
Preventing Vitamin C Photooxidation in Beverage Model System by
Virgin Coconut Oil-Rice Bran Oil Nanoemulsion
Yuli Perwita Sari, Sri Raharjo*, Umar Santoso and Supriyadi
Department of Food and Agricultural Product Technology, Faculty of Agricultural Technology, Universitas Gadjah Mada,
Bulaksumur, Yogyakarta 55281, Indonesia
Keywords: Vitamin C, Photooxidation, Nanoemulsion, Model Beverage.
Abstract: Vitamin C (L-ascorbic acid) is a water-soluble vitamin and frequently added in a beverage. This research
aimed to investigate the effect of virgin coconut oil-rice bran oil (VCO-RBO) nanoemulsion on vitamin C
photooxidation in beverage model system. The oil phase was VCO: RBO (3:7, v/v), surfactant (Tween 80) to
oil ratio was 2.5:1 and distilled water was used as the aqueous phase by emulsion phase inversion method.
One and 5% (v/v) of VCO-RBO nanoemulsion were added to a system containing vitamin C (450 and 1800
ppm), erythrosine (0-120 ppm) and citric acid (to adjust pH 2.3 and 3.2) in distilled water. The presence of
light and erythrosine can degrade vitamin C in beverage model system at 1 ppm vitamin C/min, effectively.
The increase in erythrosine concentration increased the vitamin C degradation in a dose-dependent manner.
By using VCO-RBO nanoemulsion (1 and 5% v/v), the degradation of vitamin C in beverage model system
can be inhibited. At pH 2.3, the addition of 5% (v/v) of VCO-RBO nanoemulsion in the beverage model
system was more effective in preventing vitamin C photooxidation than that at pH 3.2. It suggests that VCO-
RBO nanoemulsion can be added in beverage model system to protect the vitamin C photooxidation.
1 INTRODUCTION
Vitamin C or L-ascorbic acid is one of the water-
soluble vitamins. It is commonly added in beverage,
especially in isotonic water, for its health benefit and
to meet the consumer demand. Vitamin C is known
as a potent antioxidant. However, it can easily be
degraded under high pH, high temperature and by
photooxidation (Huang et al., 2004; Jeney-nagymate
and Fodor, 2008; Yang and Min, 2009; Sheraz et al.,
2015). Photooxidation is one of the main problems in
food and beverage. It was induced by the presence of
sensitizer such as food colorant (FD&C Red number
3) or erythrosine, riboflavin, chlorophyll, etc. (Lee et
al., 1997; Yettela and Min, 2008; Yang and Min,
2009). These compounds naturally present or
deliberately added to improve the appearance and
functional value of products. The reaction rate of
photooxidation is a lot faster than autooxidation. It
can produce oxidation products that contribute to off-
flavor or degradation of beneficial components like
vitamin C, vitamin D, amino acid, etc.
Photooxidation can be prevented by singlet oxygen
quencher or antioxidant. Unfortunately, many
antioxidants are lipid-soluble like β-carotene, α-
tocopherol, γ-oryzanol etc. It is very challenging to
use it in beverage product.
Solid lipid nanoparticle, nanostructured lipid
carrier and nanoemulsion are nano-lipid based
delivery systems. Some studies reported that
nanoemulsion can be incorporated into beverages to
increase the value of products. Zhang et al. (2020)
used docosahexaenoic acid (DHA) and
eicosapentaenoic acid (EPA) nanoemulsion in apple
juice. This nanoemulsion didn’t affect basic
properties such as pH, soluble solids, titratable acid
and reducing sugar of apple juice. Even though it
influenced the transparency of product, the addition
of DHA/EPA nanoemulsion in apple juice was still
acceptable by sensory test (Zhang et al., 2020). Buriti
(Mauritia flexuosa L.) oil nanoemulsion was also
potential as natural colorant replacer in isotonic sport
drink (Bovi et al., 2017). Fish oil and rice bran oil was
also used to be part of oil-in-water nanoemulsion
before incorporating with yoghurt as reported by
Zhong et al. (2018). This nanoemulsion gave some
significant impacts on reduction in acidity, syneresis
and peroxide value with maximum retention of EPA
and DHA.
Sari, Y., Raharjo, S., Santoso, U. and Supriyadi, .
Preventing Vitamin C Photooxidation in Beverage Model System by Virgin Coconut Oil-Rice Bran Oil Nanoemulsion.
DOI: 10.5220/0010541900003108
In Proceedings of the 6th Food Ingredient Asia Conference (6th FiAC 2020) - Food Science, Nutrition and Health, pages 113-121
ISBN: 978-989-758-540-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
113

Rice bran oil is rich in a specific antioxidant
named γ-oryzanol up to 2. 6g/100 g of oil (Pokkanta
et al., 2019). These compounds have been attractive
because it was only found in rice bran products,
especially in rice bran oil. Virgin coconut oil is
known as oil which rich in lauric acid. The medium-
chain fatty acid is the main ingredient to produce
nanoemulsion by a low-energy method.
