on
the number of nodes in the decision tree.
3.2 Circuits
We can represent a recognizer’s logical statement as a
circuit in the following way (Shannon, 1938; Barnett,
2009). Let every input wire be connected to a con-
straint. Then following the logical statement inside
out from each predicate, connect the wires through
logic gates of the same type. For example, if the two
predicates or statements are connected by ∧ then feed
both wires into an AND gate, for ∨ use an OR gate.
Any ¬ in the circuit is represented by a bubble on the
wire before the next gate or after the last gate to indi-
cate the inversion.
Theorem 2. There exists a circuit for every finite set
of constraints.
Proof. Recall that every set of constraints may be ex-
pressed as a logical statement. The result then fol-
lows immediately by strong induction on the number
of logical connectives in the constraints’ logical state-
ment.
Corollary 2.1. There exists a minimal circuit for ev-
ery finite set of constraints.
Proof. Use the same logic as Corollary 1.1, except
with logic gates instead of nodes.
Minimal Complexity Requirements for Proteins and Other Combinatorial Recognition Systems
73

4 INFORMATION CONSTRAINTS
By casting a recognition task in an information-
theoretic search framework we can find a lower bound
for the information needed to perform the task. In the
same way, we can use Abu-Mostafa’s formula (Abu-
Mostafa and St. Jacques, 1985) to find an upper bound
on the information capacity of a recognizer based on
its size. Finally, we use these two results to present
an inequality from which we can determine the mini-
mum number of parts a recognizer would need to per-
form the task.
4.1 Information Burden
Every recognizer must have enough memory to store
its constraint set—this is its information burden. We
discuss a technique for finding a lower bound on a
recognizer’s information burden by reframing the task
as a search problem.
Consider an arbitrary finite constraint set C. It is
possible that other constraint sets exist besides C that
categorize items in the exact same way. All such con-
straint sets fall in the equivalence class C
eq
.
Following the discussion in Section 2.2 and using
arguments similar to those from Corollaries 1.1 and
2.1, it is clear that every constraint set C has at least
one minimal representation, C
min
. Note that C
min
does
not have to be unique, but all possible selections of
C
min
must be of the same size |C
min
| = d.
We approach the problem of finding the infor-
mation cost of C
min
by using the algorithmic search
framework (Monta
˜
nez, 2017). Let S be the set of all
possible constraint sets. The collection R
d
⊆ S is then
all constraint sets in S with size d. Thus C
eq,d
⊆ R
d
,
where C
eq,d
contains all constraint sets in C
eq
of size
d.
Assuming we know d, we can determine how
much information the recognizer would need to lo-
cate an element of C
eq,d
from the space R
d
, which
gives us a lower bound on the amount of information
needed to find an element of C
eq,d
within the larger
space S. In the language of algorithmic search, R
d
is
our search space and C
eq,d
is our target set. Assum-
ing a search space partitioned into sets of size |C
eq,d
|
and no prior knowledge of target locations, the num-
ber of bits needed to identify the target set among the
partitions is
−log
2
|C
eq,d
|
|R
d
|
(1)
which is equivalent to the minimum number of yes/no
(binary) questions one would need to ask to reduce
the search space to a set containing only the target
elements, under those assumptions.
Some will recognize the quantity in (1) as the
Shannon surprisal of a random outcome belonging
to the target set under a uniform distribution on the
search space, and may be tempted to protest that this
does not take into account cases where the elements of
the search space are not equally likely. Indeed, when
target elements are very likely, they require fewer bits
of information to identify (Shannon, 1948). However,
the restriction of side-information and prior knowl-
edge prohibits us from adopting anything other than
a maximum entropy (maximum uncertainty, or zero-
knowledge) uniform distribution to represent our un-
certainty concerning target locations (Jaynes, 2003;
Dembski and Marks, 2009). Furthermore, recent re-
sults in machine learning (Monta
˜
nez, 2017; Montanez
et al., 2019) show that any distribution which makes
target elements more likely (thus reducing the infor-
mation cost of locating them) must itself be propor-
tionally unlikely, incurring its own information bur-
den that must be added to the total. Specifically, find-
ing a distribution (called a strategy) which reduces the
information cost by b
0
bits requires at least b
0
bits,
so nothing is gained by positing such a favorably bi-
asing distribution once its own information cost is
taken into account (Monta
˜
nez, 2017). Thus, the Shan-
non surprisal absent of side-information gives a lower
bound on the actual information burden b, satisfying
−log
2
|C
eq,d
|
|R
d
|
≤ b
where b is the true information burden in bits.
