
Using the Virtual Chemical Laboratories in Teaching the Solution of
Experimental Problems in Chemistry of 9th Grade Students While
Studying the Topic “Solutions”
Pavlo P. Nechypurenko
1 a
, Tetiana V. Selivanova
1 b
, Maryna P. Chernova
1
, Olga O. Evangelist
1
,
Yevhenii O. Modlo
2
c
and Vladimir N. Soloviev
1 d
1
Kryvyi Rih State Pedagogical University, 54 Gagarin Ave., Kryvyi Rih, 50086, Ukraine
2
State University of Economics and Technology, 5 Stepana Tilhy Str., Kryvyi Rih, 50006, Ukraine
Keywords:
Experimental Problems in Chemistry, Virtual Chemical Laboratories, Solutions, Learning Research Activity.
Abstract:
The article discusses the importance of student research activities for the effective formation of the key com-
petencies of a future specialist in the field of chemistry, the importance of the skills of primary school students
to solve experimental problems in chemistry and the conditions for the use of virtual chemical laboratories in
the process of the formation of these skills. The concept of “experimental chemical problem” was analyzed.
The essence of the concept of “virtual chemical laboratories” is considered and their main types, advantages
and disadvantages that define the methodically reasonable limits of the use of these software products in the
process of teaching chemistry, in particular, to support the educational chemical experiment are described.
The main advantages and disadvantages of the virtual chemical laboratories on the modeling of chemical pro-
cesses necessary for the creation of virtual experimental problems in chemistry are analyzed. The features of
the virtual chemical laboratory VLab, the essence of its work and the creation of virtual laboratory work in
it are described. It is determined that to support students’ research activities, two types of virtual chemical
laboratories are used: distance and imitation. The combination of these types of virtual chemical laboratories
in the study of the topic “Solutions” provides an opportunity to take advantage of each of them and increase
the level of support for learning research activities of students. Examples of developed virtual chemical works
and their essence are given. Based on the implementation of virtual chemical laboratories in the educational
process of various educational institutions, it is justified the assumption about the effectiveness of using the
developed virtual experimental chemical problems to develop students’ research activities when studying the
topic “Solutions”.
1 INTRODUCTION
Electronic learning tools are widely used in the edu-
cational process of teachers from different disciplines,
but it is in the chemistry lessons of their use that is
perhaps the most appropriate. A chemist should not
so much accumulate knowledge as discover some-
thing new. Electronic learning tools, in particular vir-
tual chemical laboratories, can bring the process of
knowledge of chemical laws to a qualitatively new
level: to facilitate the involvement of all participants
in the educational process in active search and re-
a
https://orcid.org/0000-0001-5397-6523
b
https://orcid.org/0000-0003-2635-1055
c
https://orcid.org/0000-0003-2037-1557
d
https://orcid.org/0000-0002-4945-202X
search activities, self-expression; to ensure the forma-
tion of critical and associative thinking, imagination;
promote the development of the ability to argue, ana-
lyze data, justify and argue the conclusions.
One of the important means of developing chem-
ical thinking and checking the strength of learning is
the experimental problems in chemistry. However,
now this kind of problems is practically not used in
the educational process at school, but it is used at high
levels olympiads in chemistry. One of the reasons for
this phenomenon is the lack of time for the organi-
zation of experimental problems, the risk associated
with possible harm to the health of students, the in-
sufficient provision of schools with chemical reagents
and equipment, and the like. Virtually all of the above
problems can be solved with the help of appropriate
means and tools of information and communication
Nechypurenko, P., Selivanova, T., Chernova, M., Evangelist, O., Modlo, Y. and Soloviev, V.
Using the Virtual Chemical Laboratories in Teaching the Solution of Experimental Problems in Chemistr y of 9th Grade Students While Studying the Topic "Solutions".
DOI: 10.5220/0010924100003364
In Proceedings of the 1st Symposium on Advances in Educational Technology (AET 2020) - Volume 1, pages 319-335
ISBN: 978-989-758-558-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
319

technology (ICT).
That is why the purpose of our work is to deter-
mine the capabilities of the virtual chemical laborato-
ries to ensure the possibility of solving experimental
problems in chemistry and developing the appropriate
set of virtual computer problems.
To achieve this goal it is necessary to solve the
following tasks:
• to analyze the concept of “experimental problem
in chemistry” and find out the meaning and place
of experimental problems in the school chemistry
course;
• analyze the opportunity of using virtual chemical
laboratories in pre-profile training;
• to find out the advantages and disadvantages of
using different types of virtual chemical laborato-
ries in the creation and implementation of virtual
chemistry problems;
• apply the results of research in practice in the form
of creating a set of virtual experimental chemistry
problems for students in grade 9;
• to analyze the results of the virtual chemical lab-
oratories introduction in the process of studying
chemistry (topics “Solutions” in 9 grades).
2 THEORETICAL
FOUNDATIONS OF USING
VIRTUAL CHEMICAL
LABORATORIES IN
TEACHING THE SOLUTION OF
EXPERIMENTAL PROBLEMS
IN CHEMISTRY AND
DEVELOPMENT OF
STUDENTS’ LEARNING
RESEARCH SKILLS WHILE
STUDYING THE TOPIC
“SOLUTIONS”
2.1 Experimental Problems as a Means
of Teaching Chemistry
Chemistry is an experimental science, and that is why
a chemical experiment in student’s develops a chem-
ical style of thinking – the ability to understand the
essence of chemical processes, their significance and
how to manage them. The modern pedagogical pro-
cess should be aimed at the child’s mastering the very
techniques, methods, ways of thinking, that is, the
student must master the technology of carrying out
appropriate mental actions.
From the studies of famous teachers, didactists,
psychologists, the formation of learning abilities is a
complex process, the essence of which is to create op-
portunities for performing work related to learning.
In particular, the competence-based approach (Modlo
et al., 2018) focuses on the acquisition of skills, expe-
rience, and practical application of acquired knowl-
edge in chemistry. Therefore, despite the fact that
the content of educational material in chemistry is di-
rected to students mastering practical skills in work-
ing with substances, provides for observation and
experiment, solving computational and experimental
problems, establishing causal relationships, the use
of algorithms helps students in solving a number of
problems, over time, develop into the ability to solve
life problems (Savchyn, 2015).
Thanks to the educational chemical experiment,
students acquire practical experience in obtaining
facts and their preliminary synthesis at the level
of empirical concepts, concepts and laws. Under
such conditions, the chemical experiment performs
the function of the method of educational cognition,
thanks to which new connections and relationships
are formed in the consciousness of the student, per-
sonal knowledge is formed. It is because of the educa-
tional chemical experiment that the activity approach
to teaching chemistry is effectively implemented. But
it is impossible to carry out an experiment without
first considering the result and not drawing up an ac-
tion plan. That is why the experimental problem solv-
ing as a kind of simulator are offered to students.
The solution of chemical problems is an impor-
tant aspect of mastering the knowledge of the basics
of chemical science. The inclusion of tasks in the ed-
ucational process allows the following didactic teach-
ing principles to be implemented: 1) ensuring the in-
dependence and activity of students; 2) the achieve-
ment of the strength of knowledge and skills; 3) im-
plementation of the connection of learning with life;
4) the implementation of polytechnic chemistry train-
ing, vocational guidance (Zarubko, 2015).
The ability to solve problems develops in the pro-
cess of learning, and this skill can be developed only
in one way – to solve problem constantly and system-
atically.
Algorithmic actions of students in solving chemi-
cal problems in most cases is not at all in strict adher-
ence to a specific procedure, guaranteed to lead to the
correct result. But the learning algorithm, according
to Savchyn (Savchyn, 2015), first of all means a cer-
tain variability of actions in search of the optimal way
to solve the problem. In many cases, this variation
AET 2020 - Symposium on Advances in Educational Technology
320

in the course of isolation is inherent in experimental
chemical problems.
Among the arsenal of chemistry teaching meth-
ods occupies a special place by the solution of exper-
imental problems in the classroom and the execution
of home experiments by students. Experimental prob-
lems are problems whose solution is accompanied by
experiments. Pak (Pak, 2015) considers experimen-
tal chemical problems as a type of cognitive problems
in chemistry. In contrast to laboratory work, students
solve experimental problems on their own without ad-
ditional instructions from the teacher. All students’
work in solving experimental problems is built on an
attempt to apply acquired theoretical knowledge and
practical skills to solve a specific problem in condi-
tions are close to real. In its content, the experimental
problems can be directed to:
• observation and explanation of phenomena;
• preparation of solutions;
• execution of characteristic and qualitative reac-
tions;
• recognition of substances.
You can also give another classification of exper-
imental problems, according to which they are based
on the activities of (Brajko and Mushkalo, 1982):
• familiarization with the properties of substances;
• determining the qualitative composition of sub-
stances;
• separation of mixtures;
• phased conversion of substances;
• determination of the quantitative composition of
substances, mixtures;
• release of substances from the mixture in its pure
form;
• quantitative problems on the laws of conservation
of mass of substances and the stability of their
composition;
• preparation of solutions of a given concentration
and determination of the concentration of an un-
known solution.