In this research, we used a combination of virgin
coconut oil and rice bran oil, which were incorporated
with Tween 80 and distilled water to make an oil-in-
water nanoemulsion. This study aimed to investigate
the effect of virgin coconut oil-rice bran oil (VCO-
RBO) nanoemulsion on photooxidation of vitamin C
in a beverage model system.
2 MATERIALS AND METHODS
2.1 Materials
Vitamin C or L-ascorbic acid for analytical grade (J.T.
Baker); Tween 80, potassium iodide and iodine were
obtained from Merck (Germany). Virgin coconut oil,
rice bran oil, sucrose, erythrosine and citric acid were
food grade that obtained from local market.
2.2 Methods
2.2.1 Preparation of Virgin Coconut
Oil-Rice Bran Oil (VCO-RBO)
Nanoemulsion
The VCO-RBO nanoemulsion formula were VCO:
RBO (3:7, v/v), surfactant (Tween 80) to oil ratio was
2.5:1 and distilled water was used as the aqueous
phase. This nanoemulsion was produced by emulsion
phase inversion according to Sari et al. (2020).
2.2.2 Preparation of Beverage Model
Systems
The beverage model systems was prepared according
to (Ariviani et al., 2011) with slight modification. The
beverage model system containing vitamin C (450-
1800 ppm, w/v), erythrosine (0-120 ppm, w/v), citric
acid (to adjust pH 2.3 and 3.2) in distilled water.
VCO-RBO nanoemulsion (0, 1 and 5%, v/v) were
added into the systems. A 10 mL of the beverages
model systems was prepared into 30-mL vial with
rubber cap and sealed with parafilm. The samples
were illuminated at + 3200 lux or stored in the dark
up to 2 hours. Vitamin C content were analyzed in
every 30 minutes.
2.2.3 Physicochemical Analysis
Vitamin C in each samples were analyzed by iodine
titrimetric according to Sudarmadji et al. (1997)
method. pH of samples were measured by Hanna
Instrument. The color of samples were determined
using a Konica Minolta Colorimeter with L*, a* and
b* parameters. The erythrosine concentration were
analyzed using spectrophotometer by (Yang and Min,
2009) with some modifications. Curva calibrations
were constructed in each sample formula. The
wavelength detection analysis according to each
maximum wavelength absorption by scanning
method (200-700 nm). Turbidity of samples were
determined at 600 nm (Zhong et al., 2017; Sari et al.,
2020).
2.2.4 Statistical Analysis
The experiment were done in duplicate. The samples
were analyzed at least duplicate in each experiment.
Data were analyzed by regression analysis with
Microsoft Excel 2013 and IBM SPSS Statistic 24.
3 RESULTS AND DISCUSSION
3.1 Characterization of VCO-RBO
Nanoemulsion
In this study, VCO:RBO (3:7, w/w) were used as the
oil phase. The surfactant (Tween 80) to oil ratio was
2.5:1. Distilled water as the aqueous phase in 80%
(v/v) of the total system. Based on the previous study,
this formula was selected because it gave the smallest
particle size of nanoemulsion (65.64 nm) with zeta-
potential was -12.16 mV (Sari et al., 2020). This
formula had slight transparency, therefore only
slightly affected the beverage model system’s
appearance.
The visual sample product can be seen in Fig 1.
Samples containing 5% nanoemulsion in 450 ppm
vitamin C were more slightly pink than models with
1800 ppm vitamin C at the same concentration of
VCO-RBO nanoemulsion and pH system (2.3).
VCO-RBO nanoemulsion up to 5% (v/v) in beverage
model systems didn’t make samples to be turbid. The
turbidities of all samples containing 1800 ppm
vitamin C were relatively small (<0.1 cm
-1
) It might
be due to small size of nanoemulsion and a small
portion of it to be incorporated in beverage model
systems (Fig.2).
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
114

(a)
(b)
(c)
(d)
Figure 1: Visual apperarance of beverage model systems containing 450 ppm vitamin C with 0 and 5% of VCO-RBO
nanoemulsion (a) and (b), respectively; 1800 ppm vitamin C with 0 and 5% of VCO-RBO nanoemulsion (c) and (d),
respectively. All pH systems were 2.3.
