
Identification of Food Natural Antimicrobe Compound from Waru
Leaves (Hisbicus tillacaeus L.) Extract by GC-MC
Dewi Sartika, Samsu Udayana Nurdin, Neti Yuliana, Susilawati and Wahyudi
THP Department, Faculty of Agriculture, University of Lampung, Indonesia
Keywords: A Natural Antimicrobial, Agent Chicken Meat, Escherichia coli, Waru Leaves.
Abstract: Chicken is the most common source of animal protein in Indonesia due to its high protein content and low
price. However, because of was not a standardized process, chicken meats sold in the traditional market is
mostly contaminated by phatogen bacteria such as Escherichia coli. Previous research indicated of waru
(Hibiscus tillaceus L.) inhibited Escherichia coli growth. Therefore in this research, had aim 1) to study
whether the antimicrobial activity of extracts of waru/hisbiscus by GCMS, 2) to find out the activity of waru
that have antimicrobial activity against Escherichia coli contaminant. The results showed that the
antimicrobial activity of waru leave extracts depends on the respective proportion in the blend. Increasing
antimicrobial activity was observed when waru leaves extract concentration was an increase in the mixture.
The results of the research showed that content of the leaves of waru was dominated by Phytol (38%); methyl
ester (22,1%); Pentadecanoic acid,14-methyl-Squalene (6,58%); Bis (2-Ethylhexyl) phthalate (5,94%); and
ester compound, that had as an antimicrobe potency.
1 INTRODUCTION
The microbes that often contaminate meat or high
protein food are pathogenic microbes, such as,
Escherichia coli and Salmonella sp. (Sartika et al.,
2019). Contamination of pathogenic microbes can
cause various diseases, such as fever, typhoid,
diarrhea, etc. or often referred to as a foodborne
disease. Djafaar and Rahayu (2007) explained that
Escherichia coli contamination can cause denatured
protein on meat and produce toxin compounds. These
toxin compounds can cause several cases of
foodborne disease, one of them as diarrhea (Jawetz et
al., 1995). Escherichia coli or pathogenic microbes
contamination needs to be inhibited to reduce the
number of pathogenic microbes contamination and
prevent damage to chicken meat.
Contamination of pathogenic microbes on high
protein foodstuffs, meat, generally, can be inhibited
by cooling treatment. The other inhibition microbe
treatments, such as the addition of salt, sugar, acid,
and preservatives with synthetic or chemical
preservatives (Usmiati, 2010). However, the methods
that use preservatives with synthetic or chemical
preservatives can have a serious impact on
consumers. So, need a natural preservative method
that has a low risk of health. Therefore, it is necessary
to develop more effective antimicrobials such as
natural antimicrobials in inhibiting pathogenic
microbes contamination on meat. Natural
antimicrobials are recommended because not cause
side effects or negative effects. The natural
preservative method can use by a natural antimicrobe
from natural resources.
Indonesia is a country that has various natural
resources, such as waru (Hisbicus Tillacaeus L.).
Waru is a popular plant in Indonesia. The leaves of
waru were presumed can act as a natural
antimicrobial because contain antimicrobial
compounds. The waru leaves contain saponins,
flavonoids, tannins, and polyphenols. Meanwhile,
teak leaves contain flavonoids, saponins, tannins,
katekat tannins, quinones, steroids/triterpenoids
(Hartati et al., 2007). The antimicrobial can be
explored from environmental such as dragon fruit
leather (Sartika et al., 2019); plant extract (katalinic
et al., (2006); kapok bananas (Ningsih and Nurmiati
(2013); gambir (Pambayun et al., (2007); red rosella
(Putri et al., (2006) and bacteriophage (Sartika et al.,
2002).
Antimicrobial compounds from waru leaves can
be isolated through extraction processes such as
maceration, soxhlet process, percolation, reflux, and
Sartika, D., Nurdin, S., Yuliana, N., Susilawati, . and Wahyudi, .
Identification of Food Natural Antimicrobe Compound from Waru Leaves (Hisbicus tillacaeus L.) Extract by GC-MC.