To solve any experimental problem, a certain
sequence of actions is characteristic (Grygorovych,
2016):
1) drawing up an experiment plan (action algorithm),
within which it is necessary to determine which
specific question should be answered and which
experiments should be carried out for this pur-
pose;
2) the implementation of the experimental part;
3) the formulation of conclusions about the possibil-
ity of using the obtained experimental data to an-
swer the question posed, and reasonable evidence
or refutation of the initial assumptions.
Experimental problems in chemistry can be solved
by the following methods: analytical-synthetic, hy-
potheses, and attempts. But mainly experimental
problems in chemistry are solved by the analytical-
synthetic method.
The use of experimental problems in the educa-
tional process allows us to solve a number of impor-
tant pedagogical problems, in particular, to develop
students’ creative abilities and the ability to analyze
the condition of the problem and select an experimen-
tal model, improve the skills of applying the laws of
chemistry, and the like (Brajko and Mushkalo, 1982).
The choice of problem solving method depends on
the students having theoretical knowledge and practi-
cal skills.
Students should be taught to choose a rational way
of solving experimental problems. At the same time,
students form the ability to analyze problems, make
plans for decisions and reports.
In the class of studying new educational material,
experimental problems can be used in various aspects:
at the beginning of a lesson, to nominate a problem
and arouse students’ cognitive activity; during the les-
son – in the study of the chemical properties of sub-
stances or substances; at the end of the lesson - to
consolidate new knowledge.
In the lesson of consolidation of knowledge and
the formation of practical skills, experimental prob-
lems can be used at its different stages in order to
teach students to apply their knowledge to solve prac-
tical problems, or to study the device and the principle
of the device and acquire the ability to use it.
In the lessons of generalization and deepening of
knowledge, solutions to experimental problems are
organized to specify the content of physical concepts
and to establish new methods for measuring physical
quantities and establishing new information about the
phenomenon studied.
In knowledge control lessons, solving experimen-
tal problems will help test students’ ability to apply
knowledge in familiar and unfamiliar situations, an-
alyze facts and take a critical look at the results of a
chemical experiment.
At the lessons of control and accounting of stu-
dents’ knowledge, as well as at the lessons of gener-
alization and deepening of knowledge, a significant
part of the lesson and even the entire lesson can be
devoted to solving experimental problems. It is ad-
visable to solve complex problems, in particular the
combined ones, which require knowledge of various
Using the Virtual Chemical Laboratories in Teaching the Solution of Experimental Problems in Chemistry of 9th Grade Students While
Studying the Topic "Solutions"
321

sections of chemistry.
The ability to solve problems is one of the main
indicators of the level of students’ mastery of knowl-
edge in chemistry. However, students often can-
not solve a difficult task, although they discover the
knowledge of theoretical material, they know the def-
inition, the basic formulas, the laws, and solve stan-
dard problems. The reason is that students are used
to solving typical tasks, and problems of an unknown
type cause them to be confused (Mukan, 2002). The
tasks are useful, as a result of which students get
new information or acquire skills, tasks that make you
think logically, based on theoretical knowledge, but
with a creative approach. These criteria are exactly
the experimental problems.
Selecting experimental problems, it is necessary
to take into account the age of students, their psycho-
logical characteristics and the level of knowledge in
chemistry. Experimental problems are highly effec-
tive when students have sufficient knowledge of the
relevant material. The form of the problem statement
should be convenient for solving at each stage of the
lesson.
Today there are many manuals and periodicals in
which you can find a selection of experimental prob-
lems on a particular topic and are ready to solve them.
However, the current trend is the introduction of infor-
mation technology training in the process of forma-
tion of the subject competence of students. It can be
said with confidence that students’ performance of ex-
perimental problems using information and commu-
nication technology tools will be more interesting for
students and more productive (Brajko and Mushkalo,
1982).
2.2 Development of Students’ Learning
Research Skills While Studying the
Topic “Solutions”
The educational institution must prepare a student
who thinks creatively, has theoretical and fundamen-
tal knowledge, appropriate skills for the independent
work and the ability to process and explain the results
of their research.
One of the most important competencies that stu-
dents acquire in the learning process is research com-
petence – it is the formed quality of personality, which
is ex-pressed in the mastery of knowledge, skills and
methods for the effective research and the ability of
independently acquire new knowledge (Mindeyeva,
2010; Nechypurenko et al., 2016; Leshchenko et al.,
2021).
The formation of students’ research competence
takes place in the process of independent creative re-
search activity and is a necessary condition for the
professional development and self-improvement of
the individual. Learning research activity is practi-
cally the only means for the formation and develop-
ment of research competencies.
Modern specialized education should initiate and
develop the individual’s ability to carry out research
activities, higher education institutions – to consoli-
date and deep these skills, as well as bring them to
the highest level – the ability to conduct independent
research.
Thus, research skills should be formed in school,
which takes place in the form of the learning research
activities. This is done by involving students to the
implementation of the educational research, projects,
introduction to the educational process the elements
of research activities.
Independent acquisition of new knowledge about
the environment is the purpose of learning research
activities, in contrast to the usual educational activi-
ties (explanatory and illustrative).
We are most impressed by the opinion of Nefe-
dova (Nefedova, 2012), who interprets the research
activities of students’ as “the process of solving a
creative problem that does not have the result, based
on mastering the features of the environment through
the scientific methods, during which the translation of
cultural values”.
Therefore, the research is characterized by an ac-
tive cognitive position which is based on the inter-
nal search for answers to any question, through com-
prehension and creative processing of data, action
through “trial and error”, the activation of critical
thinking.
The work on the formation of research skills in
chemistry lessons can be divided into four interrelated
areas (Zabolotnyi, 2007):
1) inclusion of research elements in the structure of
the lesson while studying new material;
2) organization of laboratory and practical work as
research, which will provide an opportunity to in-
crease the level of interest of students in obtaining
and interpreting the results of these works;
3) formulation of homework in the form of research
can diversify this form of work and make it more
interesting;
4) planning and conducting extracurricular activities
(research group, project work), using problems
with active research activities.
The current state of the most schools in Ukraine
does not allow students to carry out research activi-
ties on a large scale – covering the whole classes, and
is implemented, as a rule, only with children in the
AET 2020 - Symposium on Advances in Educational Technology
322

category of “gifted” and, mainly, in the form of ex-
tracurricular activities.
Solutions is the most common objects of students’
research in chemistry. Because the solutions surround
a person in nature, everyday life, industry and other
areas of activity, students get acquainted with them
in childhood. In the course “Natural Science” (5th
grade) this acquaintance is more substantive and sci-
entific. Solutions be-come the main object of study
and research in the 9th grade during the study of the
relevant topic in the course of chemistry (Velychko
et al., 2017).
The chemistry curriculum in 9th grade (Velychko
et al., 2017) provides for solving experimental prob-
lems at this topic, as well as the equations of reac-
tions using solutions with a certain mass fraction of
solute; using of demonstration experiments, labora-
tory experiments, practical work, preparation and de-
fense of educational projects.
Most of these forms of work directly or indi-
rectly contribute to the development and improvement
of learning research skills of students. However, it
should be noted that a number of planned laboratory
experiments and practical work will be performed in
an abbreviated or demonstration form. If we talk
about the development of research skills of students,
then there is a need for additional chemical experi-
ments, which aim to reveal the essence of the phe-
nomena studied, to provide students with a creative
approach to solving research problems, to consoli-
date theoretical knowledge through multiple empiri-
cal confirmation.
The most important and most complex semantic
parts of this topic are the solubility of substances,
its dependence on various factors; saturated and un-
saturated, concentrated and diluted solutions; ther-
mal phenomena accompanying the dissolution of sub-
stances; the concept of crystal hydrates; electrolytic
dissociation. Therefore, the learning research activi-
ties should be directed to the study of these semantic
parts of the topic.
The topic “Solutions” is the central in the study of
chemistry, because it is inter-twined with important
sections of inorganic and organic chemistry, chemi-
cal technology; the processes of dissociation, ion ex-
change reactions and other types of reactions are also
somehow related to this topic.
The prevalence and availability of solutions also
makes them as the unique object for students’ learning
research activities. A significant number of classes at
this topic can be organized in the form of educational
research, both laboratory and home (applied).
While studying the topic “Solutions”, students
acquire skills in working with chemicals, chemical
equipment (including measuring equipment), the abil-
ity to observe, measure, calculate. At the same time,
learning research activities provide an opportunity to
do this at a better level, while developing the ability to
make assumptions, build algorithms for testing them,
conduct experiments and formulate conclusions.
The problems of effective organization of the
learning research activities of students while studying
the topic “Solutions” are:
• insufficient time to conduct a large number of
different learning experiments (especially long-
term);
• imperfections in the material support of school
chemical laboratories (lack of scientific equip-
ment, potentially dangerous substances and pre-
cursors, insufficient number of utensils, etc.);
• limitations related to the physical abilities and
health of individual students, features of psychical
and mental development, cognitive activity, etc.
2.3 Virtual Chemical Laboratories as a
Tools of Teaching Chemistry
When studying chemistry at school, one of the most
difficult tasks facing the teacher is to familiarize stu-
dents with real chemical objects and processes. This
difficulty is due to the simplicity and lack of equip-
ment in school chemical laboratories, restrictions on
the use of certain chemical compounds in them, re-
duction of time to study certain topics in curricula,
and the like.