4.2 Information Capacity
In broad terms, we may also think of a recognizer as a
collection of parts placed together as some structure,
some or all of which performs the recognition task.
Let M represent the number of distinct parts in
some nontrivial recognizer. Then, let p represent the
proportion of those parts that are actually involved in
the recognition task. (Note that 0 < p ≤ 1, with an ex-
clusive lower bound since at least one part is involved
in nontrivial recognition.) We can see that the number
of parts involved in recognition is Mp = n.
Now, if we let k denote how many types of parts
can be arranged to form a recognizer, then we can see
that k
n
is an upper bound on the total number of rec-
ognizers that use n parts for the recognition task, since
there are at most k choices for each of its n parts.
If we consider these k
n
recognizers as a whole, they
demonstrate at most k
n
distinct behaviors (that is, they
accept/reject configurations according to distinct cri-
teria).
Now recall from Abu-Mostafa (Abu-Mostafa and
St. Jacques, 1985) that the information capacity of
BIOINFORMATICS 2020 - 11th International Conference on Bioinformatics Models, Methods and Algorithms
74
a structure that can distinguish between d items is
log
2
(d). The information capacity c of our space of
k
n
recognizers satisfies
c ≤ log
2
(k
n
) ∈ O(n)
since the collection can distinguish between at most
k
n
behaviors.
Since the information burden must be less than the
capacity, we know that if b > f (n) for all functions
f (n) linear in n, then our recognizer does not have
enough parts or capacity to perform the task. Con-
versely, assuming it can perform the task requires that
its capacity is no less than the information burden for
that task, namely, that b ≤ c.
Chaining our bounds, we obtain
−log
2
|C
eq,d
|
|R
d
|
≤ b ≤ c ≤ log
2
(k
n
) (2)
or more simply
−log
2
|C
eq,d
|
|R
d
|
≤ n log
2
k. (3)
5 PROTEINS AS RECOGNIZERS
Biologists believe there are at least tens of thou-
sands of different proteins in the human body (Pono-
marenko et al., 2016), each with a specific set of
tasks (Branden and Tooze, 1999). Some of these tasks
involve the protein binding to another molecule, such
as an enzyme binding to substrate. For restriction en-
zymes and some other families of proteins, the sub-
strate molecule is DNA. In these cases, the enzymes
have a distinct set of DNA sequences that can be
bound based upon affinities between amino acids in
the enzyme and the base pairs within the DNA.
Let us consider proteins binding to a specific set
of DNA sequences as a recognition task. In this sce-
nario, the protein is the recognizer while DNA se-
quences are the configurations. We can determine
the information burden and capacity of the abstracted
view of a protein, using the bounds established earlier.
Since there are 20 frequent amino acids, let k = 20.
The lower bound term is specific to the protein’s task,
so we cannot simplify it any further. Using Inequal-
ity (3) we then obtain,
−log
2
|C
eq,d
|
|R
d
|
≤ n log
2
20 < 5n.
Thus we can determine the minimum number of
sector (functional) amino acids (McLaughlin Jr et al.,
2012), denoted n, required for a protein to perform a
specific task (i.e., bind to specific subset of all DNA
sequences) as
n > −
1
5
log
2
|C
eq,d
|
|R
d
|
which, given binary predicates as constraints (imply-
ing |R
d
| ≥ 2
d
), can be rewritten as
n >
1
5
d − log
2
|C
eq,d
|
. (4)
In the case that the minimum constraint set is unique
(namely, |C
eq,d
| = 1), then we obtain the simplified
n >
1
5
d, (5)
where d is the number of constraints in the minimal
set.