DOI: 10.5220/0010529300003108
In Proceedings of the 6th Food Ingredient Asia Conference (6th FiAC 2020) - Food Science, Nutrition and Health, pages 69-74
ISBN: 978-989-758-540-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
69

so on. Maceration is an extraction method that is
simple, easy to perform, and does not require high
costs. The macerated extract of hibiscus leaves was
effective in inhibiting the growth of Bacillus subtilis
and Escherichia coli. In the fact, the content of waru
leaves does not found exactly. So, this research aimed
was to find the waru content with use by GCMS.
2
MATERIAL
AND
M
ET
HO
D
2.1 Material
Materials used in this research were waru leaves that
were collected from Lampung Timur of Lampung
province, ethanol 70%, polar dissolvent, aqua
distillate, and alcohol 70%. type of Equipment used
included knife, basin, blender, filter paper, macerator,
beaker tube, Erlenmeyer tube, vacuum rotary
evaporator, scale tube, stirrer, Gas Chromatography-
Mass Spectrometry (GC-MS).
2.2 Study Area
Waru leaves were obtained from Sribahwono Village,
Bandar Sribhawono District, East Lampung Regency,
Lampung Province. Selected samples of
waru/hisbiscus that were not too old, not too young,
healthy, and not moldy were included (Figure 1).
Figure 1: Location of Desa Sribahwono indicating the
sampling sites of Hibiscus tiliaceus L.; 5°18' 40.76" S | 105°
44' 31.24" E.
2.3 Method
This research was conducted in two stages. The first
stage was the preparation and extraction process of
waru leaves sample at The Laboratory of Chemistry,
Faculty of Sciences at Lampung University. The
second stage of the research was to test the chemical
compounds that contained the waru leaves by using
Gas Chromatography-Mass Spectrometry (GC-MS)
which was conducted in the Integrated Laboratory of
Lampung University.
2.3.1 Powder Making and Leaf Extract
(Ningsih et al., 2013)
The leaves are cleaned first using clean water to
remove dirt, then cut into small pieces. Furthermore,
it is oven using 500C temperature for 24 hours. The
use of temperature and time of the oven is intended to
prevent the active compound in the leaves from being
damaged (Putri et al., 2014). After that, the dry leaves
are ground using a blender to produce leaf powder.
Then the leaf powder is sieved using a 40 mesh sieve
to obtain a uniform leaf powder. Sembiring et al.
(2006) reported that 40 mesh powders can produce a
high yield of active substances after the extraction
process. The powdered leaves of waru and teak leaves
that have been obtained are then mixed according to
the proportions for each treatment. After that, the
powder was immersed in boiling distilled water (1000
C) for 10 minutes. The use of boiling distilled water
(1000 C) as a solvent aims to improve the solubility
of the extract (Pambayun et al., 2007). Furthermore,
it is filtered using filter paper to obtain leaf extract.
2.3.2 Total Microbial Decrease Test
The total microbial reduction test was carried out in
several stages. First, the Escherichia coli bacteria
were rejuvenated (Suwandi, 2012) and made a
turbidity standard of 0.5 Mc Farland (Sutton, 2011).
Then the bacterial suspension was made, where the
bacterial suspension was compared to the standard
0.5 Mc Farland using a spectrophotometer with a
wavelength of 600 nm. After that, total Escherichia
coli testing was carried out on fresh fillet chicken
meat, tested the antimicrobial inhibition of each
treatment using disc paper (Suwandi, 2012), and
decreased total Escherichia coli in chicken meat.
2.3.3 Data Analysis
Antimicrobial inhibition test data, total reduction test
for Escherichia coli in chicken meat, test for phenol
content of chicken meat, and test for the degree of
acidity (pH) were further tested with the descriptive
method. Then the test data for the application of
antimicrobial activity and sensory tests were further
tested using the Microsoft Excel application (Anova
and LSD).
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
70

3 RESULTS AND DISCUSSION
3.1 Waru Characteristic by GC-MS
Method
The preparation process of this research was the waru
leaves were taken in the morning, washed, and dried
to remove dust on the material (figure 2). It was
shredded into smaller sizes. Then, the small slice of
waru was dried to remove water content using an
oven. The waru leaves dry powder, 500 grams of dry
weight, was taken to make extraction by using ethanol
70% solvent.
Figure 2: Extraction of Waru leaves.
