
Investigation and Comparison of Physicochemical Characteristics of
Non-aged and 4-month Aged Mulberry Wine Prepared from
Three Different Wine Making Techniques
Resha Shrestha
1
, Siriwan Panprivech
2
, Kamolnate Kitsawad
1
and Viyada Kunathigan
1,*
1
Department of Food Biotechnology, Faculty of Biotechnology, Assumption University, Bangkok, Thailand
2
Department of Agro-Industry, Faculty of Biotechnology, Assumption University, Bangkok, Thailand
Keywords: Mulberry, Cold Pressed, Physicochemical Analysis, Antioxidant, Anthocyanin.
Abstract: Winemaking techniques and the aging process of wine are important to the quality of wine. This study was
investigated and compared the effect of different winemaking practices on physicochemical characteristics
among non-aged and 4-month aged of Morus alba Linn (cv. Chiang Mai 60) wines. Hand press juice (HPJ),
hand press juice with pulp (HPP) and cold press juice (CPJ) were prepared from fresh mulberry fruit which
were fermented using Saccharomyces cerevisiae (Premier rouge) and then bottled and aged for 4 months in
the cold room (12±2˚C). The pH, total titratable acidity (TTA), alcohol content, reducing sugar, color
measurements, total phenolic content (TPC), total flavonoid content (TFC), antioxidant capacity and total
anthocyanin content (TAC) were observed. The results showed significant differences on the chemical
parameters (TTA, alcohol content, reducing sugar, TPC, TFC and TAC) among three treatments of mulberry
wines without aging. Among all wine making techniques used, HPP and CJP method produced wine that
have similar level of TFC, and color intensity which was higher than the wine prepared by HPJ method. The
HPJ method resulted in the wine that have significantly lower level of TPC, TAC, and TFC than the other two
techniques. In addition, after aged the mulberry wines for 4 months, the wine made with different technique
had significantly lower level of TPC, antioxidant capacity, TAC, TFC and color hue than the non-aged
mulberry wine made from the same technique.
1 INTRODUCTION
Mulberry has unique flavour, texture, and color.
(Vijayan et al., 2011) considered that mulberry fruits
have well balanced sweetness and tartness. It is highly
perishable therefore it is used in manufacturing
different types of food for example sauce, fruit tea,
ice-creams, supplements, syrup, vinegar and also
alcoholic beverages (Juan et al., 2012). Mulberry is a
potential plant due to the presence of bioactive
ingredients (Ercisli and Orhan, 2007). Moreover,
Yang and Tsai (1994) found nutritional components
such as sugars, organic acids, free amino acids,
vitamins and micronutrients in mulberry. Thus, it has
significant importance inhuman health. Moreover,
Nomura et al. (1976) found that the parts of mulberry
such as twigs, leaves, fruits and root bark contain
phenolic compounds which can be used to treat
various diseases. The mulberry contains functional
components such as phenolic, flavonoids and
ascorbic acid (Bae and Suh, 2007) which add both
interest and value to it. It also contains anthocyanin.
According to Kim and Lee (2020) major anthocyanin
component found in mulberry are cyanidin-3-O-
glucoside and cyanidin-3-O-rutinoside. Due to the
presence of the ascorbic acid, flavonoids and
anthocyanin, mulberry possess antioxidant
characteristics. Besides, anti-microbial, anti-
inflammatory and anti-cancer (Butt et al., 2008) and
antiradical (Suh et al., 2004) properties are also found
in mulberry fruit extract. In addition, Mattivi et al.
(2006) and Mcdougall et al. (2005) reviewed anti-
atherosclerotic, anti-carcinogenic, and anti-
inflammatory properties in mulberry. These
significant therapeutic qualities in mulberry may be
one of the reasons for its consumption in various
possible forms of food and beverages.
The raw materials, environment, temperature,
yeasts, different processing techniques, fermentation
and aging have significant impact on the physical,
chemical and organoleptic properties in wine. Iland et
Shrestha, R., Panprivech, S., Kitsawad, K. and Kunathigan, V.
Investigation and Comparison of Physicochemical Characteristics of Non-aged and 4-month Aged Mulberry Wine Prepared from Three Different Wine Making Techniques.
DOI: 10.5220/0010529500003108
In Proceedings of the 6th Food Ingredient Asia Conference (6th FiAC 2020) - Food Science, Nutrition and Health, pages 79-87
ISBN: 978-989-758-540-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
79

al. (2000) considered pre- fermentation, fermentation
and post fermentation as vital processes in wine
making. For example, Wang et al. (2020) experiment
on apple juice extracted by three different mechanical
juice extraction methods such as squeezed juice,
spiral juicer and broken juice have greater impact on
the quality of apple juice. The quality of juice
depends upon parameters such as pressing time, flow
rate, pressure and heat. Moreover, the processes such
as crushing, pressing and filtration can affect the
yield, texture and quality of juice. The proper
mashing and filtration results in less viscous juice.
One of the challenging part in making mulberry wine
is high viscosity of juice. The fruits contain fibre of
11.75 ± 1 g/100 g fresh weight (Imran et al., 2010)
making juice thicker and syrupy resulting in
prolonged filtration time. The quality, time and cost
due to extraction may add or reduce the value to
winemaking. Chen et al. (2015) suggested that there
are lesser information on influence on the quality of
fruit and vegetable juice due to processing
technologies. Thus, there may be a debate on the
quality of fruit juice extracted from different
methods. Perez-Cacho and Rouseff (2008) stated that
juice processing methods can affect the juice flavour.