A solution to these problems is to use information
and communication technologies in the educational
process, in particular spreadsheets (Semerikov et al.,
2018), augmented reality tools (Nechypurenko et al.,
2018; Kharchenko et al., 2021; Midak et al., 2021)
and virtual chemical laboratories (VCL) (Nechy-
purenko et al., 2019; Lytvynova and Medvedieva,
2020).
According to Trukhin (Trukhin, 2002), a virtual
laboratory “is a hardware-software complex that al-
lows experiments to be carried out without direct con-
tact with a real installation or in the complete absence
of it. In the first case, we are dealing with a so-called
laboratory setup with remote access, which includes
a real laboratory, software and hardware to control
the installation and digitization of the data, as well
as means of communication. In the second case, all
processes are modeled using a computer”.
So, under the virtual laboratories understand two
types of software and hardware systems (Trukhin,
2002):
Using the Virtual Chemical Laboratories in Teaching the Solution of Experimental Problems in Chemistry of 9th Grade Students While
Studying the Topic "Solutions"
323

• laboratory installation with remote access (remote
laboratories);
• software that allows to simulate laboratory exper-
iments – virtual laboratories (in the narrow sense).
Thus, we can distinguish two types of virtual lab-
oratories: remote and simulation.
Remote virtual chemical labs provide remote ac-
cess to real lab equipment either in real time or by
playing relevant videos. The remote virtual labora-
tory includes:
1) a real laboratory with real equipment and
reagents;
2) software and hardware for control of the corre-
sponding equipment and digitization of the re-
ceived data;
3) tools of communication to connect users with the
first two components.
Virtual laboratories, in which the relevant equip-
ment, substances and processes are modeled using a
computer or other gadgets, are a set of programs de-
signed to simulate laboratory work in the laboratory
(Trukhin, 2002). Simulation virtual chemical labora-
tories can be represented by a set of immutable mod-
els, as well as mathematical interactive models that
can adequately reflect the effects of various user ac-
tions associated with changes in the conditions of the
experiment, in its results. The main advantage of such
virtual chemical laboratories is the ability to imple-
ment a creative approach to the implementation of vir-
tual experiments by users and the formation of users
a more holistic view of the simulated processes and
phenomena.
Both types of VCL have common advantages:
• no need to purchase expensive equipment and
reagents. Due to inadequate funding, many
school chemical laboratories have old equipment
installed that can distort the results of experiments
and serve as a potential source of danger for stu-
dents. Also, in addition to equipment, consum-
ables and reagents are required, the cost of which
is quite high. It is clear that computer equipment
and software are also expensive, but the univer-
sality of computer equipment and its wide distri-
bution and availability somewhat compensate for
this disadvantage.
• the possibility of modeling processes, progress or
observations of which are fundamentally impos-
sible in the laboratory. Modern computer tech-
nologies by means of visualization on the moni-
tor screen provide an opportunity to observe pro-
cesses that cannot be observed in real conditions
without the use of additional equipment, for ex-
ample, due to the small size of the observed par-
ticles or difficult to achieve conditions (ultra high
or ultra low temperatures, pressure, etc.).
• the possibility of penetrating into the subtleties
of processes and observing the details of a phe-
nomenon that occurs on a different time scale,
which is important for processes occurring in a
fraction of a second or, on the contrary, last for
several years.
• no immediate danger to the lives and health of stu-
dents. Safety is an important advantage of using
VCL, especially in cases where the work involves,
for example, the use of hazardous chemicals or
devices associated with the use of high tempera-
tures, pressures, electric current, etc.
• saving time and resources for transferring the re-
sults into electronic format.
• the possibility of using VCL for informal educa-
tion and distance learning, is to ensure the possi-
bility of performing laboratory work in chemistry
for the lack of access to school laboratories, in-
cluding when working with children with limited
physical abilities who miss classes due to illness
or under quarantine time.
• the development of skills to find the optimal so-
lution, the ability to transfer the real problem in
model conditions and vice versa.
Perhaps the disadvantage of using virtual chemi-
cal laboratories is that the model objects created by
the computer are completely supplanted by the ob-
jects of the child in the real world. But working with
sign systems is the basis of analytic-synthetic activity,
that is, thinking does not exist outside of abstraction
and symbolization. Also, significant shortcomings of
the VCL are the limited information that they transmit
to various users’ senses, and the inability of students
to develop skills in working with real laboratory ob-
jects.
By the way of visualization, laboratories are dis-
tinguished using two- and three-dimensional graphics
and animation.
Also, virtual laboratories are divided according to
the way they represent knowledge of the subject area.
In one case, virtual laboratories are based on individ-
ual facts, limited to a set of pre-programmed exper-
iments. They represent a specific set of laboratory
studies, compiled in accordance with the curriculum.
Experiments in such virtual laboratories can only be
viewed. Intervention in their course is impossible
(Derkach, 2008).
Otherwise, conducting virtual laboratory experi-
ments is based on a mathematical model of a real
AET 2020 - Symposium on Advances in Educational Technology
324

chemical process. Such virtual laboratories provide
for the possibility of changing the experimental con-
ditions within certain limits and adequately reflecting
these changes in its results. Licensed versions of such
programs, as a rule, provide an opportunity to create
your own laboratory work. Such virtual laboratories
contribute to independent knowledge of the world by
students and provide an opportunity for the teacher to
realize their creative abilities regarding the chemistry
learning process.
The development of VCL, based on mathematical
modeling of real chemical processes, is more com-
plex and time-consuming, but significantly expands
the possibilities of their application (Derkach, 2008).
Examples of such VCL are Yenka Chemistry
(Yenka, 2017), Model ChemLab (Model Science,
2019) and Virtual Lab (VLab) (ChemCollective,
2018). The only virtual chemical laboratory that
meets these requirements and is freely available is the
Virtual Lab, so we decided to implement the develop-
ment of a set of experimental problems in it.
Any of the VCL is only a model of the real world,
and therefore, like any other model, there is a cer-
tain limitation, simplicity. Different virtual chemical
laboratories have a different level of simplicity com-
pared to real chemical laboratories: different in de-
tail graphic display of objects, lack of transmission of
smells and tactile sensations of objects manipulated
in a virtual environment (Nechypurenko, 2012).
ChemCollective Virtual Lab software currently
covers more than 50 exercises and problems that help
in mastering chemical concepts, mainly related to the
study of solutions and the processes that take place in
them (Chemcollective.org, 2018b).
On the other hand, the use of remote virtual lab-
oratories provides an opportunity to observe high-
quality visualization of relevant processes occurring
with real objects – it is possible to conduct high-
quality chemical experiments and perform practical
work or experimental problems of a qualitative na-
ture. However, this type of virtual laboratories, at
least those that are publicly available, do not provide
the opportunity to interfere in the process and perform
quantitative experiments.
Remote virtual laboratories should be used in the
same types of lessons as other virtual chemical labo-
ratories: at the stage of learning or consolidating new
material, as independent or home research, in classes
of relevant electives or groups, and to test students’
knowledge (in the form of experimental problems).
Simulation virtual laboratories have the advantage
over remote ones in the ability to change the experi-
mental conditions many times and perform all the ex-
perimental operations almost instantly (saving time),
the advantage of remote virtual laboratories is a more
realistic reproduction of all details of the experiment.
Thus, in our opinion, it is possible to qualitatively
support the learning research activities of chemistry
students in the study of the topic “Solutions” by com-
bining the capabilities of two types of virtual chemi-
cal laboratories – remote (for qualitative experiments)
and simulation (for quantitative experiments).
In both cases, there is a need to develop their own
laboratory works, which will be implemented through
virtual chemical laboratories and will be adapted to
the content of the curriculum for secondary schools
in chemistry (topic “Solutions”, grade 9).
3 METHODICAL BASIS FOR THE
DEVELOPMENT OF A SET OF
EXPERIMENTAL PROBLEMS
IN CHEMISTRY FOR
STUDENTS IN GRADE 9 IN
THE CLOUD-ORIENTED
VIRTUAL CHEMICAL
LABORATORY VLAB
3.1 Features of the Virtual Chemical
Laboratory VLab
The most accessible of the modern VCL, providing
the ability of the user to intervene in the course of a
virtual experiment, as well as the possibility of devel-
oping their own virtual laboratory work is the Virtual
Lab (VLab).
The goal of the VLab virtual chemistry lab, which
is a ChemCollective product, is to create flexible, in-
teractive learning environments in which students can
approach chemistry as practicing scientists.
ChemCollective began with work on the IrYdium
Project’s Virtual Lab in 2000. The project was to cre-
ate training exercises designed to provide interactive,
interesting materials that link chemical concepts with
the real world.
The project leader is Dr. David Yaron, Professor
of Chemistry at Mellon College of Science. Most of
the original exercises included in this virtual lab were
developed by a team at Carnegie Mellon University,
including Yaron, experienced software engineers, stu-
dent programmers, educational consultants, and edi-
tors (Chemcollective.org, 2018a).