The step of Chemical Compounds research was 3
μl of the sample (waru leaves extract) which had been
washed with an ethanol solvent that was taken by
using a gas chromatograph inlet. The sample was
injected into a gas chromatograph by the following
conditions: Gas Chromatograph with AutoSampler
(Agilent Technologies 5973 N) and Mass Selective
Detector 5873 I; Capillary Column (Innowax) with
dimension of 60 m length, 0.2mm wide, 0.25 mm film
thickness; 290
o
C injector temperature; 290
o
C
temperature detector; temperature program of
90
o
C(150 minutes) - 290
o
C (20 minutes); carriage gas
– Helium 1 ml/min with the constant flow; 1uL Split
(ratio 50:1) injection volume; ethanol solvent. The
analysis of chemical compounds contained in waru
leaves extract was conducted by using Gas
Chromatography-Mass Spectrometry (GC-MS).
Chromatograph results are presented in Figure 3.
Figure 3: Chromatograph graphic of the waru leaves
extract.
The result of chromatograph graphic showed that
the waru had characteristic content from high to low
rating, as follows, Phytol (38,70%); Pentadecanoic
acid,14-methyl-,methyl ester (21,11%); 9,12,15-
Octadecatrienoic acid,methyl-ester, (Z,Z,Z)- (8,24%);
8,11-Octadecadienoic acid, methyl ester (5,59%). The
other compound of waru leaves content by GCMS was
summarized completely in Table 1.
Table 1: Waru Chemical compounds.
The Chemical Compound Area
%
Area
1. 4-((1E)-3-Hydroxy-1-
p
ro
p
en
y
l
)
-2-methox
yp
henol 16.978 1,39
3,7,11,15-Tetramethyl-
2-hexadecen-1-ol 9.722 0,80
2H-Pyran,2-(7-
heptadecynyloxy)tetrahydro- 7.685 0,63
3,7,11,15-Tetramethyl-
2-hexadecen-1-ol 6.447 0,53
11-Hexadecenoica cid,
methyl este
r
2.510 0,21
Pentadecanoic acid,14-
methyl-,methyl este
r
258.045 21,11
n-Hexadecanoic acid
58.626 4,80
n-Hexadecanoic acid
8.092 0,66
Heptadecanoic
acid,meth
y
l este
r
12.992 1,06
8,11-Octadecadienoic
acid,meth
y
l este
r
68.372 5,59
9,12,15-
Octadecatrienoic
acid,meth
y
l ester,
(
Z,Z,Z
)
- 100.746 8,24
Phytol
472.954 38,70
Octadecanoic
acid,methyl este
r
29.407 2,41