0
30’
90’
60
’
120’
0
30’
90’
60’
120
’
Preventing Vitamin C Photooxidation in Beverage Model System by Virgin Coconut Oil-Rice Bran Oil Nanoemulsion
115
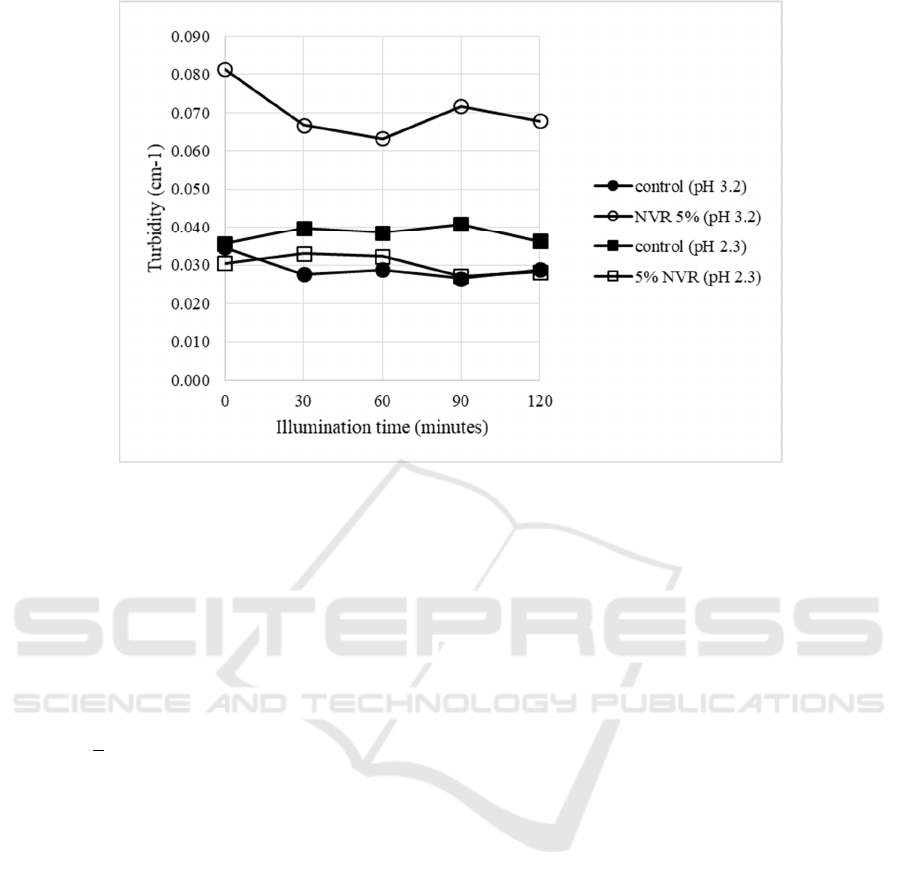
Figure 2: The changes of turbidity in photooxidation of beverage model systems (vitamin C 1800 ppm) (NVR=VCO-RBO
Nanoemulsion).
3.2 The Effect of Light and Sensitizer
on Vitamin C
To investigate the cause of vitamin C degradation, we
used some sets of samples that were illuminated or
stored in the dark, with and without of erythrosine as
a sensitizer. According to Fig 3, the samples
containing 120 ppm or erythrosine and held in a
lightbox at + 3200 lux up to 2 hours gave a vitamin C
degradation almost 1 ppm of vitamin C/min (y = -
0.9724x + 1863.1; R
2
= 0.8732; p<0.05). Meanwhile,
the relatively stable vitamin C content was performed
by samples stored in the dark (y = -0.2182x + 1926.9;
R
2
= 0.6749; p>0.05) or without sensitizer and stored
under light (y = -0.6682x + 1833.4; R
2
= 0.3489;
p>0.05). By hypothesis null analysis in regression
statistic, these two latter slopes were almost 0. It
means that photooxidation can only occur by a
combination of sensitizer and light. This study was
similar to previous studies. At pH 4, 5.6 and 7, the 50
and 100 ppm of ascorbic acid were declined in the
photooxidation in the presence of food colorant red nr
3. Meanwhile, ascorbic acid content was relatively
stable under dark for one hour (Yang and Min, 2009).
The degradation of riboflavin was also faster under
the light than in the dark (Huang et al., 2004). It
suggests that singlet oxygen was involved in these has
sensitizer, light and triplet oxygen can produce singlet
oxygen that can degrade vitamin C. The reaction
between ascorbic acid with singlet oxygen produces
unstable hydroperoxide of ascorbic acid (Choe and
Min, 2005).
The effect of the initial concentration of vitamin
C before photooxidation was also studied. From Fig.4
showed that the 450 and 1800 ppm of ascorbic acid
content gives a relatively same of vitamin C
degradation rate for about 1 ppm/min (p>0.05). At
different pH (2.3 and 3.2), the degradation rate of
vitamin C with 1800 ppm as initial content was also
relatively same (0.97 and 1.02 ppm vitamin C/min,
respectively, p>0.05).