Virtual chemical laboratory Virtual Lab is free to
install, use and distribute. It can be used both online
(by running the virtual lab plugin from the Chem-
Using the Virtual Chemical Laboratories in Teaching the Solution of Experimental Problems in Chemistry of 9th Grade Students While
Studying the Topic "Solutions"
325

Collective website using any browser) or locally by
downloading the installation files and installing the
program on the computer.
Virtual Lab can also be integrated with the Moodle
system using a special plugin. This makes it possible
to apply the individual tasks of the virtual lab directly
to the specific topics of the Moodle course (Nechy-
purenko and Semerikov, 2017).
In each assignment of the virtual chemical labo-
ratory VLab, access to chemical reagents, which may
include general purpose reagents or compounds spe-
cific for a given job, as well as chemical glassware
(beakers, conical flasks, graduated cylinders, pipettes,
volumetric flasks of various volumes, also a 50 ml bu-
rette and plastic cup) and equipment (Bunsen burner,
weighing hook and scales).
A separate panel of the program window is de-
signed to provide information about a substance or a
mixture: name, volume, state of aggregation, amount
of substance (mol or g), concentration (mol/l or g/l),
spectrometer data, pH meter, and thermometer. Some
of these tools can be disabled if this is required by
the condition of the problem, which is solved in this
virtual laboratory (figure 1).
All actions with dishes and substances in it are
performed in drag and drop mode, that is, by sim-
ply dragging objects with the left mouse button. The
same operations, as well as some specific actions, can
be carried out through the menu that appears when
you click on an object with the right mouse but-
ton(Yaron et al., 2010).
The essence of the program is to download cer-
tain problems and solve them experimentally or cal-
culated with the subsequent experimental verification
of the result. There are no restrictions on the number
of attempts to perform experience on restrictions on
the use of certain quantities of reagents and materials.
Using the exercises of the virtual laboratory VLab,
according to its developers, provide the ability to:
• help students who have missed class work in the
laboratory to do an experiment from their personal
computer, without the need to do work under the
supervision of a teacher;
• supplement current work and homework on paper
with exercises that allow students to use chemical
concepts to design and perform their own experi-
ments;
• monitor the correctness of the assignments of stu-
dents (students use a virtual laboratory to check
the results of their own calculations or qualitative
forecasting without risk to their own health);
• to supplement the demonstration experiment con-
ducted in the classroom (teachers first carry out
a demonstration in the classroom so that students
can see the actual chemical processes, and the stu-
dents then study the chemical system and pro-
cesses independently, guided by the problems in
the virtual laboratory).
Virtual Lab software currently includes more than
50 exercises and problems that are designed to assim-
ilate chemical concepts, mainly related to the study of
solutions and processes in them: moths, stoichiome-
try and limiting reagents (problems for excess), den-
sity, dilutions, dissociation constant, acids and bases,
thermochemistry, solubility, chemical equilibrium,
redox processes (Chemcollective.org, 2018b).
The installation package of Virtual Lab contains
thirteen launch files for this program in different lan-
guages, among which Ukrainian since 2014 has been.
Running the local version of the program, as well
as the old online version, required the presence of a
Java plug-in. Recently, this plugin has been blocked
by most browsers and antivirus programs, it requires
separate settings on the system, therefore, in 2017,
the HTML5 version of the VLab was launched on
the ChemCollective website in 2017, which currently
supports only three languages: English, Spanish and
Italian.
On the old version of the ChemCollective site
(http://collective.chem.cmu.edu), you can download
a special problem editor, the Virtual Lab Authoring
Tool, which allows you to both modify existing prob-
lems and develop your own from scratch for the local
version of the program.
In the problem set, included in the standard ver-
sion of the VLab program, most of the virtual works
are oriented to a level higher than the level of the
basic school – core, or college and university. The
content of a certain number of problems is struc-
tured in such a way that all of them are full-fledged
study and research problems (Nechypurenko and Se-
merikov, 2017). Our work was thus aimed at devel-
oping problems that can be classified as experimental
chemical problems on the “Solutions” topic, were co-
ordinated with the curriculum, and at the same time
were available for primary school students in terms of
complexity.
3.2 Creation of Laboratory Work in a
Virtual Laboratory VLab
In order to create your own laboratory work, you need
to understand how this virtual lab works. The virtual
laboratory is launched by running the default.xml file
(or default uk.xml for the Ukrainian version), which
is located in the assignments directory. This is the
default virtual lab file. This file contains individ-
AET 2020 - Symposium on Advances in Educational Technology
326
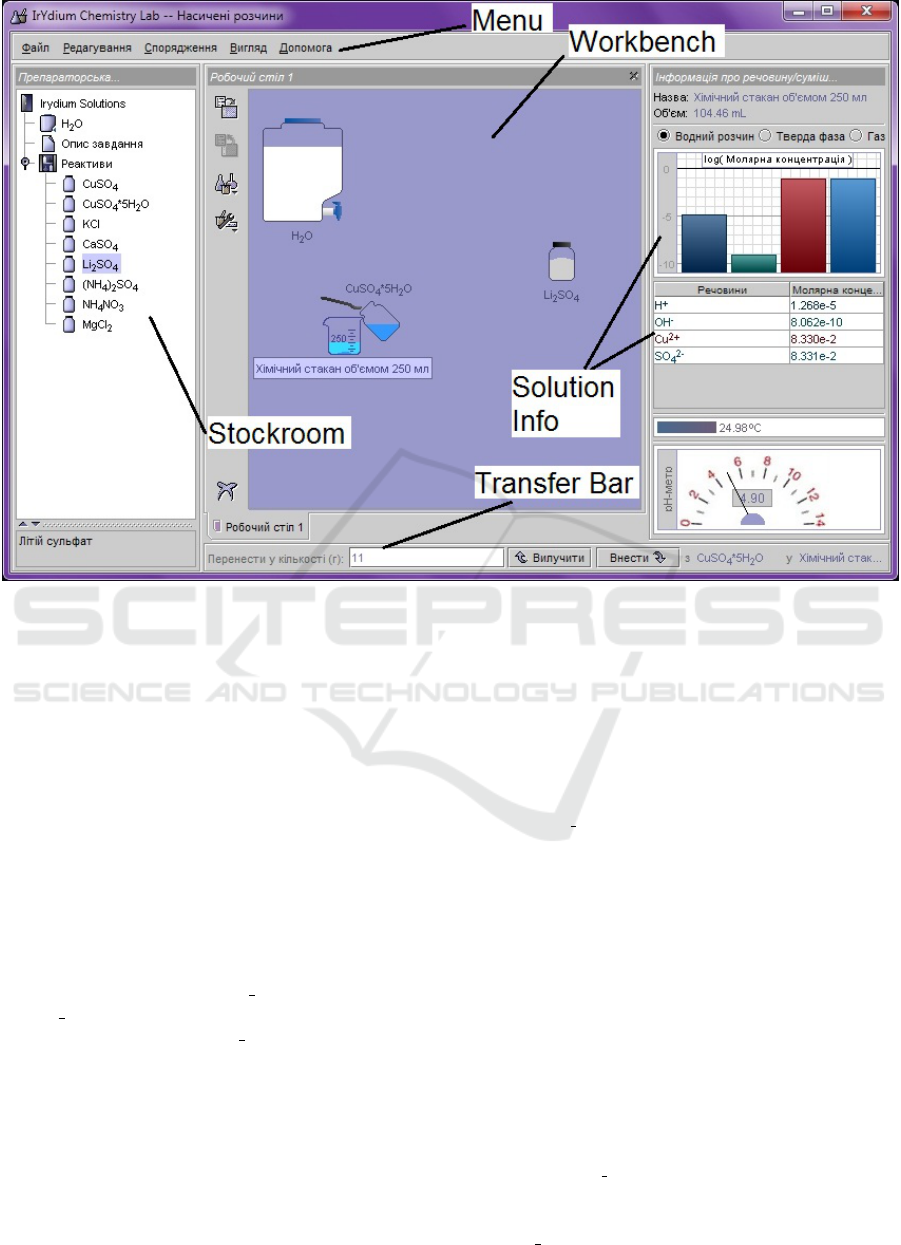
Figure 1: VLab window with virtual laboratory work.
ual properties of the program’s working area: the
availability of tools (thermometer, pH meter, win-
dows with information about the chemical compo-
sition of substances and solutions) and the available
modes of substance transfer (accurate transfer, trans-
fer of rounded quantities and realistic transfer). These
tools and transfer modes can be either available for
work, all or some of them can be turned off depend-
ing on the needs of the problem scenario. Also in this
file are the ways in which the working area of the pro-
gram is filled with reagents, possible physicochemi-
cal processes with their participation, a description of
the work problem, and the like. These default paths
lead to files that are in a subdirectory with the same
name as the control xml file — that is, the files to work
with, are guided by the default uk.xml file, are in the
default uk directory (the path to it is in the program
directory assignments/default uk). The directory ref-
erenced by the control xml file contains typically four
files:
• filesystem.xml – contains information about the
solutions (reagents) planned for use in this vir-
tual laboratory work and the dishes in which they
are contained, their volume or mass, and a brief
description of this reagent (name, concentration,
etc.);
• reactions.xml – contains information on all pos-
sible (planned) chemical reactions with a specific
set of substances in this virtual laboratory work;
• species.xml – contains information on all sub-
stances available in this virtual laboratory work
and their properties (color, state of aggregation,
thermodynamic parameters, molar mass, etc.);
• problem description.html – contains a text de-
scription of the problem and instructions for per-
forming virtual lab work.