5.1 Discussion
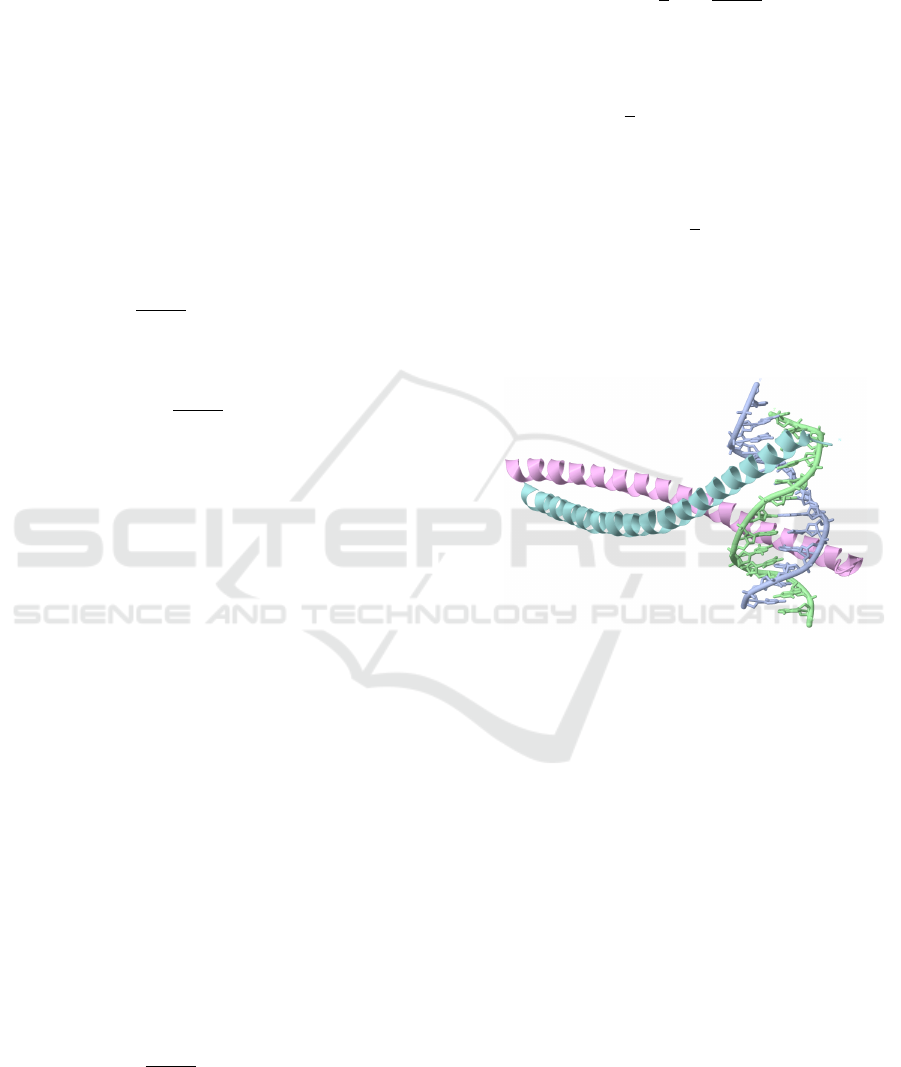
Figure 1: [Example] Crystal structure of leucine zipper bzip
transcription factor PAP1 bound to DNA, requiring multi-
turn alpha helix components. (PDBid 1GD2/PD0180, (Fujii
et al., 2000).)
Inequality (4) gives a lower bound for protein
recognition systems based on a completely abstract
view of proteins as combinatorial recognition sys-
tems. Given that the median protein length is roughly
300 residues (Brocchieri and Karlin, 2005), and as-
suming that roughly 20% of the residues are sector
residues (McLaughlin Jr et al., 2012), this bound tells
us that proteins would be able to accomplish recogni-
tion tasks requiring up to 300 constraints, which ap-
pears to be a generous overestimate. Keeping a com-
pletely abstract view of recognition systems appears
to come at a cost, but not one without remedy. We
can tighten the bound by putting back into our model
some of the specifics concerning DNA-binding pro-
teins.
Our initial overestimation is partially due to the
abstract model allowing for cases where the choice
of each amino acid transmits log
2
(20) ≈ 4.32 bits
of information, potentially allowing a single sector
Minimal Complexity Requirements for Proteins and Other Combinatorial Recognition Systems
75

residue to process and determine the outcome of four
distinct binary constraints. Real proteins, however,
regularly require secondary structure in their con-
straint handling, such as alpha helices and beta sheets,
which are substructures within proteins formed by
the three-dimensional arrangement of multiple amino
acids (Pauling et al., 1951; Pauling and Corey, 1951).
For example, DNA-binding domains such as leucine
zippers, helix-turn-helix and winged-helix structures
require multiple alpha helices (Korasick and Jez,
2015; Fujii et al., 2000), which themselves require
3.6 amino acids per turn. Thus, an alpha helix with
10 turns requires 36 amino acids, and if just two al-
pha helices of this size are needed to process a spe-
cific constraint, this increases the residue count by a
factor of 72. Rather than a single amino acid han-
dling many constraints, it is more typical that a single
constraint requires multiple amino acids working to-
gether to process it.