Comparatively, the increasing of erythrosine
concentration gave a significant effect on decreasing
of vitamin C in a dose-dependent manner (Fig.5). At
40 and 80 ppm, the degradation rate of vitamin C
were 0.62 and 0.69 ppm/min, respectively.
Meanwhile, by using 120 ppm in the reaction system,
the degradation rate of vitamin C up to 1.04 ppm/min.
From these results, it was concluded that the
difference of initial concentration or pH (2.3 and 3.2)
didn’t give any significant difference in the declining
rate of vitamin C. Erythrosine concentration gave
significant effect on the degradation rate of vitamin C
in photooxidation system. The higher erythrosine
concentration induced more singlet oxygen.
Therefore, vitamin C will be degraded more
frequently.
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
116
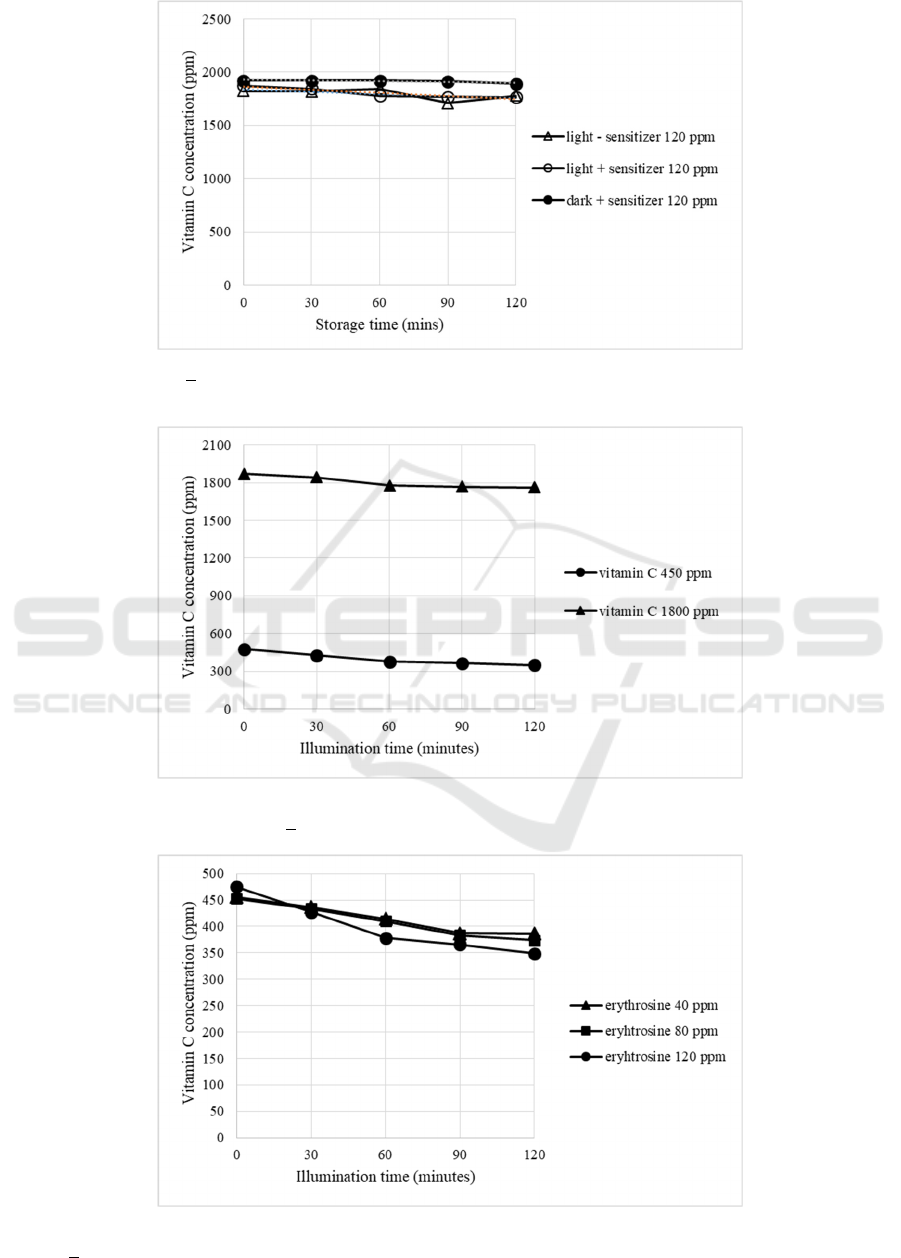
Figure 3: Effect of light (+ 3200 lux) and sensitizer (120 ppm erythrosine) on vitamin C changes in beverage model system
up to 120 minutes stored.
Figure 4: Effect of initial vitamin C concentration (450 and 1800 ppm) on degradation of vitamin C in photooxidation of
beverage model system (light intensity + 3200 lux at room temperature).