VLab versions higher than 2.1.0 may also contain
the spectra.xml file, which contains the spectral char-
acteristics of the substances that will be displayed in
the photocolorimeters window, if it is available for use
in this work.
Other laboratory works are started on the same
principle, only the control xml-files are located in sep-
arate thematic sub-subdirectories in the subdirecto-
ries of language localization, for example, the con-
trol xml-file of the localized Ukrainian work “Deter-
mining the solubility of CuCl2 at different tempera-
tures” CuClSolu.xml is located along the path assign-
ments/problems
uk/solubility.
The list of control xml files with the path to them
and a brief description of the work is in the Proble-
mIndex uk.xml file (ProblemIndex.xml for the stan-
Using the Virtual Chemical Laboratories in Teaching the Solution of Experimental Problems in Chemistry of 9th Grade Students While
Studying the Topic "Solutions"
327

dard English version) in the root directory of the pro-
gram. From this file that the list of laboratory works
available for execution is called up via the menu
“File” → “Load problem”.
Any of these files can be edited using Notepad (it
is important to save changes in the UTF-8 encoding)
or any xml file editor. But a more optimal option is
to use the special editor Virtual Lab Authoring Tool.
There are several options for creating a new labora-
tory work: from scratch, editing and saving the de-
fault xml file, and based on another work. The second
way is faster and more rational, since it allows par-
tially (and in some cases, possibly completely) using
those reagents, equipment and other necessary param-
eters of work, since they have already been entered
and are guaranteed to work. To make this change,
open the control xml file in the Virtual Lab Author-
ing Tool editor and select “Save As ...” in the “File”
menu, specify the new file name and its location. In
our case, it was the School catalog, which we created
specifically for this set of works. A directory with
content files is automatically generated.
Henceforth control xml-file in the editor Virtual
Lab Authoring Tool need to edit. The editor window
has several tabs, each of which changes a certain part
of the work data (figure 2):
• General – contains fields for entering the title of
the work, the last name of the author and a brief
description of the content of the work.
• Permissions – contains two tabs: Viewers to spec-
ify the tools for viewing the properties of sub-
stances and their chemical composition will be
available during the work; and Transfer Bars to
determine the substance transfer parameters avail-
able in the job.
• Species – contains tools for creating and editing
substances needed in this work. In addition to the
formula, the molar mass and the name of the sub-
stance, the state of aggregation, as well as its col-
oring parameters, its standard enthalpy of forma-
tion and entropy are obligatory characteristics –
these data will be used to simulate chemical reac-
tions between the corresponding substances.
• Reactions – contains tools for planning the flow
of physicochemical processes, by defining reac-
tive particles as reagents or reaction products, set-
ting appropriate coefficients.
• Stockroom – provides the ability to create and
edit the contents of the “Stockroom” in the virtual
laboratory – add cabinets, dishes with reagents,
accompanying files (description of the problem,
etc.).
At the end of the work in the editor Virtual Lab
Authoring Tool you need to save the changes and
make the created work in the registry of works so
that it becomes available for use. To do this opera-
tion, a block is created in the ProblemIndex uk.xml
file (editing with a notepad or xml editor):
<DIRECTORY name="The name of the block
of laboratory works">
<PROBLEM url="assignments/problems_uk/
school/File_name.xml">
<TITLE>Problem title</TITLE>
<AUTHOR>Autors</AUTHOR>
<DESCRIPTION>
A brief description of the problem
</DESCRIPTION>
</PROBLEM>
</DIRECTORY>
A block limited by <DIRECTORY> ...
</DIRECTORY> tags can contain as many in-
dividual works as desired, each of which is separated
by <PROBLEM> ... </PROBLEM> tags.
Created or edited works become available after the
next program launch.
3.3 A Set of Experimental Chemical
Problems in a Virtual Chemistry
Lab VLab for Use in School When
Studying the Topic “Solutions”
The chemistry curriculum in grade 9 (Velychko et al.,
2017) provides for the solution of experimental prob-
lems on this topic, as well the computational problems
using solutions with a certain mass fraction of solute;
use of demonstration experiments (thermal phenom-
ena during dissolution: dissolution of ammonium ni-
trate and concentrated sulfuric acid in water, studies
of substances and their aqueous solutions for elec-
trical conductivity, exchange reactions between elec-
trolytes in aqueous solutions) conducting laboratory
studies (detection of hydrogen and hydroxide ions
in solutions, established approximate pH values of
water, alkaline and acidic solutions (sodium hydrox-
ide, hydrochloric acid) using a universal indicator, pH
studies search and cosmetic products, the exchange
reaction between electrolytes in aqueous solutions,
accompanied by precipitation, the exchange reaction
between electrolytes in aqueous solutions, accompa-
nied by the evolution of gas, the exchange reaction
between electrolytes in aqueous solutions, followed
by water absorption, the detection of chloride, sulfate
and carbonate ions in solution) carrying out practical
work (ion exchange reactions between electrolytes in
aqueous solutions) of executing a home experiment
AET 2020 - Symposium on Advances in Educational Technology
328
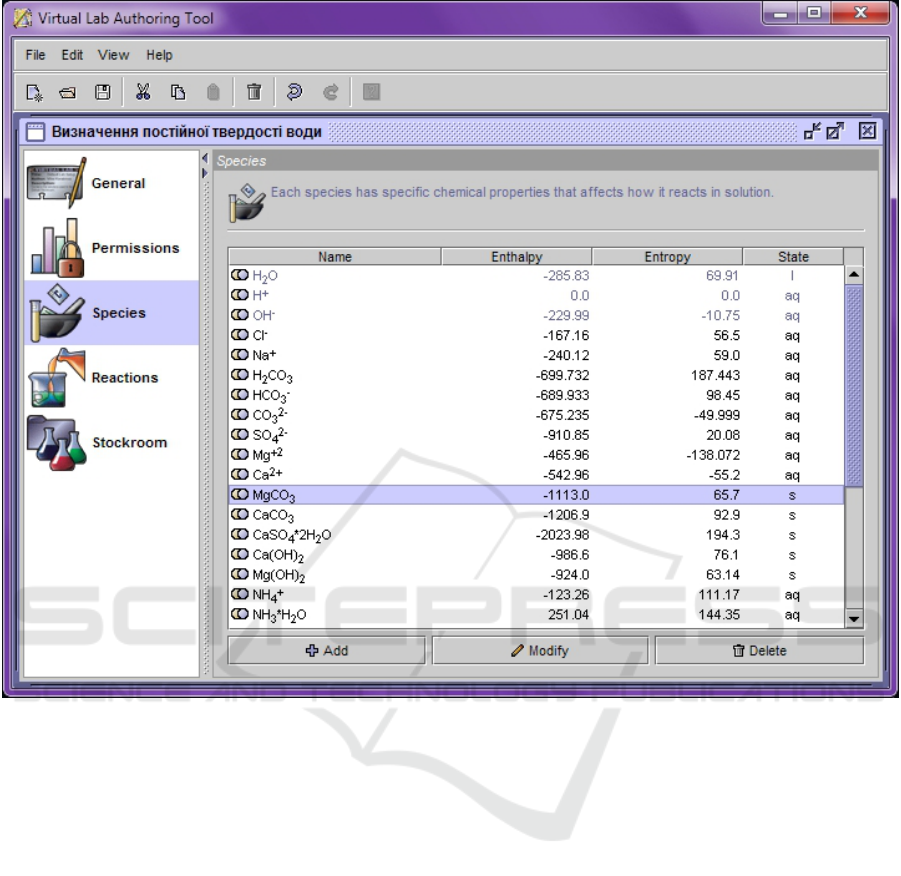
Figure 2: Editor Virtual Lab Authoring Tool window.
(preparing colloidal solutions (jelly etc.)), preparation
and protection of educational projects (“Electrolytes
in modern accumulators”, “Growing of crystals of
salts”, “Production of solutions for provision of med-
ical assistance”, “Research of soil pH of the area”,
“Investigation of the influence of acidity and alkalin-
ity of soils on plant development”, “Research pH of
atmospheric precipitation and their influence on var-
ious materials in the environment”,“Investigation of
natural objects as acid-basic indicators”, “Investiga-
tion of the pH of the mineral water of Ukraine”).
The most important and most complex parts of
this topic are the solubility of substances, its depen-
dence on various factors. Saturated and unsaturated,
concentrated and diluted solutions. Thermal phenom-
ena accompanying the dissolution of substances, dis-
solution as a physical and chemical process, the con-
cept of hydrates, electrolytic dissociation etc. There-
fore, experimental problems should be directed to the
study of precisely these substantive parts of the topic.