9,12,15-
Octadecatrienoic acid,2,3-
dihydroxypropyl ester,
(Z,Z,Z)- 5.547 0,45
9,12-Octadecadienoic
acid,meth
y
l ester,
(
E,E
)
- 2.505 0,20
Pentatriacontane 5.673 0,46
Cedran-diol,8S,14-
2.878 0,24
Bis(2-ethylhexyl)
p
hthalate 72.592 5,94
Squalene
80.480 6,58
3.2 Antimicrobial Inhibition
The results of the research showed that the proportion
of hibiscus leaves gave an effect on the inhibition
zone width. The increase of concentration would
increase the zone inhibition. The highest of inhibition
Identification of Food Natural Antimicrobe Compound from Waru Leaves (Hisbicus tillacaeus L.) Extract by GC-MC
71
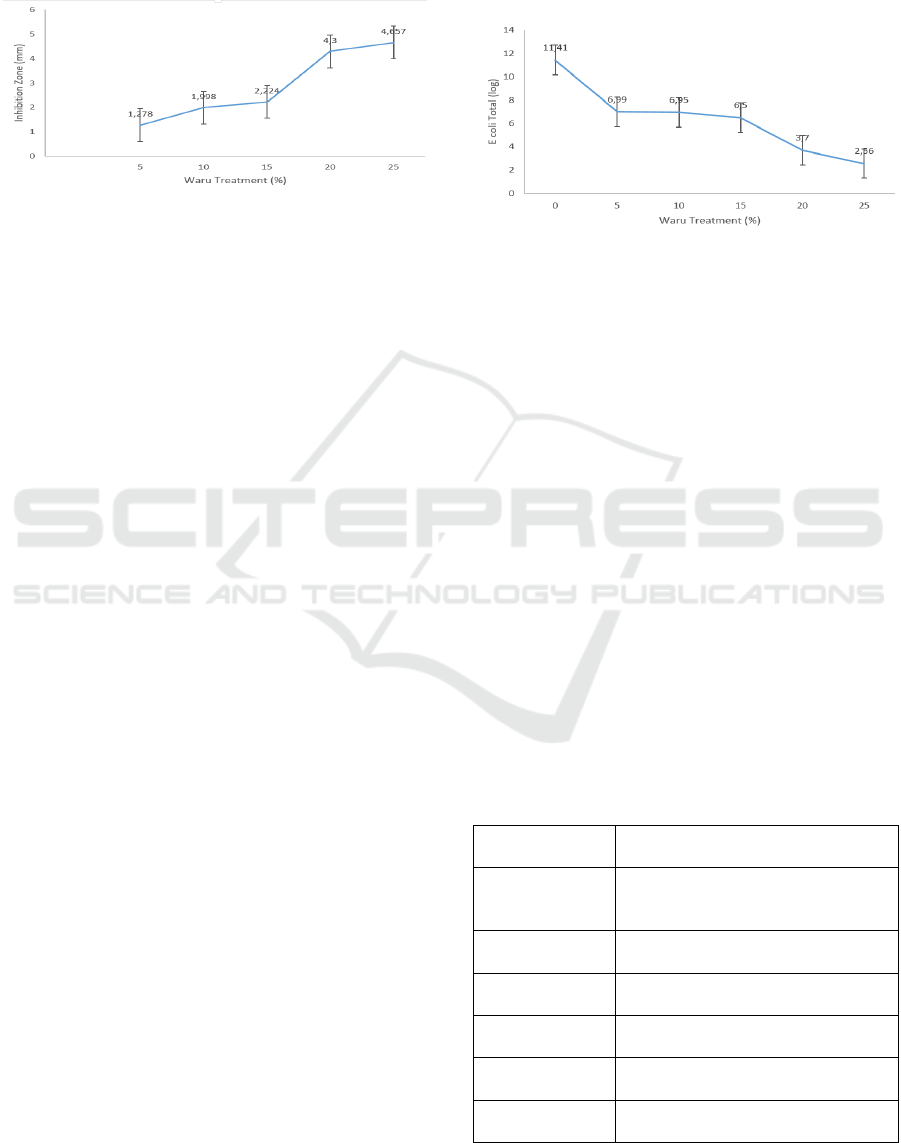
zone was reached at the 25% (4,567 ± 0,320) and the
lowest was at the 0% (1,278 ± 0,298) concentration
level. The following figure describes that the effect of
hibiscus extract concentration (Figure 4).
Figure 4: The effect of the hibiscus extract on Escherichia
coli inhibition.
The research result showed that the concentration
of waru/hisbiscus leaves affected inhibition zone
width, which described that waru was effective against
Escherichia coli bacteria. The best treatment was
reached out at a 25% concentration level This
happened probably because the highest of waru
concentration had the highest antimicrobial
compounds. According to Oktavia (2018), hibiscus
leaf extract at a concentration of 25% has a flavonoid
level of 21.7398%. Meanwhile. Reducing Escherichia
coli by Waru or hisbiscus on chicken meat meat.
The total yield of Escherichia coli in chicken
fillets from Pasar Tempel, Rajabasa Raya, Bandar
Lampung was 2,775 ± 0.775 x 104 colonies/gram.
This case indicates that Escherichia coli
contamination on the fillet chicken meat upper than
the maximum contamination limit set by SNI-7388-
2009 (1x101 colony/gram). Escherichia coli
contamination can be caused by the unhygienic
handling process. Therefore, Escherichia coli
contamination in chicken meat can be prevented by
performing hygienic handling with the best possible
process or added preservative.