Figure 5: Effect of erythrosine concentration degradation of vitamin C in photooxidation of beverage model system (light
intensity + 3200 lux at room temperature).
Preventing Vitamin C Photooxidation in Beverage Model System by Virgin Coconut Oil-Rice Bran Oil Nanoemulsion
117

3.3 Effect of VCO-RBO Nanoemulsion
on Photooxidation of Vitamin C in
Beverage Model Systems
Containing Various Erythrosine
Concentration
In this study, we added VCO-RBO nanoemulsion at
1 and 5% (v/v) in beverage model system. The 450
ppm vitamin C was relatively stable in beverage
model systems containing various erythrosine
concentration (40-120 ppm) and 5% of VCO-RBO
nanoemulsion (Table 1). It might be due to the
capability of nanoemulsion to maintain erythrosine in
photooxidation. By using 5% VCO-RBO
nanoemulsion, the color of the beverage model
system was more slightly pink than the control (Fig.
1). It was concluded that nanoemulsion could
maintain the stability of erythrosine; therefore, less
singlet oxygen was produced and 450 ppm vitamin C
was relatively constant for photooxidation reaction
time (Table 1). Adding 1% of VCO-RBO
nanoemulsion could avoid vitamin C degradation
almost 33 and 60% at pH system was 3.2 and 2.3,
respectively. By using regression statistical analysis,
5% of VCO-RBO nanoemulsion could protect
vitamin C degradation (slope ≈ 0) at 1800 ppm of
vitamin C as an initial concentration in pH 2.3 and
3.2. The natural antioxidant in oil phase such as α-
tocopherol was suspected responsible to protect
vitamin C avoid photooxidation. Some researchers
found that rice bran oil contained 13.2-29.95 mg α-
tocopherol /100 g oil (Pestana et al., 2008;
Dhavamani et al., 2014; Yang et al., 2018). It was also
known as effective singlet oxygen quencher with the
singlet oxygen quenching rate was 4.9 x 10
7
up to
3.54 x 10
8
/M/s (Nishida et al., 2007; Kim et al.,
2009; Ouchi et al., 2010).
Comparatively with photooxidation in vitamin C
450 ppm, the preventing mechanism by VCO-RBO
nanoemulsion in beverage model systems containing
1800 ppm of vitamin C at pH 2.3 and 3.2 is not still
clearly understood. Although 1 and 5% of VCO-RBO
nanoemulsion can protect vitamin C degradation by
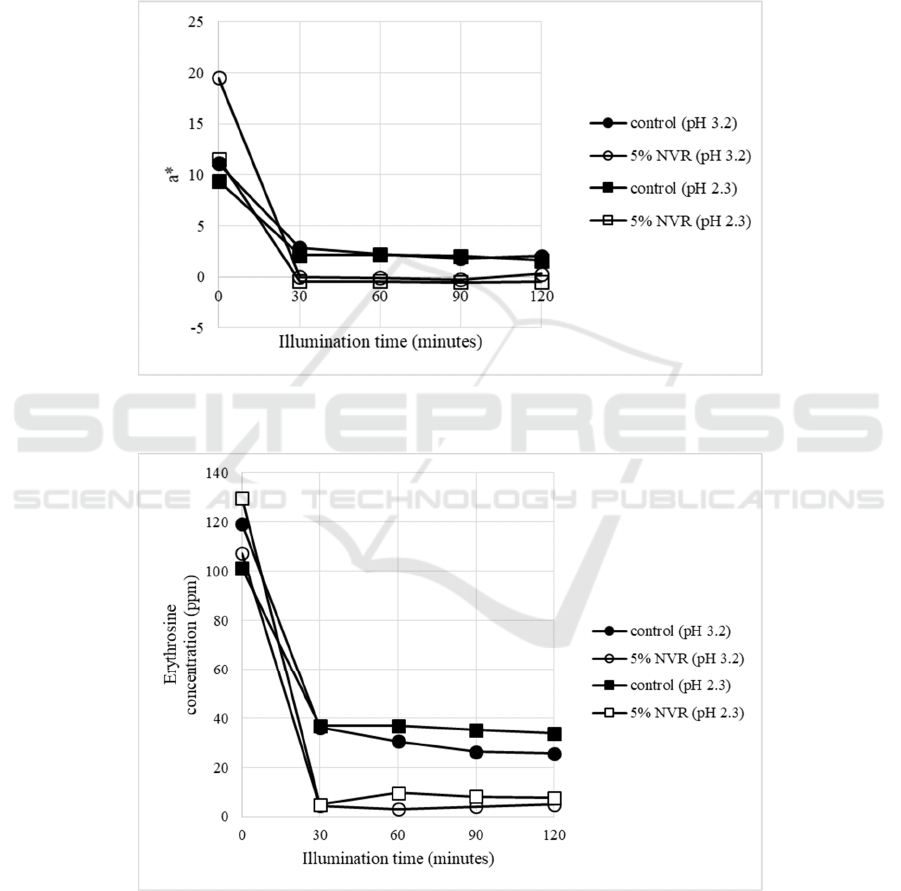
photooxidation (Table 2), the a* values samples
containing 5% VCO-RBO nanoemulsion at pH 2.3
and 3.2 were lower than the control (Fig. 6). This
value was positively correlated with erythrosine
concentration (Fig. 7). Therefore, VCO-RBO
nanoemulsion couldn’t maintain erythrosine in high
concentration of vitamin C beverage model systems.