Plugging such biology-specific factors into the
model will yield tighter lower bounds on residue
counts. We can add a protein-specific scaling factor
α which for each sector residue required by the gen-
eral model will add a multiplicative factor reflecting
the biological need for secondary support structures,
taking into account the additional amino acids each
sector residue carries with it to fulfill its role. The
modified bound then becomes
n >
α
5
d − log
2
|C
eq,d
|
(6)
with corresponding simplification
n >
α
5
d (7)
when C
min
is unique.
As an example, if we again assume two alpha
helices of ten turns are required for each constraint,
this gives a scaling factor of α = 72. By Inequal-
ity (7), a protein testing four constraints will require
at least 57 sector residues, which corresponds to a
protein of 285 total residues (assuming sector amino
acids account for around 20% of all residues in a typ-
ical protein). This biology-specific estimate agrees
with known median protein lengths for eukaryotes
and prokaryotes (Brocchieri and Karlin, 2005), sug-
gesting the utility of attaching domain-specific con-
siderations to our general model, while still allowing
us to abstract away many of the intricacies of protein-
DNA interactions such as how proteins deform DNA
structure or how specific proteins and DNA sequences
do not always interact in the same way.
6 ALTERNATIVE MODELS OF
PROTEIN-DNA INTERACTION
Just as using biology-specific details can help im-
prove analysis, one might be tempted to consider us-
ing other, less-abstract, models of computation to an-
alyze the minimal complexity requirements for DNA-
binding recognition tasks. Such an idea is not without
merit, but we find that if we use other common models
of computation difficulties quickly arise. While they
can model the specific functions of DNA-binding pro-
teins in some ways, they suffer as we try to encompass
additional aspects of the interactions. We outline two
such models with a discussion of their application and
shortcomings below.
6.1 Deterministic Finite Automaton
Following the standard construction (Sipser et al.,
2006), a deterministic finite automaton (DFA) is a
five-tuple (Q, Σ, δ, q
0
, F). Here, Q is a set of states
and q
0
∈ Q is the starting state; Σ is a set of symbols
that may be strung together as inputs to the DFA; the
DFA reads in its input left-to-right one symbol at a
time and moves between states according to the tran-
sition function δ : Q ×Σ → Q; and F ⊆ Q is the set of
accepting states.
6.1.1 Shortcomings
The DFA model fails to capture the way that DNA-
binding proteins read in their input. The model reads
its input left-to-right in one direction. In actual-
ity, proteins move probabilistically along DNA as
they read it; at any point, protein may move left,
move right, jump along the DNA, or even disconnect
entirely from the DNA to end computation prema-
turely (Stanford et al., 2000).
6.2 Sliding Window Protein Automaton
A Sliding Window Protein Automaton (SWPA) is
a tuple (Q, Σ, δ,A, P, w). Recalling other automaton
constructions, Q is a set of states; A ⊆ Q is the set of
accepting states; and the alphabet Σ is a set of sym-
bols. The automaton reads a finite tape containing
symbols in Σ using a sliding window that views a con-
tinuous block of w symbols at a time. The window
begins at a random spot on the tape. P = {p
l
, p
r
, p
x
}
contains the probabilities that, at each step of the au-
tomaton’s run, the automaton’s window will slide one
symbol left, one symbol right, or fall off. (If the au-
tomaton reaches the right end of the tape and slides
further right, or vice-versa, it simply falls off.) Note
BIOINFORMATICS 2020 - 11th International Conference on Bioinformatics Models, Methods and Algorithms
76

that p
l
+ p
r
+ p
x
= 1. Finally, the transition function
δ : Q × {L, R} × Σ → Q describes the state to which
the automaton moves at each step of the computation,
given the direction its window slides and which new
symbol enters the window as it slides. If the protein
ever enters an accepting state, then the computation
stops and accepts. Otherwise the computation runs
until the window falls off.
6.2.1 SWPAs and Distinguishability
The notion of language distinguishability can be used
to find a lower bound on the number of states in a
DFA. We discuss why the traditional distinguishabil-
ity formulation is not well-suited for SWPAs and pro-
vide a new notion of distinguishability more relevant
to SWPAs.