The results of the analysis of variance showed that
the proportion of the mixture of hibiscus leaves and
teak leaves had a significant effect on the total
reduction of Escherichia coli bacteria. The results of
further testing with the least significant difference
(LSD) at the 5% level showed that the control
treatment was not significantly different from the
W6J6 treatment (0% of waru leaves and 25% of teak
leaves), but was significantly different from other
treatments. The treatments of W3J3 (15% waru
leaves and 10% teak leaves), W4J4 (10% waru leaves
and 15% teak leaves), and W5J5 (5% waru leaves and
20%) were not significantly different. Then the W2J2
treatment (20% waru leaves and 5% teak leaves) was
significantly different from the W1J1 treatment (25%
waru leaves and 0% teak leaves) and the two
treatments were significantly different from other
treatments. The graph of the effect of mixing the
extract of hibiscus leaves and teak leaves on the
decrease in total Escherichia coli.
Figure 5: The effect of the extract of hibiscus/waru leaves
on the Escherichia coli total.
Figure 5 described that the highest proportion of
hibiscus/waru leaves gave an effect to the Escherichia
coli total was highest too. Thus, the results of the total
microbial reduction test were in line with the
antimicrobial inhibition zone test. The concentration
level at 25% hibiscus leaves treatment showed the
highest total reduction of Escherichia coli on chicken
meat with a decreasing percentage of 76.12%.
Meanwhile, the resulting research showed that the
lowest treatment (0%) tend to decrease of Escherichia
coli on chicken meat was lowest too (log E. coli total
was 6.59%). So, Extract waru leaves can be
categorized as an antimicrobial or a bacteriostatic
compound. According to Pelezar and Chan (1988),
antimicrobials can work bacteriostatically (inhibit
microbial growth) and bactericidal (kill microbes).
Characteristics of Escherichia coli colonies that grow
on Eosin Methylene Blue (EMB) media are described
in Table 1.
Table 2: Characteristics of Escherichia coli colonies
growing on Eosin Methylene Blue (EMB) media.
Treatment
Colony Characteristics of
Escherichia coli
Control
Darkcore purple with metallic green
color. Grows on the base, middle,
and surface of the media
25%
Dark purple core. Grows on the base
of the mediu
m
20%
Dark purple core. Grows in the
middle, and base of the mediu
m
15%
Dark purple core. Grows in the
middle, and base of the mediu
m
10%
Darkcore purple with metallic green
on the base, middle, and surface
5%
Darkcore purple with metallic green
on the base, middle, and surface
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
72

In Table 2, it can be seen that the characteristics
of Escherichia coli colonies that grow on Eosin
Methylene Blue (EMB) media are different. The
results showed that the addition of hibiscus leaf
extract with a greater proportion could prevent the
formation of the metallic green color of Escherichia
coli colonies on Eosin Methylene Blue (EMB) media.
This is evidenced by the growth of the Escherichia
coli bacteria forming colonies with dark purple cores
without metallic green on EMB media treated by a
25% waru leaves, 20% waru leaves, and 15% hibiscus
leaves. While the EMB medium was treated with 0%
waru leaves, 5% waru leaves, 0% waru leaves formed
Escherichia coli bacteria colonies which are dark
purple with a metallic green color. According to
Connie et al. (2015) on EMB media, Escherichia coli
bacteria can ferment lactose quickly and produce
acid. As a result of acidic conditions, eosin will
change color from clear to dark purple which is
usually accompanied by metallic green. This is
supported by the statement by Molita (2017) which
states that bacteria that ferment lactose from EMB
media produce colonies with dark cores with black
dots and a metallic green sheen. Escherichia coli
colonies that did not form a metallic green color on
EMB media were treated with the extract with more
mixture of leaves indicating that the lactose
fermentation process was not running optimally.
4 CONCLUSIONS
The Extract waru leaves had an antimicrobe potency
and can be categorized as an antimicrobial or a
bacteriostatic compound, it can be seen on the
resulting test using by GC-MS and inhibition zone
method. The GC-MS test result showed that the waru
had characteristic content from high to low rating, as
follows, Phytol (38,70%); Pentadecanoic acid,14-
methyl-,methyl ester (21,11%); 9,12,15-
Octadecatrienoic acid,methyl ester,(Z,Z,Z)- (8,24%);
8,11-Octadecadienoic acid,methyl ester (5,59%). The
highest proportion of hibiscus/waru leaves gave an
effect to the Escherichia coli total was highest too.