According to Nadulski et al. (2017) depending on raw
material and operation type, different juice extraction
techniques are applied. Hydraulic, basket, belt and
screw press are popular in food industry for pressing
(Jaeger et al., 2012). Cautela et al. (2010) stated that
the juice processing method can affect the
phytochemical content. However, less information
about various wine making techniques and their
comparative study in terms of mulberry wine can be
found till date
In one of the recent study done by Chang (2020)
on cold pressed mulberry wine, the temperature for
fermentation of wine has significant impact on
physiochemical properties. The same study showed
that the alcohol production is faster in room
temperature (30±2°C) whereas the higher amount of
phenolic (2319.44±2.94 mg/L cyanidin-3-glucoside)
was obtained in lower temperature (12±2˚C).
Therefore, temperature can also play an important
role affecting the phenolics. The cold pressed
mulberry wine can have high phenolic contents.
Aging of wine can be another factor that has great
impact on its chemical composition. Balga et al.
(2015) specified that the phenolic compounds
undergo various chemical reactions during aging. The
alteration in phenolic affects the quality of wine.
Somers (1971) expressed that the phenolic play an
important role in the taste and color of wine during
maturation, aging and storage. Maturation and aging
of wine can change the taste, aroma, texture and
flavour of a wine due to the chemical reactions among
sugar, phenolic and acids. The wine become smoother
creating the complex aroma and taste.
The objective of this study is to analyse and
compare the non-aged and 4-month aged mulberry
wines which were prepared from three different wine
making techniques.
2 MATERIALS AND METHODS
2.1 Wine Preparation
The fresh and ripe mulberry (Morus alba Linn.cv.
Chiang Mai 60) fruits were sorted and directly
proceed for hand pressed and cold pressed juice. The
juice was prepared using three different processing
techniques namely manual mashed mulberry juice
without pulp (HPJ), manual mashed mulberry Juice
with pulp (HPP) and cold-pressed mulberry juice
without pulp (CPJ). HPJ and HPP were hand mashed.
The difference between the two treatments was
filtration of pulp in HPJ whereas pulp was included
in HPP. However, CPJ was prepared using cold press
machine (Slow juicer Tefal model ZC150838). Each
treatment was then added with distilled water in the
ratio 4:1 (v/v) (juice: water). The pH was adjusted to
3.7 using acid blend (citric: malic = 1:1.5 w/w). The
˚Brix was adjusted to 22˚ with granulated cane sugar.
Then the pectinase enzyme (L.D. Carlson, OH, USA)
was added (0.67 g/L), followed by addition of
potassium meta-bisulphite (KMS) (150mg/L). After
10-12 hours, Saccharomyces cerevisiae (Premier
Rouge UCD#904) was added (7.5x10
6
cell/ml) along
with 2 g/L diammonium phosphate (DAP). After
complete fermentation (6 days), all treatments of
wines were filtered, bottled and aged for 4 months at
12±2 ˚C. All treatments were sampled after
completed fermentation and 4-month aged then
centrifuged (3000 rpm for 2 min) and transferred
supernatant into new tubes and subsequently frozen
at -20˚C until analysis.
2.2 Chemical Analysis
The chemical compositions of each sample were
analyzed in triplicate. The pH was analyzed using the
pH meter (pH 211 Microprocessor pH meter, China).
The alcohol content was measured using
Ebulliometer (160000-complete traditional
Ebulliometer, Laboratories Dujardin Salleron
TM
,
France). The official method of analysis (AOAC
926.12, 1990) was used to measure total titratable
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
80

acidity (TTA) and expressed percentage of citric acid.
The 3, 5-dinitrosalicylic acid (DNS) assay (Miller,
1959) was followed to measure the amount of
reducing sugar in all wine samples which was
expressed in g/L.
2.3 Total Phenolic Content (TPC)
TPC was quantified using Folin-Ciocalteu micro
method (Waterhouse, 2002). Wine sample (0.02 mL)
was added to 1.58 mL distilled water. To it, 0.1 mL
F-C reagent was added. After 5 min, 0.3 mL 20%
sodium carbonate (Na
2
CO
3
) was added, mixed well
then incubated for 1.5 hours at 30±2 ºC. The
absorbance was read at 765 nm in triplicate. TPC was
calculated from the calibration curve and expressed
as mg/L of gallic acid equivalent.
2.4 Total Flavonoids Content (TFC)
TFC was determined using aluminium chloride
colorimetric method (Ivanova et al., 2010, Zhishen et
al., 1999) in triplicate. Wine sample (1 mL) was
mixed with distilled water (4 mL) in a tube. Then 0.3
mL of 5% sodium nitrite (NaNO
2
) was added and
rested for 5 min at room temperature. Then 0.3 mL of
10% aluminium chloride (AlCl
3
) was added and
reacted for 5 min. Then 2 mL of 1M sodium
hydroxide (NaOH) was added and the total was made
up 10 mL with distilled water. The solution was
mixed well, and the absorbance was read at 510 nm
in triplicate. TFC was calculated from the calibration
curve and expressed as mg/L of Rutin equivalent.
2.5 Total Anthocyanin Content (TAC)
TAC was determined in triplicate by using the pH
differential method described by AOAC Official
method 2002.02. TAC was determined as mg/L of
cyanidin-3-glucoside equivalent according to the
following equation (1):
TAC = (A × MW × DF × 10
3
) / (ɛ × L)
(1)
Where A is absorbance [(A520nm – A700nm) pH
1.0 – (A520nm – A700nm) pH 4.5]; MW is the
molecular weight of cyanidin-3-glucoside (449.2
g/mol); DF is the dilution factor (100); L is the path
length in cm (1); and ε is the molar extinction
coefficient of cyanidin-3-glucoside (26900 L/mol
cm).
2.6 Antioxidant Content
Antioxidant was determined by using DPPH radical
scavenging method described by Šimić et al. (2017).