Traditionally, two strings a, b are distinguishable
with respect to a language L if there exists some (po-
tentially empty) string z such that L contains az but
not bz, or vice-versa. Intuitively, this formulation is
useful because if a and b are distinguishable with re-
spect to L, then any DFA accepting L must reach a
different state when it reads a as opposed to b. Thus,
distinguishability lets us establish a lower bound on
the number of states in L.
Distinguishability relies on the assumption that
our automaton reads in strings without ever chang-
ing its read direction. Since this assumption holds for
DFAs, distinguishability is a powerful tool for DFA
analysis; however, the assumption does not hold for
SWPAs, so this form of distinguishability is not use-
ful for that context. Intuitively, if az is in L while bz is
not, then just knowing that a string ends with z is not
enough to say whether or not it is in L. So, as a DFA
for L reads in the string az moving left to right, the
DFA must somehow “remember” that the string be-
gan with a instead of b, but it cannot read the string in
reverse to check. The DFA “remembers” by encoding
a and b in different states. However, SWPAs do not
read in strings in one direction: at every step of com-
putation, their sliding window may move left or right,
and this movement is probabilistic. Thus, we cannot
distinguish two strings by appending a common suf-
fix to them both—in general, we cannot guarantee that
the SWPA will read the suffix! Since SWPAs do not
read strings in one direction, we must reformulate our
notion of distinguishability to apply it to SWPAs.
For an SWPA, two strings a, b are distinguishable
if there exists a list of SWPA instructions i ∈ {(d, σ) |
d ∈ {l, r}, σ ∈ Σ} such that, if we begin with our win-
dow containing a and perform i in order, we end up in
an accepting state, whereas if we perform the same
process starting with our window containing b, we
end up in a rejecting state (or vice-versa).
6.2.2 Shortcomings
This model aims to capture information about the
complexity of proteins’ binding sites. However, in its
present form, it fails to encode information available
to the binding site, and it acts upon information that
the binding site does not have access to.
The model is capable of storing information that
the binding site cannot. In general, after a protein’s
binding site tests and rejects a segment of DNA, the
protein does not store a “memory” of this rejection.
On the other hand, memory is a central aspect of our
SWPA model. Whenever the SWPA slides along its
tape, it undergoes a state transition. Unless every state
in the automaton has a transition to every other state,
and those transitions are all be taken regardless of the
sliding window’s current contents (which, by SWPAs’
determinism, cannot hold unless SWPAs only have
one state), then the automaton’s present state will al-
ways contain some information about where and/or
in what order the sliding window has been on the
tape previously. That is, the SWPA always has ac-
cess to more “memory” than the binding site unless
the SWPA only contains one state—in which case it
either accepts every string (if the state is accepting)
or rejects every string. It seems, then, that although
the SWPA model captures the way proteins move be-
tween steps of computation, it does not suitably de-
scribe how the proteins perform each step of the com-
putation.
7 CONCLUSION
Our work here concerns recognition problems, in
which recognizers evaluate configurations according
to constraints. The constraints are equivalent to sets of
logical statements, and as such, recognition problems
can also naturally be framed in terms of structures
such as decision trees or circuits. Using information-
theoretic results related to search and general memory
capacity, we derived bounds on the complexity of rec-
ognizers, bounds which limit the complexity of tasks
they can perform. The information capacity of recog-
nizers must at least equal the information burden of a
task for the recognizer to be able to perform it. Thus,
we can use the information burden to lower-bound the
combinatorial complexity of an abstract recognition
system, if we know that the task is performed by the
system in question. Finally, we suggest DNA-binding
as a simple biological application of our framework,
and explain how other computational models fail in
various ways for this particular application.
Minimal Complexity Requirements for Proteins and Other Combinatorial Recognition Systems
77

ACKNOWLEDGEMENTS
This work was financially supported by the NSF un-
der Grant No. 1659805, Harvey Mudd College, the
Rose Hills Foundation, and by a grant from the Walter
Bradley Center for Natural and Artificial Intelligence.
We would like to thank Karl Haushalter, Steve
Adolph, Eliot C. Bush, Jae Hur, Douglas Axe, Joe
Deweese, and Matina Donaldson-Matasci for their in-
sights and suggestions on early iterations of this work.
REFERENCES
Abu-Mostafa, Y. and St. Jacques, J. (1985). Information
capacity of the Hopfield model. Information Theory,
IEEE Transactions on, 31(4):461–464.
Barnett, J. H. (2009). Applications of Boolean Alge-
bra: Claude Shannon and Circuit Design. Available
from the webpage http://www.cs.nmsu.edu/historical-
projects.