After analyzing the technical and visual capabili-
ties of the Virtual Lab, we determined that it would be
most appropriate to create virtual experimental prob-
lems related to the dissolution process (its energy and
quantitative characteristics), the dissociation process
of substances in a solution and determine its pH, as
well as the use of some qualitative reactions, indica-
tors and the like. The problems associated with the
study of the properties of colloidal solutions, the flow
of certain exchange reactions, the extraction of crys-
tals, the study of the analytical effects of qualitative
reactions associated with the formation of precipita-
tion cannot be realized either due to the limited possi-
bilities of modeling chemical phenomena in the VLab
and due to the limitations of visual accompaniment
(for example, to conduct qualitative reactions with the
formation of sediment among the equipment in the
VLab there are not enough test tubes, and the pres-
ence of sediment and its color become noticeable in
a glass x on the desktop of the virtual laboratory only
in quantities of a few grams or more, does not comply
with the principles of qualitative chemical analysis).
Using the Virtual Chemical Laboratories in Teaching the Solution of Experimental Problems in Chemistry of 9th Grade Students While
Studying the Topic "Solutions"
329

Based on all the above, we have created a trial set
of experimental problems on the topic “Solutions”,
which contains seven problems. The works contain
instructions for solving problems and a number of
questions that students need to answer.
For example, the laboratory work “Precursor”
suggests that the student present himself as a labora-
tory technician and carry out dilutions of concentrated
sulfuric acid, which is on the list of precursors. The
task is to prepare equal volumes of solutions with the
indicated concentrations.
In the work “Separation of salt mixture”, it is nec-
essary to separate the mixture of crystalline potassium
chlorate and sodium chloride by recrystallization of
potassium chlorate, based on the difference in the sol-
ubility of these salts. The problem contains the order
of actions that will help to perform the work. The pur-
pose of this problem is to familiarize students with the
methods of purification and separation of substances,
the dependence of the dissolution of salts on temper-
ature.
To demonstrate the preparation of saturated solu-
tions, you can use the work “Preparation of saturated
solutions of various chemical compounds”. Here the
student will be able to prepare solutions by changing
the temperature, and on the basis of the data obtained,
construct curves for the concentration of a saturated
solution of a substance on temperature. The aim of
the work is to study the change in the solubility of
substances from temperature, the formation of skills
in the preparation of saturated solutions, the analysis
of the experimental data.
The study of thermal effects of dissolution can be
carried out in the work “Thermal effects of dissolu-
tion”. In the description, it is reported that during
the dissolution of the substance various physical and
chemical processes take place with both the solute and
the solvent. One of the external indicators that can
be easily fixed is the thermal effect observed when
various substances are dissolved. The task is to in-
vestigate the thermal effects of dissolution of various
crystalline compounds in water and to draw appropri-
ate conclusions and assumptions regarding the pro-
cesses leading to the occurrence of these effects. The
purpose of the work is to form an understanding of
the thermal phenomena that accompany the process
of dissolution and test them in practice, consolidating
knowledge about exo- and endothermic processes.
The overwhelmingly developed problems contain
a sufficient number of hints so that the student can
experiment in a virtual laboratory independently, for
example, on a home computer, and some of the prob-
lems are quite realistic to reproduce in a real school
chemistry laboratory, given the time and possibilities
(in this case problem solving in a virtual laboratory
can be used as a training option to verify the correct-
ness of theoretical calculations and repeat the order
necessary action).
A set of these laboratory works are posted
on the website of the Department of chem-
istry and methods of learning chemistry at the
Kryvyi Rih State Pedagogical University (https:
//kdpu.edu.ua/khimii-ta-metodyky-ii-navchannia/
tsikava-khimiia/dlia-vseznaiok/
5928-virtualna-khimichna-laboratoriia.html) with
the aim of further introducing schools into the educa-
tional process and receiving feedback on improving
the quality and expansion of this set.
4 CREATION AND TESTING OF
A SET OF VIRTUAL
LABORATORY WORKS FOR
THE ORGANIZATION OF
LEARNING RESEARCH
ACTIVITIES OF STUDENTS IN
CHEMISTRY IN THE STUDY
OF THE TOPIC “SOLUTIONS”
Most of the problems in the set developed for the
topic “Solutions” in VLab are formulated in a re-
search (problem) style – the student has a task:
1) to obtain a certain practical result;
2) to study processes and phenomena, the exact
properties of which are unknown to him in ad-
vance.
In the first case, the student has the opportunity
to create their own algorithms and check their ade-
quacy in practice, but in a virtual environment. The
use of trial and error method is not ruled out. In
the second case, completing the problem will mean
for the student the discovery of subjectively new pat-
terns, properties, and so on. That is why, the student
has the opportunity to independently, based on the re-
sults obtained in the virtual chemical laboratory, to
draw conclusions about the influence of the certain
factors on the dissolution process, and only then com-
pare them with those in textbooks described, heard
from the teacher’s story, etc.
Most of the problems contain enough prompts for
the student to experiment in a virtual laboratory on
their own, for example, at a home computer, and some
of the problems can be reproduced in a real educa-
tional chemical laboratory of the school if time and
opportunity (in this case the problem in the virtual
AET 2020 - Symposium on Advances in Educational Technology
330
laboratory can be used as a training option to check
the correctness of theoretical calculations and repeat
the order of necessary actions).
The VLab virtual chemical laboratory provides
the possibility of independent repeated experimenta-
tion with various substances and their solutions, with
the involvement of accurate measuring instruments,
but it is not designed to perform qualitative reactions.
Most qualitative reactions do not require accurate cal-
culations and measurements, but they do require as
clear an analytical effect as possible, not distorted
by the imperfection of the object’s appearance in its
model. For the virtualization of qualitative experi-
ments, qualitative visualization is often more desir-
able than the ability to make accurate measurements.
Since in the topic “Solutions” a certain amount of stu-
dent research is related to qualitative chemical exper-
iments (performing qualitative reactions, determining
the acidity of the environment using indicators, etc.),
there is a need to create a resource to support of quali-
tative chemical experiments. The most realistic trans-
mission of visual information about an object is a
video recording. The essence of the developed remote
virtual chemical laboratory is to provide users with
remote access to a set of substances that can be used
to perform high-quality laboratory experiments. At
the same time, we tried to anticipate various options
for user actions, including those that could have been
done accidentally, without logical justification. To do
this, the program interface is organized in such a way
that the user has two sets of reagents. Any reagent
from the first set can be mixed with any reagent from
the second. Selecting the appropriate pair of reagents
triggers a short video recording of the mixing of these
reagents in a real chemical laboratory. The user can
not change the number of reagents or the order of
their addition, but has the opportunity many times to
observe high-quality visualization, accompanied by a
textual description of the nature of the reaction that
occurs.
The availability of such a virtual chemical labora-
tory can be ensured by placing it on the Internet on
the pages of the site. The window interface of such
a remote virtual chemical laboratory is essentially the
html-page of the site. For the operation of a laboratory
installation with remote access, it is necessary that
the site page contains a set of elements of JavaScript,
video, codes, etc. that relate to a separate laboratory
work (figure 3).
The operation of the remote virtual chemical lab-
oratory created by us is provided by a number of ob-
jects located in different directories:
• the favicons folder contains favicon elements, ie
site icons for different browsers;
Figure 3: Elements of the site of the laboratory installation
with remote access.
• js folder is a folder for saving java script files that
provide dynamic interactivity on the site;
• scss folder contains style files that form the exter-
nal design and stylization of the site page;
• all videos of the experiments that we recorded for
running on the site are saved in the videos folder;
• the index file is the main one, because the main
startup code of the laboratory is written in it.
The following online page of the virtual chemical
laboratory involves the execution of certain program
code, which can be edited by connecting to an FTP
server and launching Notepad++ or xml-editor.
The general principle of the first virtual laboratory
with remote access on the topic “Indicators” is to se-
lect buttons from the upper left corner – the indicator,
and the lower left corner of the solution with a cer-
tain level of acidity, such a combination of pressing
“show” allows you to run videos where the first reac-
tion, change of color of solution is shown (figures 4
and 5).
To return to the indicator and solution selection,
press the “Clear” button in the middle on the left and
start the selection again.
The following laboratory work No 2, created on
the basis of the site, is based on an experimental prob-
lem on “Qualitative reactions to the most common an-
ions”. The general principle of operation is similar to
the virtual chemical laboratory “Indicators” and con-
sists in selecting the buttons from the upper left cor-
ner – solutions of reagents AgNO
3
, Pb(NO
3
)
2
, BaCl
2
,
and the lower left corner - a solution containing an un-
known anion to be determined by students. When you
press the “Show” button, a video is launched, which
shows the course of the chemical reaction between the
selected solutions.