Table 1: The regression equations of beverage model systems containing VCO-RBO nanoemulsion (0 and 5% v/v) and
various erythrosine concentration (40-120 ppm) in photooxidation reaction system.
Erythrosine
conc. (ppm)
VCO-RBO
nanoemulsion
% (v/v)
Regression equation R
2
Degradation rate
(ppm vitamin C/min)
p-value*
40 0 Y = - 0.6234x + 453.24 0.9524 0.6234 0.004
40 5 Y = - 0.2875x + 504.44 0.5852 0.2875 ≈ 0 0.132
80 0 Y = - 0.69x + 452.02 0.9851 0.69 0.001
80 5 Y = - 0.0146x + 499.5 0.0021 0.0148 ≈ 0 0.941
120 0 Y = - 1.0451x + 461.37 0.9263 1.0451 0.009
120 5 Y = - 0.1651x + 453.55 0.678 0.1651 ≈ 0 0.087
*p-value<0.05 means the slope was si
g
nificantl
y
different from 0.
Table 2: The regression equations of beverage model systems containing VCO-RBO nanoemulsion (0-5% v/v) at pH 2.3 and
3.2 in photooxidation reaction system.
pH VCO-RBO nano-
emulsion % (v/v)
Regression equation R
2
Degradation rate
(
p
p
m
vitamin C/min)
p-value* p-value**
2.3 0 Y = -0.9724x + 1863.1 0.8138 0.9724 0.02 0.885
3.2 0 Y = -1.0258x + 1933.8 0.8732 1.0258 0.036
2.3 1 Y = -0.401x + 1895 0.8072 0.401 0.038 0.110
3.2 1 Y = -0.6696x + 1883.3 0.9507 0.6696 0.005
2.3 5 Y = -0.1303x + 1818.9 0.0358 0.1303 ≈ 0 0.761 1
3.2 5 Y = -0.3796x + 1821 0.5096 0.3796 ≈ 0 0.176
* p-value<0.05 means the slope was si
g
nificantl
y
different from 0
** p-value>0.05 means the two slopes were not significantly different at different pH and same amount of
VCO-RBO nanoemulsion
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
118
It seems like there is a behind mechanism by this
nanoemulsion to prevent photooxidation of 1800 ppm
vitamin C in beverage model systems. In another
studies, vitamin C fortification (40-80 mg/100mL)
can degrade anthocyanin and color loss in cranberry
juice because the high concentration of vitamin C
could increase oxidation products of vitamin C that
degrade anthocyanin (Li et al., 2014; Roidoung et al.,
2016, 2017). Meanwhile, in this study, samples
containing 450 and 1800 ppm of vitamin C and 120
ppm of sensitizer without nanoemulsion gave the
same trend of a* values during photooxidation (Fig.
8). Only samples containing 1800 ppm of vitamin C
with VCO-RBO nanoemulsion that had transparent
appearance and lower a* than the 450 ppm of vitamin
C (Fig. 9).
Figure 6: The changes of a* (redness) in photooxidation of beverage model systems (vitamin C 1800 ppm). (NVR=VCO-
RBO Nanoemulsion).
Figure 7: The changes of erythrosine concentration in photooxidation of beverage model system (vitamin C 1800 ppm).
(NVR=VCO-RBO Nanoemulsion).