The DPPH radical scavenging activity is calculated in
terms of percentage inhibition of DPPH activity
according to the following equation (2);
% inhibition = [(AC - AA) / AC] x 100 (2)
Here AA and AC are the absorbance values of the
samples and the control, respectively. The percentage
inhibition of DPPH activity of Trolox solution was
also tested. The calibration curve was constructed
with the Trolox solution concentrations versus the
percent inhibition of DPPH activity. The antioxidant
capacity of the samples were quantified and
expressed as Trolox equivalents mg/L from the
calibration curve.
2.7 Color Measurement
The absorbance of the wine samples at 420, 520, and
620 nm was measured directly by use of a
spectrophotometer with an optical path length of 10
mm. The color intensity was calculated as the sum of
the absorbance at 420, 520, and 620 nm (A
420
+ A
520
+ A
620
). Hue was obtained as the ratio of absorbance
measured at 420 and 520 nm (A
420
/A
520
).
2.8 Statistical Analysis
All the physicochemical parameters of non-aged and
4-month aged wines were carried out in triplicates.
All the data were analysed using Statistical Analysis
System (SAS) program version 9.4 (SAS Institute,
Cary, NC and USA). The statistical difference
between the means was evaluated using least
significant difference (LSD) test. T-test was used to
compare the difference in the mean between each
parameter of non-aged and 4-month aged wines.
3 RESULTS AND DISCUSSION
3.1 Chemical Compositions
The chemical composition of mulberry wines
prepared by three different wine making techniques
(HPJ, HPP, and CPJ) was determined before and after
4 months aging period (Figure 1). The HPJ has given
significantly higher alcohol content in all three
treatments. HPJ and CPJ contained only juice making
Investigation and Comparison of Physicochemical Characteristics of Non-aged and 4-month Aged Mulberry Wine Prepared from Three
Different Wine Making Techniques
81
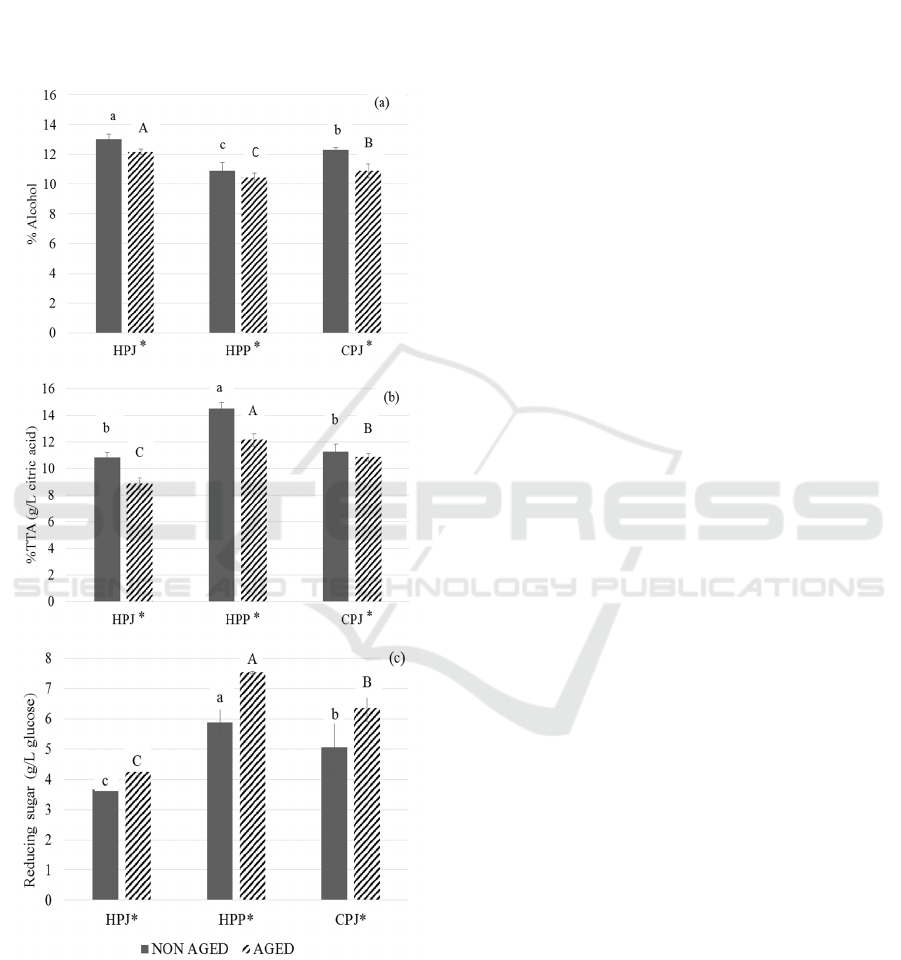
them lesser viscous than HPP. In this study, the
alcohol content in HPJ with lesser viscous fluid is
higher. The amount of sugar was well utilized by
Saccharomyces cerevisiae in HPJ and CPJ resulting
in higher alcohol content in them. According to
Abdullah et al. (2007), the use of pectinase aid in the
hydrolysis of soluble polysaccharides (high viscosity)
to soluble sugars and short chain (low viscosity).
Figure 1: The changes in chemical composition of non-aged
and 4 months aged mulberry prepared from three different
wine making techniques (HPJ, HPP and CPJ).: (a) alcohol
content, (b) acidity (TTA) percentage, and (c) reducing
sugar. The data are expressed as mean ±SD (n=3). The
different letters indicate significant difference (p < 0.05).
‘*’ represents the significant difference.