It should be noted that both laboratory works can
be used as research: the “Indicators” lab contains not
only the indicators described in the textbook, but also
non-standard for the school curriculum – bromocresol
purple, congo red, red cabbage juice, and therefore
work with them is easy to organize as a research. The
work ”Qualitative reactions of some anions” is gen-
erally an experimental problem for the recognition of
Using the Virtual Chemical Laboratories in Teaching the Solution of Experimental Problems in Chemistry of 9th Grade Students While
Studying the Topic "Solutions"
331
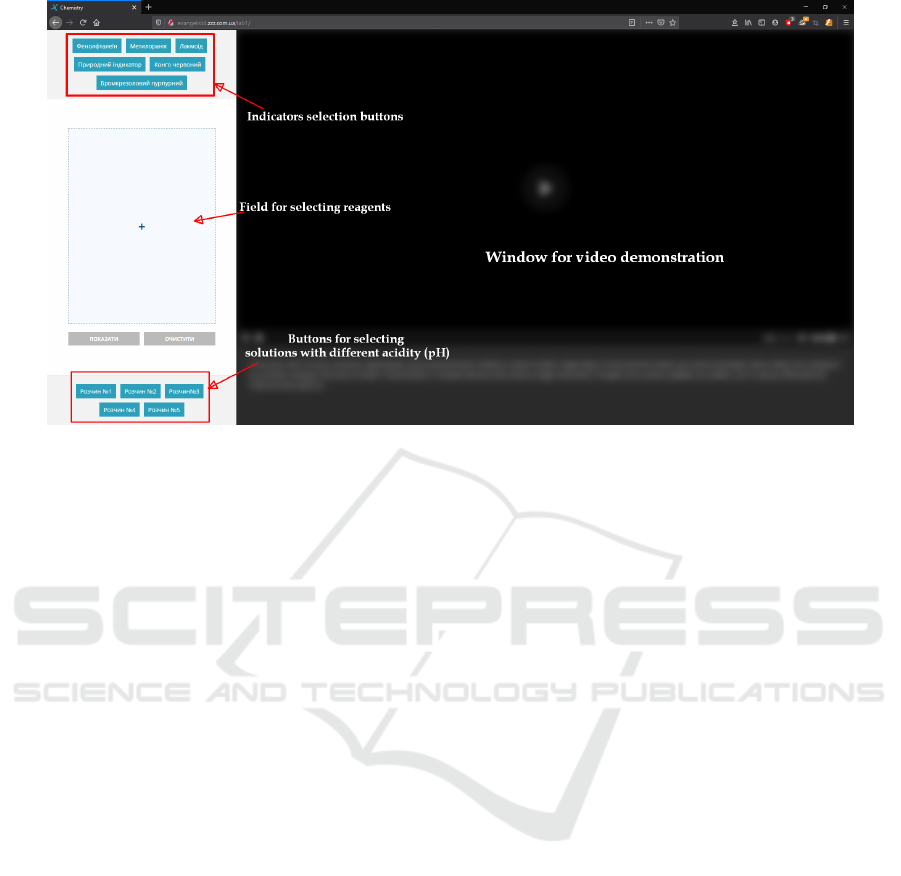
Figure 4: Location of the buttons of the main elements of the remote laboratory.
anions.
Both laboratory works are posted on the
website http://distvlab.easyscience.education/,
where they are available at the links
http://distvlab.easyscience.education/Lab1 and
http://distvlab.easyscience.education/Lab2.
5 RESULTS
The created virtual laboratory works were tested dur-
ing chemistry lessons and optional classes in several
educational institutions of the city of Kryvyi Rih dur-
ing 2019: Kryvyi Rih Central City Lyceum, Kryvyi
Rih Central City Gymnasium, schools No 66, No 21
and Kryvyi Rih College of National Aviation Univer-
sity of Ukraine. To do this, teachers used personal
computers and netbooks, SMART Board interactive
whiteboards, and smartphones and tablets.
Chemistry teachers especially noted the conve-
nience of using virtual chemical laboratories to pre-
pare for laboratory work or their partial replacement,
and to organize effective independent work of stu-
dents.
Students were asked a questionnaire with the fol-
lowing questions:
1. “Were you interested in using virtual chemical
laboratories?”
2. “Was it easy for you to use virtual chemical labo-
ratories?”
3. “Will virtual experiments help you better under-
stand the theoretical material of the topic?”
4. “Did virtual chemistry labs help you better pre-
pare for classroom practice work?”
5. “What did you like most about using virtual
chemistry labs while studying chemistry?”
144 students took part in the survey. The results
of the survey are shown in table 1.
The fifth question with an open answer was of-
ten answers by students, which can be formulated
as: “non-standard approach to the organization of
lessons”, “unusual and novelty of the use of virtual
chemical laboratories”, “the possibility to make ex-
periments without time or strict responsibility for the
quality of individual actions”, “the possibility to inde-
pendently make experiments as you want or interest-
ing”, “the possibility to prepare at home, especially
if you missed the lesson”. According to the obser-
vations of teachers involved in the experiment, the
use of virtual chemistry laboratories increased stu-
dents’ desire to experiment and reduced their fear of
making mistakes during the experiment, making erro-
neous conclusions, and so on. This was evidenced by
the high results demonstrated by students in perform-
ing practical work and experimental problems within
the topic “Solutions”.
Thus, the majority of students noted a positive ef-
fect from the use of VCL primarily for practical work
preparation, as well (a slightly lower percentage) in
the acquisition of theoretical knowledge. For the vast
majority of students, VCL was an interesting means
of chemistry learning (perhaps due to novelty and
non-standard), but a smaller percentage of students
noted the ease of use of VCL, due to the same nov-
elty for students, and therefore lack of skills in using
AET 2020 - Symposium on Advances in Educational Technology
332
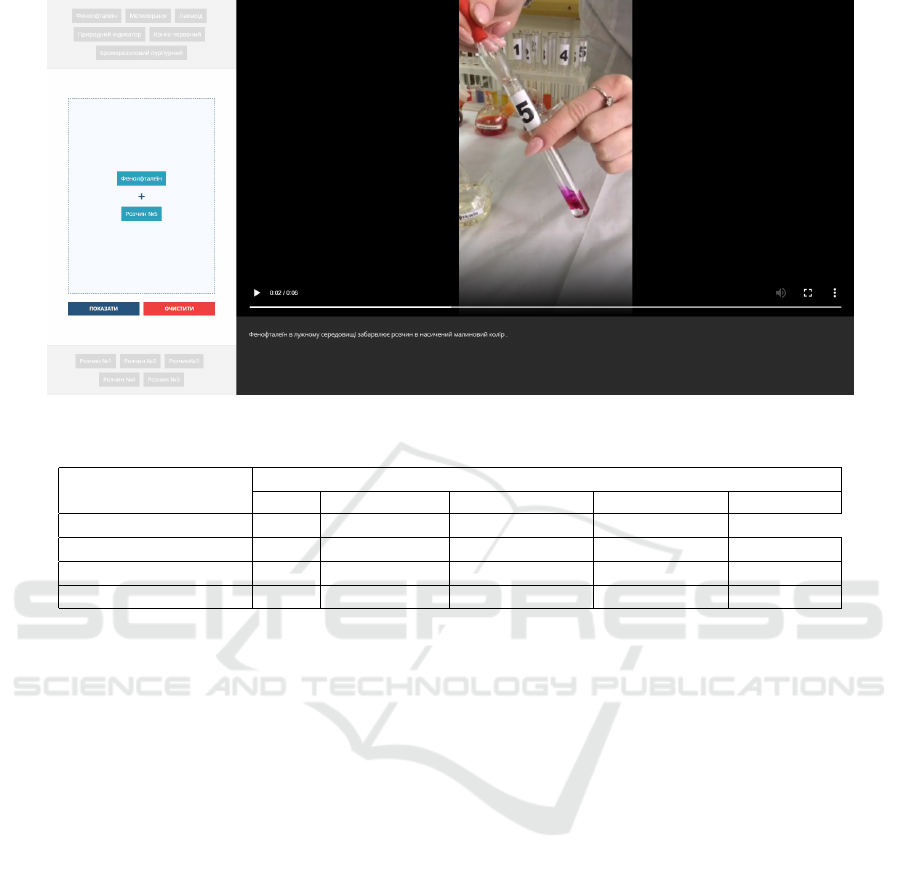
Figure 5: Remote chemical laboratory operation: selected buttons “Phenolphthalein” and “Solution No 5”.
Table 1: The results of student surveys.
Answers to questions
Number of question
“No” “Rather no” “Hard to say” “Rather yes” “Yes”
1 0 11 (7.6%) 52 (36.1%) 81 (56.3%)
2 0 3 (2.1%) 18 (12.5%) 56 (38.9%) 67 (46.5%)
3 0 6 (4.2%) 14 (9.7%) 45 (31.2%) 79 (54.9%)
4 0 4 (2.8%) 13 (9%) 38 (26.4%) 89 (61.8%)
these teaching tools.
6 CONCLUSIONS
1. The learning research activities are an integral part
of a quality educational process, especially in the
study of natural sciences. The learning research
activity differs from ordinary learning in that it
requires an active cognitive position based on the
internal search for answers to any question related
to the understanding and creative processing of in-
formation, action through “trial and error”, and
from scientific research it differs, first of all, in
the results – the acquisition of subjectively new
knowledge, the formation of research skills and
other personality traits of students.
2. One of the varieties of learning research activities
of students in chemistry is experimental chemical
problems – a separate type of chemical problems,
the solution of which is necessarily accompanied
by the practical implementation of a chemical ex-
periment.
3. Experimental chemical problems are character-
ized by the methodological feasibility of their use
in various types of lessons, at different stages of a
lesson and in extracurricular work.