Preventing Vitamin C Photooxidation in Beverage Model System by Virgin Coconut Oil-Rice Bran Oil Nanoemulsion
119
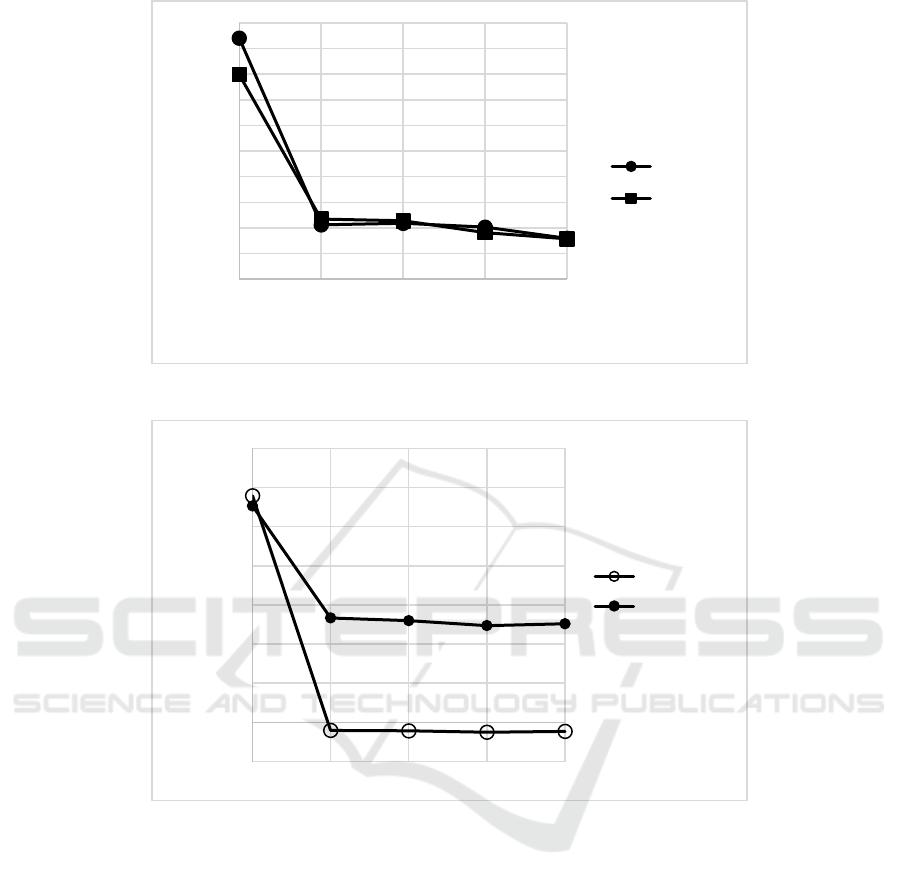
Figure 8: The changes of a* values of beverage model systems (vitamin C 450 and 1800 ppm) without nanoemulsion.
Figure 9: The changes of a* values of beverage model systems (vitamin C 450 and 1800 ppm) with 5% of VCO-RBO
nanoemulsion during illumination.
4 CONCLUSIONS
The presence of light and erythrosine can degrade
vitamin C in beverage model system at 1 ppm vitamin
C/min, effectively. The increasing of erythrosine
concentration affected on decreasing of vitamin C in
a dose-dependent manner. By using VCO-RBO
nanoemulsion (1 and 5% v/v), the degradation of
vitamin C in beverage model system can be
prevented. In pH 2.3, the 5% (v/v) of VCO-RBO
nanoemulsion in beverage model system was more
useful to avoid vitamin C photooxidation than in pH
3.2. It suggests that VCO-RBO nanoemulsion can be
added in beverage model system to avoid the
photooxidation of vitamin C.
ACKNOWLEDGEMENTS
The authors thank to Ministry of Research and
Technology / National Agency for Research and
Innovation, Republic of Indonesia for financial
support.
REFERENCES
Ariviani, S., Raharjo, S., and Hastuti, P. 2011. Potensi
Mikroemulsi β -Karoten dalam Menghambat
Fotooksidasi Vitamin C Sistem Aqueous. Jurnal
Teknologi dan Industri Pangan XXII (1): 33–39.
0
1
2
3
4
5
6
7
8
9
10
0 30 60 90 120
a*
Illumination time (min)
1800 ppm
450 ppm
-2
0
2
4
6
8
10
12
14
0 306090120
a*
Illumination time (min)
1800 ppm
450 ppm
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
120

Bovi, G. G., Petrus, R. R., and Pinho, S. C. 2017. Feasibility
of incorporating buriti (Mauritia flexuosa L.) oil
nanoemulsions in isotonic sports drink. Internatioal
Journal of Food Science and Technology 1–9.
Choe, E., and Min, D. B. 2005. Chemistry and Reactions of
Reactive Oxygen Species in Foods. Journal of Food
Science 70 (9): 142–159.
Dhavamani, S., Poorna, Y., Rao, C., and Lokesh, B. R.
2014. Total antioxidant activity of selected vegetable
oils and their influence on total antioxidant values in
vivo: A photochemiluminescence based analysis. Food
Chemistry 164: 551–555.
Huang, R., Choe, E., and Min, D.B. 2004. Kinetics for
Singlet Oxygen Formation by Riboflavin
Photosensitization and the Reaction between
Riboflavin and Singlet Oxygen. Journal of Food
Science 69 (9): C726–C732.
Jeney-nagymate, E., and Fodor, P. 2008. The stability of
vitamin C in different beverages. British Food Journal
110 (3): 296–309.