However, the alcohol percentage significantly
decreased [Figure 1(a)] during 4 months aging in all
three treatments. During fermentation or aging of
wine, the ethyl esters formation such as ethyl esters
of lactic, malic and tartaric acid occurs developing
odor and taste of wine (Shinohara et al., 1979). These
might be the reason for decrease in the percentage of
alcohol in all three treatments after 4 months aging.
When the acidity of all three treatments were
observed [Figure 1(b)] in 4-month aged mulberry
wine, the result showed significantly reduction in the
acidity in all treatments. Shinohara et al. (1979) stated
that during aging, ethyl esters acid are formed rapidly.
The acid in wine reacts with alcohol to form esters.
Ancin-Azpiicueta et al. (2008) stated that during
aging the ethyl esters of organic acid increased
leading to decrease in acidity.
The reducing sugar content [Figure 1 (c)] of the
three treatments was compared. HPP had
significantly higher reducing sugar. Comparatively,
HPJ had the least reducing sugar content (3.66 g/L
glucose). This indicated that the most of the sugar was
well consumed by yeast and converted to alcohol in
HPJ. However, after 4-month of aging, the reducing
sugar content was increased in all the treatments. As
stated by Butt et al. (2008), mulberry fruit contains a
good amount of proteins, carbohydrates, fats, fiber
and vitamins. Carbohydrates from mulberry may
have been remained in the wine especially in the HPP
treatment. Chapman et al. (1991) stated that pectin in
fruits can be hydrolyzed into free sugars by a
pectinase enzyme. As the pectinase enzymes has been
added to the fermentation from the beginning,
remaining activity from the pectinase could have
slowly released these sugars into wine cause the
increase in reducing sugar during 4-months aging.
3.2 TPC
The TPC of all the treatments were analyzed [Figure
2 (a)]. The results showed that there was a significant
difference (p < 0.05) between all the treatments.
Mulberry contains phenolic and polysaccharides
(Chen et al., 2015) which might give higher phenolic
content in HPP. The wine with filtered juice (HPJ)
with absence of whole fruit had significantly lesser
phenolic in it. Martinez-Lapuente et al. (2017) stated
that winemaking step such as filtration causes
decrease in wine polysaccharide content which is why
HPJ had the lesser phenolic content. However, after
4-months aging, the decreased in TPC in all the
treatments was observed. Mulberry is rich in
phytochemicals such as phenolic compounds,
flavonoids, anthocyanin and ascorbic acid (Bae and
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
82
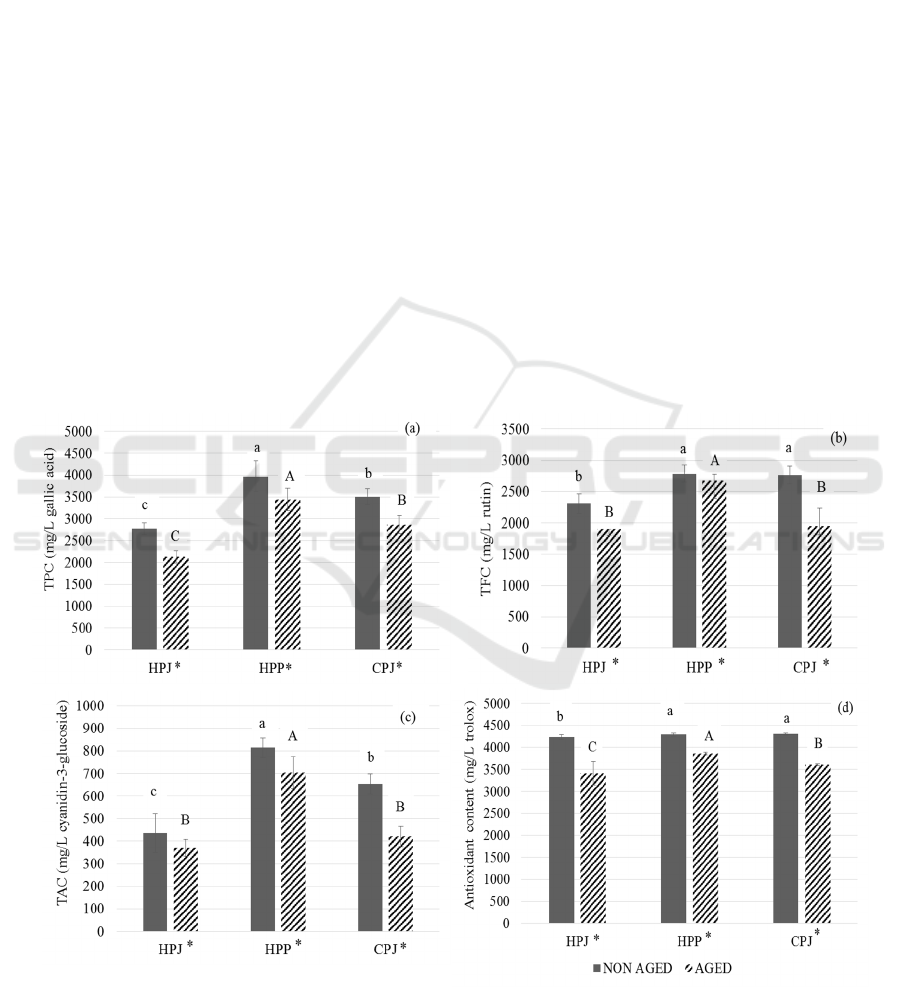
Suh, 2007). According to Ribereau-Gayon et al.