Bishop, C. M. (2006). Pattern Recognition and Ma-
chine Learning (Information Science and Statistics).
Springer-Verlag New York, Inc., Secaucus, NJ, USA.
Branden, C. I. and Tooze, J. (1999). Introduction to Protein
Structure. Garland Science. 2nd Edition.
Brocchieri, L. and Karlin, S. (2005). Protein length in eu-
karyotic and prokaryotic proteomes. Nucleic acids re-
search, 33(10):3390–3400.
Dembski, W. A. and Marks, R. J. (2009). Bernoulli’s princi-
ple of insufficient reason and conservation of informa-
tion in computer search. In 2009 IEEE International
Conference on Systems, Man and Cybernetics, pages
2647–2652. IEEE.
Fujii, Y., Shimizu, T., Toda, T., Yanagida, M., and
Hakoshima, T. (2000). Structural basis for the diver-
sity of dna recognition by bzip transcription factors.
Nature Structural & Molecular Biology, 7(10):889.
Ho, Y.-C. and Pepyne, D. L. (2002). Simple explana-
tion of the no-free-lunch theorem and its implica-
tions. Journal of optimization theory and applications,
115(3):549–570.
Ho, Y.-C., Zhao, Q.-C., and Pepyne, D. L. (2003). The No
Free Lunch theorems: Complexity and Security. IEEE
Transactions on Automatic Control, 48(5):783–793.
Jaynes, E. T. (2003). Probability theory: The logic of sci-
ence. Cambridge university press.
Korasick, D. and Jez, J. (2015). Protein domains: Structure,
function, and methods. In Bradshaw, R. A. and Stahl,
P. D., editors, Encyclopedia of cell biology. Academic
Press.
McLaughlin Jr, R. N., Poelwijk, F. J., Raman, A., Gosal,
W. S., and Ranganathan, R. (2012). The spatial ar-
chitecture of protein function and adaptation. Nature,
491(7422):138.
Milo, R. and Phillips, R. (2015). Cell Biology by the Num-
bers. Garland Science.
Mitchell, M. (2009). Complexity: A Guided Tour. Oxford
University Press.
Monta
˜
nez, G. D. (2017). The Famine of Forte: Few Search
Problems Greatly Favor Your Algorithm. In Systems,
Man, and Cybernetics (SMC), 2017 IEEE Interna-
tional Conference on, pages 477–482. IEEE.
Monta
˜
nez, G. D. (2017). Why Machine Learning Works.
PhD thesis, Carnegie Mellon University.
Montanez, G. D., Hayase, J., Lauw, J., Macias, D.,
Trikha, A., and Vendemiatti, J. (2019). The Futil-
ity of Bias-Free Learning and Search. arXiv preprint
arXiv:1907.06010.
Pauling, L. and Corey, R. B. (1951). Atomic coordinates
and structure factors for two helical configurations
of polypeptide chains. Proceedings of the national
academy of sciences of the United States of America,
37(5):235.
Pauling, L., Corey, R. B., and Branson, H. R. (1951). The
structure of proteins: two hydrogen-bonded helical
configurations of the polypeptide chain. Proceedings
of the National Academy of Sciences, 37(4):205–211.
Ponomarenko, E. A., Poverennaya, E. V., Ilgisonis, E. V.,
Pyatnitskiy, M. A., Kopylov, A. T., Zgoda, V. G.,
Lisitsa, A. V., and Archakov, A. I. (2016). The size
of the human proteome: the width and depth. Interna-
tional journal of analytical chemistry, 2016.
Quinlan, J. R. (1986). Induction of Decision Trees. Ma-
chine learning, 1(1):81–106.
Shannon, C. E. (1938). A Symbolic Analysis of Re-
lay and Switching Circuits. Electrical Engineering,
57(12):713–723.
Shannon, C. E. (1948). A Mathematical Theory of Com-
munication. Bell system technical journal, 27(3):379–
423.
Sipser, M. et al. (2006). Introduction to the Theory of Com-
putation, volume 2. Thomson Course Technology
Boston.
Stanford, N. P., Szczelkun, M. D., Marko, J. F., and Halford,
S. E. (2000). One-and three-dimensional pathways for
proteins to reach specific dna sites. The EMBO Jour-
nal, 19(23):6546–6557.
BIOINFORMATICS 2020 - 11th International Conference on Bioinformatics Models, Methods and Algorithms
78