Kim, J. I., Lee, J. H., Choi, D. S., Won, B. M., Jung, M. Y.,
and Park, J. 2009. Kinetic study of the quenching
reaction of singlet oxygen by common synthetic
antioxidants (tert-Butylhydroxyanisol, tert-di-
Butylhydroxytoluene, and tert-Butylhydroquinone) as
compared with α-Tocopherol. Journal of Food Science
74 (5): 362-369.
Lee, K. H., Jung, M. Y., and Kim, S. Y. 1997. Quenching
Mechanism and Kinetics of Ascorbyl Palmitate for the
Reduction of the Photosensitized Oxidation of Oils. 74
(9): 1053–1057.
Li, J., Song, H., Dong, N., and Zhao, G. 2014. Degradation
Kinetics of Anthocyanins from Purple Sweet Potato (
Ipomoea batatas L .) as Affected by Ascorbic Acid.
Food Science and Biotechnology 23 (1): 89–96.
Nishida, Y., Yamashita, E., and Miki, W. 2007. Quenching
Activities of Common Hydrophilic and Lipophilic
Antioxidants against Singlet Oxygen Using
Chemiluminescence Detection System. Carotenoid
Science 11: 16–20.
Ouchi, A., Aizawa, K., Iwasaki, Y., Inakuma, T., Terao, J.,
Nagaoka, S.I., and Mukai, K. 2010. Kinetic study of the
quenching reaction of singlet oxygen by carotenoids
and food extracts in solution. development of a singlet
oxygen absorption capacity (SOAC) assay method.
Journal of Agricultural and Food Chemistry 58 (18):
9967–9978.
Pestana, V. R., Zambiazi, R. C., Mendonça, C.R.B.,
Bruscatto, M. H., Lerma-García, M. J., and Ramis-
ramos, G. 2008. Quality Changes and Tocopherols and
γ-Orizanol Concentrations in Rice Bran Oil During the
Refining Process. Journal of American Oil Chemistry
Society 85: 1013–1019.
Roidoung, S., Dolan, K. D., and Siddiq, M., 2016. Gallic
acid as a protective antioxidant against anthocyanin
degradation and color loss in vitamin-C fortified
cranberry juice. Food Chemistry 210: 422–427.
Roidoung, S., Dolan, K. D., and Siddiq, M., 2017.
Estimation of kinetic parameters of anthocyanins and
color degradation in vitamin C fortified cranberry juice
during storage. Food Research International 94: 29–35.
Pokkanta, P., Sookwong, P., Tanang, M., and Setchaiyan,
S. 2019. Simultaneous determination of tocols, -
oryzanols, phytosterols, squalene, cholecalciferol and
phylloquinone in rice bran and vegetable oil samples.
Food Chemistry 271 (June 2018): 630–638.
Sari, Y.P., Raharjo, S., Santoso, U., and Supriyadi. 2020.
Formulation, characterization and stability of o/w
nanoemulsion containing rce bran oil prepared by
emulsion phase inversion. Food Research 4 (August):
1024–1029.
Sheraz, M. A. L. I., Khan, M. F., Ahmed, S., Kazi, S. H.,
and Ahmad, I. 2015. Stability and Stabilization of
Ascorbic Acid. Household and Personal Care Today
10 (3): 22–25.
Sudarmadi, S., Haryono, B., and Suhardi. 1997. Prosedur
Analisa untuk Bahan Makanan dan Pertanian.
Yogyakarta: Liberty.
Yang, T.S., and Min, D.B. 2009. Quenching mechanism
and kinetics of ascorbic acid on the photosensitizing
effects of synthetic food colorant FD & C Red Nr 3.
Journal of Food Science 74 (9): 718–722.
Yang, R., Zhang, L., Li, P., Yu, L., Mao, J., and Wang, X.
2018. A review of chemical composition and nutritional
properties of minor vegetable oils in China. Trends in
Food Science & Technology 74 (May 2017): 26–32.
Yettela, R. R., and Min, D. B. 2008. Quenching
mechanisms and kinetics of trolox and ascorbic acid on
the riboflavin-photosensitized oxidation of tryptophan
and tyrosine. Journal of Agricultural and Food
Chemistry 56: 10887–10892.
Zhang, L., Han, C., Liu, M., Yang, H., Zhang, F., Liu, B.,
and Meng, X. 2020. The formation, stability of DHA /
EPA nanoemulsion prepared by emulsion phase
inversion method and its application in apple juice.
Food Research International 133 (February): 109–132.
Zhong, J., Yang, R., Cao, X., Liu, X., and Qin, X. 2018.
Improved Physicochemical Properties of Yogurt
Fortified with Fish Oil/γ-Oryzanol by Nanoemulsion
Technology. molecules 23: 1–11.
Preventing Vitamin C Photooxidation in Beverage Model System by Virgin Coconut Oil-Rice Bran Oil Nanoemulsion
121