(2006), the chemical reactions such as thermal and
oxidative degradation of anthocyanin, interaction of
tannins with proteins and polysaccharides,
polymerization of procyanidin and copigments and
formations of anthocyanindins are the reasons for
changes in level of phenolic compounds. Whereas
Yıldırım et al. (2015) emphasized on the subsequent
transformation of wine phenolic in bottled (i.e.
anaerobic) condition where slow polymerization and
condensation reaction occurs. This process can cause
the decrease in TPC. Moreover, the similar reduction
of phenolic in mulberry juice was seen in a study done
by Zou et al. (2017). However, the reason for the
reduction in the same study was the formation of
sediments in mulberry juice during storage in which
was not a case in this study as the sediments was not
observed.
3.3 TFC
The TFC of the treatments were analyzed [Figure 2
(b)]. The HPP resulted in the higher flavonoid content
(2785.24 ± 136.53 mg/L rutin) before aging. The
fermentation with pulp in HPP may account for the
higher flavonoid content due to the increasing time
for extraction. The fruit of mulberry is rich in
phytochemicals such as phenolic, flavonoids,
anthocyanin and ascorbic acid (Bae and Suh, 2007).
Thus, the addition of pulp in the juice may promote
the higher flavonoids in wine. After 4-month of
aging, the reduction in TFC was seen in all the three
treatments. This result may show the importance of
presence of pulp in the juice for the higher flavonoid
content in wine.
The flavonoids and non-flavonoids are polyphenolic
contents (Lachman et al., 2009) which get affected by
factors such as pH, temperature and aging. The total
flavonoid phenols tended to decrease with aging of
wine. Furthermore, the flavonol, a major class of
flavonoids in wine can react with sugar to form
flavonol glycosides (Hertog et al., 1993). During
wine aging the formation of flavonol glycosides
occurs which undergo self-hydroxylation (Somers,
1971). High antioxidant properties and free radical
scavenging characteristics are due to flavonoids
(Scherer and Godoy, 2009) thus the degradation in
TFC may affect the antioxidant content.
Figure 2: The changes in phenolic of non-aged and 4 month aged mulberry prepared from three different wine making
techniques (HPJ, HPP and CPJ).: (a) total phenolic content, (b) total flavonoids content, (c) total anthocyanin content, and (d)
antioxidant content. The data are expressed as mean ±SD (n=3). The different letter indicates significant difference (p < 0.05).
‘*’ represents the significant difference between non aged and 4 months aged mulberry wines.
Investigation and Comparison of Physicochemical Characteristics of Non-aged and 4-month Aged Mulberry Wine Prepared from Three
Different Wine Making Techniques
83
3.4 TAC
The comparative study of TAC in all the treatments
[Figure 2(c)] showed that HPP had the higher
anthocyanin content (813.79±44.73 mg of cyanidin-
3-glucoside per L) before aging whereas HPJ had the
lower TAC. According to Hunjaroen and
Tongchitpakee (2010), the young mulberry has total
anthocyanin content of 2.6-6.8 mg of cyanidin-3-
glucoside. Chen et al. (2006) states that anthocyanin
is an important constituent of mulberry fruit. Thus the
presence or absence of pulp in juice affects the TAC
in juice and wine. However, slight decrease in the
TPC was observed in three treatments after 4-months
aging. The TAC of non-aged wine showed significant
difference (p < 0.05) with that of 4-month aged wines.
The reduction may be due to the conversion of
anthocyanin into non-pigmented compounds. The
anthocyanin undergoes oxidative polymerization
resulting in oligomeric and polymeric pigments
which convert red wine color to brown (Somers,
1971). Wang et al. (2015) states that the anthocyanin
possesses low stability and factors such as pH,
temperature and oxygen can affect it. Anthocyanin
are more stable at low pH resulting in red pigment
(Wahyuningsih et al., 2017). In this study, the
reduction in acidity during aging may alter the
anthocyanin content as according to (Khoo et al.,
2017), the anthocyanin pigments decreases when
there is lower acidity. These can be the reason for
anthocyanin reduction after 4 months aging.
3.5 Antioxidant Content
The analysis of antioxidant content of all three
treatments was done [Figure 2(d)]. The antioxidant
content of all three treatments ranged from 4297.54 to
4306.09 mg of gallic acid/L. According to Rice-
Evans et al. (1996), polyphenolic components in
higher plants give antioxidant activity. The presence
of other functional bioactive components such as
ascorbic acid (Ercisli and Orhan, 2007) and niacin
(Imran et al., 2010) besides anthocyanins, phenolic
and flavonoids might be the reason for similar amount
of antioxidant content in all the treatments.
However, the decrease in all three treatments was
seen after 4 months of aging. The abundant bioactive
compounds in mulberry give it great antioxidant
properties (Castrejon et al., 2008). The antioxidant
capacity is due to phenolics (Wu et al., 2013) thus
change in phenolics can change the antioxidant
capacity. The decrease in antioxidant content is due to
the reduction of phenolic content, the similar result can
be previously seen in mulberry juice (Zou et al., 2017).
This lower antioxidant is corelated with the result
of TPC, TFC and TAC presented earlier. The wine
made with HPP demonstrated the highest amount of
TPC, TFC, TAC and also antioxidant content which
could be a contribution of longer pulp extraction time
during fermentation.