Thus, the results of the total microbial reduction test
were in line with the antimicrobial inhibition zone
test. The concentration level at 25% hibiscus leaves
treatment showed the highest total reduction of
Escherichia coli on chicken meat with a decreasing
percentage of 76.12% (inhibition zone method).
ACKNOWLEDGEMENTS
The authors would like to thank Kemenristek DIKTI-
BRIN.
REFERENCES
Connie, R. 2015. Textbook of Diagnostic Microbiology 5th
Edition. Philadelphia. Saunders Elsevier. 181-420 hlm.
Djaafar, T.F. S. Rahayu. 2007. Cemaran Mikroba Pada
Produk Pertanian, Penyakit yang ditimbulkan dan
Pencegahannya. Jurnal Litbang Pertanian. 26(2): 67-
75.
Hartati, R.S.A. Gana dan K., Ruslan. 2007. Telaah
flavonoid dan Asam Fenolat Daun Jati (Tectona
grandis L. f., verbenaceae). Institut Teknologi
Bandung. Bandung.
Jawetz, E. J. L. Melnick, E. A. Adelberg, G. F. Brooks, J.
S. Butel, L. N. Ornston. 1995. Mikrobiologi Kedokteran
ed. 20. University of California. San Francisco.
Katalinic, V. Milos, M. Kulisic, T. and Jukic, M. 2006.
Screening of 70 Medicinal Plants Exstracts for
Antioxidant Capacity and Total Phenolics. Food
Chemistry 94:550-557.
Ningsih, A.P. dan Nurmiati, A. A. 2013. Uji Aktivitas
Antibakteri Ekstrak Kental Tanaman Pisang Kepok
Kuning (Musa Paradisiaca Linn.) terhadap
Staphylococcus aureus dan Echerichia coli. Jurnal
Biologi Universtas Andalas. 2(3): 207-213.
Pambayun, R. Murdijati G. Slamet, S. dan Kapti, R. 2007.
Kandungan Fenol Dan Sifat Antibakteri Dari Berbagai
Jenis Ekstrak Produk Gambir (Uncaria gambir Roxb).
Majalah Farmasi Indonesia. 8(3), 141-146.
Putri, D. D. Nurmagustiana, D. E. dan Chandra, A.A. 2014.
Kandungan Total Fenol dan Aktivitas Antibakteri
Kelopak Buah Rosela Merah dan Ungu Sebagai
Kandidat Feed Additive Alami Pada Broiler. Jurnal
Penelitian Pertanian Terapan. Vol. 14 (3): 174-180.
Rafi, M. Widyastuti, N. Elly, S. dan Latifah, D.K. 2012.
Aktivitas Antioksidan, Kadar Fenol, dan Flavonoid
Total dari Enam Tumbuhan Obat Indonesia. Jurnal
Bahan Alam Indonesia. Vol. 8 (3).
Sartika D, Novita H dan Suci NK. 2019. Aktivitas
Antimikroba Ekstrak Kulit dan Jantung Pisang Muli
(Musa Acuminata) terhadap Bakteri Escherichia coli. J.
Agritech: 39. ISSN: 2527-3825. http://
https://doi.org/10.22146/agritec.
Sartika, D, Budiarti S, and Mirnawati. 2012. Safety The
Effect Of Indigenous Salmonella P38 Phage (Phage
Fr38) On Sprague Dawley Strain Rat. J HAYATI J. of
Biosci. Vol. 19 No. 3, p 131-136.
Sutton, S. 2011. Measurement of Microbial Cells by
Optical Density. J. Of Validation Technology. XVII (I):
46-49.
Suwandi, T. 2012. Pengembangan Potensi Antibakteri
Kelopak Bunga Hibiscus Sabdariffa L. (Rosela)
Terhadap Sterptococcus Sanguinis Penginduksi
Gingivitis Menuju Obat Herbal Terstandar. (Disertasi).
Identification of Food Natural Antimicrobe Compound from Waru Leaves (Hisbicus tillacaeus L.) Extract by GC-MC
73

Program Doktor Ilmu Kedokteran Gigi Universitas
Indonesia. 170 pp.
Usmiati, S. 2010. Pengawetan Daging Segar dan Olahan.
Balai Besar Penelitian dan Pengembangan Pascapanen
Pertanian, Bogor. Jurnal Teknologi Sains. 9(3):46-51.
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
74