4. One of the most important and integral topics in
the school course of chemistry is the topic “Solu-
tions” – while studying this topic, students consol-
idate knowledge of general and inorganic chem-
istry, acquire skills to perform experiments, gain
theoretical and practical basis for further study of
chemistry.
5. Pre-profile chemistry training contains a signifi-
cant amount of experimental activity of students,
and one of the ways to overcome the contradiction
between the need to carry out a training chemical
experiment and the lack of sufficient time, nec-
essary equipment and reagents, the use of virtual
chemical laboratories — special computer pro-
grams that make it possible to simulate the phys-
ical chemical phenomena or to conduct experi-
ments without direct contact with a real chemicals
set or the complete absence thereof.
6. Virtual chemical laboratories are, first of all,
unique simulators – tools that allow users to test
the algorithm of actions, to trace the logic of
certain laboratory operations during the experi-
ment, to practice skills of collecting and record-
ing the necessary data, experimental results and
Using the Virtual Chemical Laboratories in Teaching the Solution of Experimental Problems in Chemistry of 9th Grade Students While
Studying the Topic "Solutions"
333

more. Remote virtual chemical laboratories have
the advantage of conducting qualitative experi-
ments, and simulation VCL – quantitative chemi-
cal experiments.
7. Virtual chemical laboratories in some cases can
be used as a replacement for a real chemical ex-
periment, if for some reason it’s implementation
is impossible.
8. Virtual chemical laboratories provide an opportu-
nity to safely and economically implement the de-
velopment of research competencies of students
through the use of experimental chemical prob-
lems, which can be performed entirely in virtual
mode or in simulator mode with subsequent im-
plementation in the form of a naturaly experiment.
9. Virtual chemical laboratories are a rather labile
learning tool that can be used at almost any stage
of the lesson: at the beginning, at the stage of
learning new knowledge, at the stage of consol-
idation of knowledge and at the stage of testing,
as well as for independent and homework. In the
case of proper organization of work with them,
the student has the opportunity to perform learn-
ing research at any time and in any place.
10. The best option for quality support of learning re-
search activities of students in chemistry by solv-
ing experimental chemical problems (including
distance learning) in the study of topic “Solu-
tions” is a combination of two types of virtual
chemical laboratories – remote (for qualitative ex-
periments) and simulation (for quantitative exper-
iments).
11. Currently, a set of virtual laboratory works has
been created, consisting of seven problems in the
simulation VCL Virtual Lab and two experimen-
tal problems in the remote VCL. Currently, a set
of virtual laboratory works has been created, con-
sisting of seven problems in the simulation VCL
Virtual Lab and two experimental problems in the
remote VCL.
12. The created virtual laboratory works were intro-
duced into the educational process of several ed-
ucational institutions in Kryvyi Rih during 2019
and received mostly positive feedback from both
chemistry teachers and students. This makes it
possible to say that virtual chemical laboratories
have a high potential for organizing and improv-
ing the learning research activities of students in
chemistry while studying the topic “Solutions”
and need further improvement taking into account
the results of its implementation in the school ed-
ucational process.
REFERENCES
Brajko, V. I. and Mushkalo, N. N. (1982). Experimental
Tasks on Inorganic Chemistry: A Manual for Teach-
ers. Radyanska shkola, Kyiv.
ChemCollective (2018). ChemCollective: Virtual Labs.
http://chemcollective.org/vlabs.
Chemcollective.org (2018a). Chemcollective: Introduction.
http://chemcollective.org/about us/introduction.
Chemcollective.org (2018b). Chemcol-
lective: Introduction for instructors.
http://chemcollective.org/teachers/introforInstructors.
Derkach, T. M. (2008). Information technologies in the
teaching of chemical disciplines. Vydavnytstvo DNU,
Dnipropetrovsk.
Grygorovych, O. V. (2016). Chemistry: A textbook for
Grade 8. Ranok, Kyiv.
Kharchenko, Y. V., Babenko, O. M., and Kiv, A. E.
(2021). Using Blippar to create augmented reality in
chemistry education. CEUR Workshop Proceedings,
2898:213–229.
Leshchenko, M. P., Kolomiiets, A. M., Iatsyshyn, A. V., Ko-
valenko, V. V., Dakal, A. V., and Radchenko, O. O.
(2021). Development of informational and research
competence of postgraduate and doctoral students in
conditions of digital transformation of science and
education. Journal of Physics: Conference Series,
1840(1):012057.
Lytvynova, S. and Medvedieva, M. (2020). Educational
computer modelling in natural sciences education:
Chemistry and biology aspects. CEUR Workshop Pro-
ceedings, 2732:532–546.
Midak, L. Y., Kravets, I. V., Kuzyshyn, O. V., Baziuk,
L. V., and Buzhdyhan, K. V. (2021). Specifics of us-
ing image visualization within education of the up-
coming chemistry teachers with augmented reality
technology. Journal of Physics: Conference Series,
1840(1):012013.
Mindeyeva, E. O. (2010). Organization of learning research
activities in the geography of students of a specialized
school. PhD thesis, Herzen State Pedagogical Univer-
sity of Russia, Sankt-Peterburg.
Model Science (2019). Model Science
Software Products – Model ChemLab.
http://modelscience.com/products.html.
Modlo, Y., Semerikov, S., and Shmeltzer, E. (2018).
Modernization of professional training of electrome-
chanics bachelors: ICT-based Competence Approach.
CEUR Workshop Proceedings, 2257:148–172.
Mukan, L. (2002). Tasks as a factor in the formation of
intelligence of students. Biolohiia i khimiia v shkoli,
(6):16–21.
Nechypurenko, P., Selivanova, T., and Chernova, M. (2019).
Using the cloud-oriented virtual chemical laboratory
VLab in teaching the solution of experimental prob-
lems in chemistry of 9th grade students. CEUR Work-
shop Proceedings, 2393:968–983.
Nechypurenko, P. and Semerikov, S. (2017). VlabEmbed
- the new plugin Moodle for the chemistry education.
CEUR Workshop Proceedings, 1844:319–326.
AET 2020 - Symposium on Advances in Educational Technology
334

Nechypurenko, P., Starova, T., Selivanova, T., Tomilina, A.,
and Uchitel, A. (2018). Use of augmented reality in
chemistry education. CEUR Workshop Proceedings,
2257:15–23.
Nechypurenko, P. P. (2012). Some aspects of simulation of
real chemical processes and systems in virtual chem-
ical laboratories. Theory and methods of e-learning,
3:238–244.
Nechypurenko, P. P., Semerikov, S. O., Selivanova, T. V.,
and Shenayeva, T. O. (2016). Information and com-
munication tools for pupils’ research competence for-
mation at chemistry profile learning. Information
Technologies and Learning Tools, 56(6):10–29.
Nefedova, T. V. (2012). The development of research
skills of students with mental retardation in chemistry
lessons. PhD thesis, Moscow Pedagogical State Uni-
versity, Moscow.
Pak, M. S. (2015). Theory and methods of teaching chem-
istry: a textbook for universities. Publishing house of
RGPU named after A. I. Gertsen, Sankt-Peterburg.
Savchyn, M. M. (2015). Use of algorithms in the course of
chemistry as a means and method of forming the sub-
ject competences of students. Scientific issues of Vin-
nytsia State M. Kotsyubynskyi Pedagogical University.
Section: Pedagogics and Psychology, 44:324–328.
Semerikov, S., Teplytskyi, I., Yechkalo, Y., and Kiv, A.
(2018). Computer simulation of neural networks us-
ing spreadsheets: The dawn of the age of Camelot.
CEUR Workshop Proceedings, 2257:122–147.
Trukhin, L. (2002). On the use of virtual laboratories in
education. Open and distance education, (4):67–68.
Velychko, L., Dubovyk, O., Kotliar, Z., Muliar, S.,
Pavlenko, V., Svynko, L., Tytarenko, N., and
Yaroshenko, O. (2017). Khimiia 7-9 klasy:
Navchalna prohrama dlia zahalnoosvitnikh navchal-
nykh zakladiv. https://mon.gov.ua/storage/app/
media/zagalna%20serednya/programy-5-9-klas/
onovlennya-12-2017/10-ximiya-7-9.doc.
Yaron, D., Karabinos, M., Lange, D., Greeno, J. G., and
Leinhardt, G. (2010). The ChemCollective — Virtual
Labs for Introductory Chemistry Courses. Science,
328(5978):584–585.
Yenka (2017). Yenka Chemistry. https://www.yenka.com/
en/Yenka
Chemistry/.
Zabolotnyi, O. V. (2007). Formuvannia doslidnyt-
skykh umin uchniv u protsesi vyvchennia syn-
taksysu ukrainskoi movy. Narodna osvita, (3).
https://www.narodnaosvita.kiev.ua/Narodna osvita/
vupysku/3/statti/2zabolotny/zabolotny.htm.
Zarubko, V. P. (2015). Development of cre-
ative thinking of students through solv-
ing problems. Chemistry teacher’s blog.
http://blog.himiya.in.ua/metodychna-robota/
rozvytok-tvorchogo-myslennja.html.
Using the Virtual Chemical Laboratories in Teaching the Solution of Experimental Problems in Chemistry of 9th Grade Students While
Studying the Topic "Solutions"
335