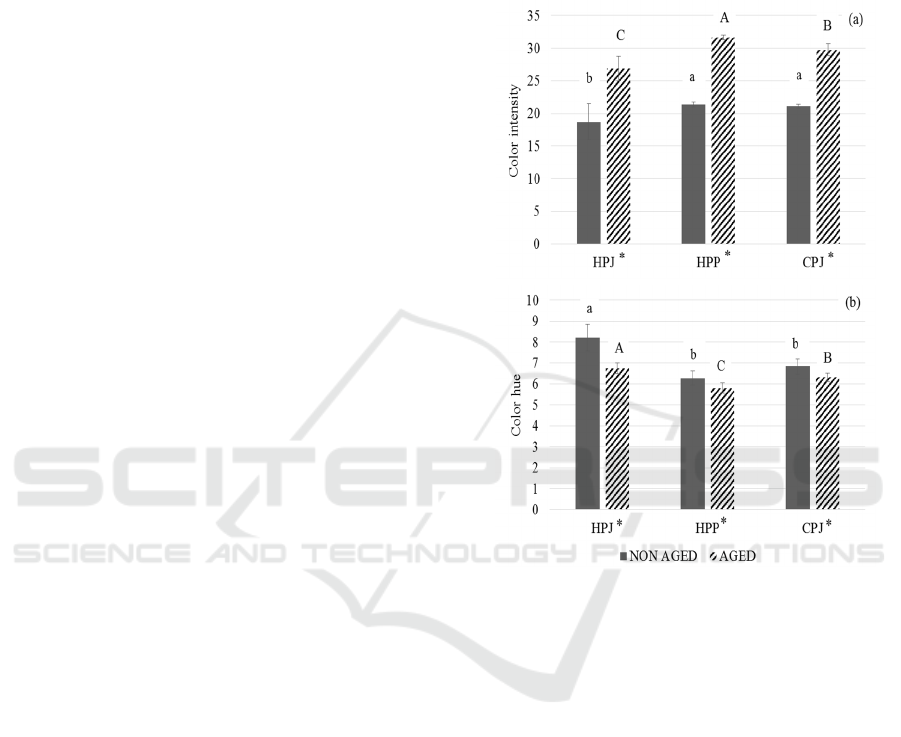
Figure 3: The changes in color measurement of non-aged
and 4 month aged mulberry prepared from three different
wine making techniques (HPJ, HPP and CPJ): (a) color
intensity, and (b) color hue. The data are expressed as mean
±SD (n=3). The different letter indicates significant
difference (p < 0.05). ‘*’ represents the significant
difference between non aged and 4 months aged mulberry.
3.6 Color Measurement
The change in the wine color measurement of three
treatments was analyzed (Figure 3). The wine color
intensity in HPJ, HPP and CPJ are found to be in
range of 18.69 to 21.34 and, the wine color hue of the
same treatments ranged from 6.28 to 8.23 before
aging. Khoo et al. (2017) suggested that the
anthocyanins are responsible for the colors of fruits,
flowers and vegetables. Mulberry is dark red fruit.
The wine color intensity and color hue measured after
4 months aging was found to be increased [Figure
3(a)] and decreased [Figure 3(b)] respectively. After
4 months aging, the color intensity of HPJ, HPP and
CPJ were increased after aging (26.90, 31.64, and
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
84

29.75, respectively). This study showed that HPP has
the highest color intensity before and after aging.
However, the color hue of HPJ, HPP and CPJ were
decreased after 4 months aging (6.75, 5.81, and 6.32,
respectively). In both wine color intensity and hue,
there found to be significant difference (p < 0.05)
between aged and non-aged mulberry wines. HPP has
the highest color intensity whereas HPJ has the
highest color hue. Color intensity shows how dark the
wine is whereas wine color hue measures the
appearance of yellow and red color present in wine.
Anthocyanin is less stable and easily degrade by
pH, storage temperature, light, oxygen,
concentration, enzyme’s presence, proteins and
flavonoids (Rein, 2005). The structure of anthocyanin
and pH has high effect on pigments of anthocyanin
(Torskangerpoll and Andersen, 2005). According to
Ummi et al. (2011), experiment on anthocyanin,
when the acidity decreases, the color changes to
purplish tones causing the decrease in color hue.
Boulton (2001) suggested that anthocyanins can react
with other molecules and convert into co-pigments
and polymerized pigments. In one of the study done
by Heras-Roger et al. (2016), the copigments
decreases hue but increases color intensity in wine
and the copigmentation increases A
520
more than in
A
420
. This condition resembles with current study
in
which A
520
had increased causing decrease in hue.
4 CONCLUSION
The comparison analysis of different treatments of
mulberry wine before aging showed that the different
wine making techniques affect the physicochemical
characteristics of wine. Among all wine making
techniques used, HPP and CJP method produced wine
that have similar level of TFC, and color intensity
which was higher than the wine prepared by HPJ
method. The 4 months aging of mulberry wine at
12±2˚C resulted in increasing level of reducing sugar,
and color intensity of wine. In contrast, the alcohol
content, acidity, phenolic, and color hue was
decreased after aged for 4 months. However, sensory
analysis of wines will be worthwhile to perform in the
future which will help to understand the sensory
characteristic along with consumer preference of
these wines from different wine making techniques.
REFERENCES
Abdullah, A. L., Sulaiman, N. M., Aroua, M. K., and Noor,
M. M. M., 2007. Response surface optimization of
conditions for clarification of carambola fruit juice
using a commercial enzyme. Journal of Food
Engineering, 81(1), pp.65-71.
Ancin-Azpilicueta, C., Gonzalez-Marco, A. and Jimenez-
Moreno, N., 2008. Current knowledge about the
presence of amines in wine. Critical reviews in food
science and nutrition, 48(3), pp.257-275.
AOAC (Association of Official Analytical Chemists).,
2002. Official methods of analysis, 15th ed. Arlington.
Bae, S.H. and Suh, H.J., 2007. Antioxidant activities of five
different mulberry cultivars in Korea. LWT-Food
Science and Technology, 40(6), pp.955-962.
Balga, I., Lesko, A., Ladanyi, M. and Kallay, M., 2015.
Influence of ageing on changes in polyphenolic
compounds in red wines. Czech Journal of Food
Sciences, 32(6), pp.563-569.
Boulton, R., 2001. The copigmentation of anthocyanin and
its role in the color of red wine: A critical review.
American Journal of Enology and Viticulture, 52(2),
pp.67-87.
Butt, M.S., Nazir, A., Sultan, M.T. and Schroën, K., 2008.
Morus alba L. nature's functional tonic. Trends in food
science & technology, 19(10), pp.505-512.
Castrejón, A.D.R., Eichholz, I., Rohn, S., Kroh, L.W. and
Huyskens-Keil, S., 2008. Phenolic profile and
antioxidant activity of high bush blueberry (Vaccinium
corymbosum L.) during fruit maturation and ripening.
Food Chemistry, 109(3), pp.564-572.
Cautela, D., Castaldo, D., Servillo, L., and Giovane, A.
(2010). Enzymes in citrus juice processing. In Enzymes
in Fruit and Vegetable Processing. pp. 211-228. CRC
Press.
Chang, P. H., 2020. Study the effect of fermentation
conditions on physiochemical of Mulberry wine.
Bachelor’s thesis, Faculty of Biotechnology.
Assumption University, Bangkok, Thailand.
Chapman Jr, G.W., Horvat, R. J., and Payne, J.A., 1991.
The non-volatile acid and sugar composition of
mayhaw fruits (Crataegusaestivalis, C. opaca, C.
rufula). Journal of food quality, 14(5), pp.435-439.
Chen, C., You, L. J., Abbasi, A. M., Fu, X., and Liu, R. H.,
2015. Optimization for ultrasound extraction of
polysaccharides from mulberry fruits with antioxidant
and hyperglycemic activity in vitro. Carbohydrate
Polymers, 130, pp.122-132.
Chen, P. N., Chu, S. C., Chiou, H. L., Kuo, W. H., Chiang,
C. L., and Hsieh, Y. S., 2006. Mulberry anthocyanin,
cyanidin3-rutinoside and cyanidin 3-glucoside,
exhibited an inhibitory effect on the migration and
invasion of a human lung cancer cell line. Cancer
letters, 235(2), pp.248-25.
Ercisli, S. and Orhan, E., 2007. Chemical composition of
white (Morus alba), red (Morus rubra) and black
(Morus nigra) mulberry fruits. Food Chemistry, 103(4),
pp.1380-1384.
Heras-Roger, J., Alonso-Alonso, O., Gallo-Montesdeoca,
A., Díaz-Romero, C. and Darias-Martín, J., 2016.
Influence of copigmentation and phenolic composition
on wine color. Journal of food science and technology,
53(6), pp.2540-2547.
Investigation and Comparison of Physicochemical Characteristics of Non-aged and 4-month Aged Mulberry Wine Prepared from Three
Different Wine Making Techniques
85

Hertog, M. G., Feskens, E. J., Kromhout, D., Hollman, P.
C. H., and Katan, M. B., 1993. Dietary antioxidant
flavonoids and risk of coronary heart disease: the
Zutphen Elderly Study. The lancet, 342(8878),
pp.1007-1011.
Hunjaroen, M., and Tongchitpakdee, S., 2010. Effects of
cultivar and maturation on anthocyanins of mulberry
fruit. Warasan Witthayasat Kaset, 41(1), pp.106-109.
Iland, P., 2000. Techniques for chemical analysis and
quality monitoring during winemaking. Patrick Iland
Wine Promotions.
Imran, M., Khan, H., Shah, M., Khan, R., and Khan, F.,
2010. Chemical composition and antioxidant activity of
certain Morus species. Journal of Zhejiang University
Science B, 11(12), pp.973-980.
Ivanova, V., Stefova, M. and Chinnici, F., 2010.
Determination of the polyphenol contents in
Macedonian grapes and wines by standardized
spectrophotometric methods. Journal of the Serbian
Chemical Society, 75(1), pp.45-59.
Jaeger, H., Schulz, M., Lu, P., and Knorr, D., 2012.
Adjustment of milling, mash electroporation and
pressing for the development of a PEF assisted juice
production in industrial scale. Innovative Food Science
and Emerging Technologies, 14, pp.46-60.
Juan, C., Jianquan, K., Junni, T., Zijian, C., & Ji, L., 2012.
The profile in polyphenols and volatile compounds in
alcoholic beverages from different cultivars of
mulberry. Journal of food science, 77(4), pp. C430-
C436.
Khoo, H. E., Azlan, A., Tang, S. T., & Lim, S. M., 2017.
Anthocyanidins and anthocyanins: colored pigments as
food, pharmaceutical ingredients, and the potential
health benefits. Food & nutrition research, 61(1),
1361779.
Kim, I. and Lee, J., 2020. Variations in Anthocyanin
Profiles and Antioxidant Activity of 12 Genotypes of
Mulberry (Morus spp.) Fruits and Their Changes during
Processing. Antioxidants, 9(3), pp.242.
Lachman, J., Šulc, M., Faitová, K. and Pivec, V., 2009.
Major factors influencing antioxidant contents and
antioxidant activity in grapes and wines. International
Journal of Wine Research, 1, pp.101-121.
Martínez-Lapuente L, Guadalupe Z, Ayestarán B., 2017.
Effect of egg albumin fining, progressive clarification
and cross-flow microfiltration on the polysaccharide
and proanthocyanidin composition of red varietal
wines. Food Research International. 96, pp.235-243.
Mattivi, F., Guzzon, R., Vrhovsek, U., Stefanini, M. and
Velasco, R., 2006. Metabolite profiling of grape:
flavonols and anthocyanins. Journal of agricultural and
food chemistry, 54(20), pp.7692-7702.
McDougall, G. J., Fyffe, S., Dobson, P. and Stewart, D.,
2005. Anthocyanins from red wine–their stability under
simulated gastrointestinal digestion. Phytochemistry,
66(21), pp.2540-2548.
Miller, G. L., 1959. Use of di-nitrosalicylic acid reagent for
determination of reducing sugar. Analytical chemistry,
31(3), pp.426-428.
Nadulski, R., Kobus, Z., Wilczyński, K., Guz, T., &
Ahmed, Z., 2017. Characterization of Selected Apple
Cultivars in the Aspect of Juice Production in the
Condition of a Farm.
Nomura, T., Fukai, T., Yamada, S. and Katayanagi, M.,
1976. Phenolic constituents of the cultivated mulberry
tree (Morus alba L.). Chemical and Pharmaceutical
Bulletin, 24(11), pp.2898-2900.
Perez-Cacho, P. R., and Rouseff, R., 2008. Processing and
storage effects on orange juice aroma: a review. Journal
of Agricultural and Food Chemistry, 56(21), pp.9785-
9796.
Rein, M., 2005. Co-pigmentation reactions and color
stability of berry anthocyanins. Dissertation. University
of Helsinki, Department of Applied Chemistry and
Microbiology. EKT series, 1331, 88, pp.10-14.
Ribéreau-Gayon, P., Glories, Y., Maujean, A., &
Dubourdieu, D., 2006. Handbook of Enology, Volume
2: The Chemistry of Wine-Stabilization and Treatments
(Vol. 2). John Wiley and Sons.
Rice-Evans, C. A., Miller, N. J., and Paganga, G., 1996.
Structure-antioxidant activity relationships of
flavonoids and phenolic acids. Free radical biology and
medicine, 20(7), pp.933-956.
Scherer, R. and Godoy, H.T., 2009. Antioxidant activity
index (AAI) by the 2, 2-diphenyl-1-picrylhydrazyl
method. Food chemistry, 112(3), pp.654-658.
Shinohara, T., Shimizu, J. I., &Shimazu, Y., 1979.
Esterification rates of main organic acids in wines.
Agricultural and Biological Chemistry, 43(11),
pp.2351-2358.
Šimić, G., Horvat, D., Dvojković, K., Abičić, I., Vuletić, M.
V., Tucak, M., & Lalić, A., 2017. Evaluation of total
phenolic content and antioxidant activity of malting and
hulless barley grain and malt extracts. Czech Journal of
Food Sciences, 35(1), pp.73-78.
Somers, T. C., 1971. The polymeric nature of wine
pigments. Phytochemistry, 10(9), pp.2175-2186.
Suh, H. J., Kim, J.M., Lee, H., Lee, S.W. and Choi, Y.M.,
2004. Thermal kinetics on antiradical capacity of
mulberry fruit extract. European Food Research and
Technology, 219(1), pp.80-83.
Torskangerpoll, K. and Andersen, Ø.M., 2005. Colour
stability of anthocyanins in aqueous solutions at various
pH values. Food Chemistry, 89(3), pp.427-440.
Ummi, K. I., Ida, I. M., and Ruzitah, M. S., 2011. The effect
of pH on color behavior of Brassica oleracea
anthocyanin. Journal of Applied Sciences, 11(13),
pp.2406-2410.
Vijayan, K., Tikader, A., Weiguo, Z., Nair, C. V., Ercisli,
S., and Tsou, C. H., 2011. Morus. In Wild Crop
Relatives: Genomic and Breeding Resources (pp. 75-
95). Springer, Berlin, Heidelberg.
Wahyuningsih, S., Wulandari, L., Wartono, M. W.,
Munawaroh, H., & Ramelan, A. H., 2017. The effect of
pH and color stability of anthocyanin on food colorant.
In IOP conference series: Materials science and
engineering. 193(1), pp.12047.
Wang, L., Sun, X., Li, F., Yu, D., Liu, X., Huang, W. and
Zhan, J., 2015. Dynamic changes in phenolic
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
86

compounds, colour and antioxidant activity of mulberry
wine during alcoholic fermentation. Journal of
Functional Foods, 18, pp.254-265.
Wang, Z., Cui, H., and Fan, S., 2020. Effect of mechanical
juice extraction method on the quality of fresh-
squeezed apple juice. In IOP Conference Series:
Materials Science and Engineering, 711(1), pp.
012051.
Waterhouse, A. L., 2002. Determination of total phenolics.
Current protocols in food analytical chemistry, 6 (1).
Wu, T., Tang, Q., Gao, Z., Yu, Z., Song, H., Zheng, X. and
Chen, W., 2013. Blueberry and mulberry juice prevent
obesity development in C57BL/6 mice. PLoS One,
8(10), p.e77585.
Yang, C. H., & Tsai, T. C., 1994. Anthocyanins in mulberry
fruit. Food Science, 21, pp.319-330.
Yıldırım, H. K., and Altındışli, A., 2015. Changes of
Phenolic Acids During Aging of Organic Wines.
International Journal of Food Properties, 18(5),
pp.1038-1045.
Zhishen, J., Mengcheng, T. and Jianming, W., 1999. The
determination of flavonoid contents in mulberry and
their scavenging effects on superoxide radicals. Food
chemistry, 64(4), pp.555-559.
Zou, B., Xu, Y. J., Wu, J. J., Yu, Y. S., and Xiao, G. S.,
2017. Phenolic compounds participating in mulberry
juice sediment formation during storage. Journal of
Zhejiang University-SCIENCE B, 18(10), pp.854-866.
Investigation and Comparison of Physicochemical Characteristics of Non-aged and 4-month Aged Mulberry Wine Prepared from Three
Different Wine Making Techniques
87
