
Profile of Sweet Potato Fermentation using Leuconostoc
Mesenteroides as a Starter
Neti Yuliana
a
, Dewi Sartika, Sutikno and Edo Jatmiko
Teknologi Hasil Pertanian (THP), Faculty of Agriculture, Universityu of Lampung,
Sumantri Brpjonegoro #1, Bandar Lampung, Inodonesia
Keywords: EPS, Fermentation Profile, Mesenteroides.
Abstract: This study aimed to know the fermentation profile of yellow sweet potato (total lactic acid bacteria, total non-
lactic acid bacteria, total lactic acid, pH, total exopolysaccharides, and morphology changing on starch
granules) using Leuconostoc mesenteroides as a starter. The sample's withdrawal was performed at 0, 24, 48,
and 72 hours. The results showed that during 72 hours fermentation time, there was a linearly decreased of
pH (minimum at pH 3.80), a linearly increased of total lactic acid (0.0023% /h), reducing sugars (0.26
mg/ml/h), crude exopolysaccharides (EPS) (0.017 g/l/h), and total Lactic Acid Bacteria (LAB) (maximum at
log 8.40 cfu/ml), as well as a decreased of non-Lactic Acid Bacteria. Leuconostoc mesenteroides had
significant effect on granule of yellow sweet potato. There was an alteration of starch granules at the end of
fermentation time (at 72 hours).
1 INTRODUCTION
Yellow sweet potato is a source of carbohydrates, so
that it has good potential to be developed in support
of food diversification programs. Yellow sweet
potato is also a beta-carotene (provitamin A) sources
(Kammona et al., 2015). Some examples of sweet
potato-based processed products are baby food, salad
dressings, cake mix (Anggraeni & Yuwono, 2014),
pickle (Oke & Workneh, 2013; Oloo, 2013; Neti
Yuliana et al., 2013), and processing based on sweet
potato flour (Sebben et al., 2017). To produce more
applicable sweet potato flour, a modification process
is required. Modification of sweet potato flour can be
done by fermentation of lactic acid (Ajayi et al., 2016,
2018; Liao & Wu, 2017; Yuliana et al., 2018; .
Yuliana et al., 2017; Yuliana et al., 2014) The
application of lactic fermentation in flour
modification will produce flour that is easy to expand
and tastes better. Besides, fermentation with the help
of specific lactic acid bacteria has the advantage of
being able to produce exopolysaccharides (EPS)
(Yuliana et al., 2020; Zubaidah et al., 2014) which
have many benefits, including improving the
properties of flour..
a
https://orcid.org/0000-0003-2759-7735
The lactic acid fermentation process can occur with
the help of a lactic acid bacteria starter (LAB). One of
the LABs that produce EPS is Leuconostoc
mesenteroides (Li et al., 2020; Taylan et al., 2019).
These bacteria include heterofermentative lactic acid
bacteria, which break down glucose and produce 50%
lactic acid, ethanol, acetic acid, glycerol, mannitol,
and CO2 (Mora-Villalobos et al., 2020). In addition
to Leuconostoc mesenteroides, a lactic acid bacterial
starter can be obtained from a pickle liquid starter
with added salt (Yuliana et al., 2018). Lactic acid
bacteria can also be obtained from a spontaneous
fermentation process with added salt. In this study,
sweet potato fermentation was carried out using the
starter Leuconostoc mesenteroides from the culture
collection unit.
The success of the lactic acid fermentation
process is strongly influenced by optimizing the
desired LAB growth factors. These factors then
provide different conditions according to the LAB
environment, which ultimately affects the
fermentation process. Each LAB starter will also
show different growth patterns, the period needed to
grow and adapt, and the resulting metabolites (Yang
et al., 2018). Information about growth patterns and
metabolites produced is needed to determine the
64
Yuliana, N., Sartika, D., Sutikno, . and Jatmiko, E.
Profile of Sweet Potato Fermentation using Leuconostoc Mesenteroides as a Starter.
DOI: 10.5220/0010514200003108
In Proceedings of the 6th Food Ingredient Asia Conference (6th FiAC 2020) - Food Science, Nutrition and Health, pages 64-68
ISBN: 978-989-758-540-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
fermentation efficiency as the growth and formation
of products affect the responsiveness of cells. During
growth, microorganisms require a substrate as the raw
material used for cell multiplication and the formation
of metabolite products (Utami et al., 2012; Zubaidah
et al., 2014) Thus, the fermentation profile related to
growth patterns, substrate consumption, and
metabolite production is needed to determine
fermentation's optimum conditions.
Fermentation of sweet potatoes using a starter of
lactic acid bacteria to improve the characteristics of
sweet potato flour has been reported (El Sheikha &
Ray, 2017). Yuliana et al., (2013) examined the
fermentation of yellow sweet potato pickles using
mixed LAB cultures, which produced the best
characteristics of pickles organoleptically with a total
lactic acid value of 0.5%, pH 3.39, and a total lactic
acid bacteria of 8.46 log CFU / mL. So far, research
on the sweet potato fermentation process has been
done a lot, but it is still limited to white sweet
potatoes. There is no information regarding the
growth patterns of lactic acid bacteria, changes in
starch granules, and exopolysaccharides during
yellow sweet potato fermentation. So that in this
study, the fermentation profile of yellow sweet potato
with the starter of lactic acid bacteria Leuconostoc
mesenteroides as a starter was studied
2 MATERIALS AND METHOD
2.1 Materials
The main ingredient used in this study was a yellow
sweet potato purchased at the traditional market in
Bandar Lampung, Indonesia. Leuconostoc
mesenteroides FNCC-0023 was from PAU Pangan
dan Gizi, University of Gajah Mada, Indonesia.
Media used were MRS broth, and MRS agar.
2.2 Method
The sweet potatoes were washed, peeled, sliced, and
added to glassware containing a boiled solution of
salt-sugar and were left at room temperature. The
sweet potato slices were fermented with Leuconostoc
mesenteroides FNCC-0023 as starters.. Observations
were performed on total LAB (Yuliana et al., 2013),
and biochemical changes: pH, total acidity as % of
lactic acid, total glucose of supernatant (phenol-
sulphuric method), and amount of crude
exopolysaccharide (Razack et al., 2013) 2013).
Sampels were withdrawal at 0 hours (H0), 24 hours
(H24), 48 hours (H48), and 72 hours (H72). A 72
hours of fermentation was selected for observation of
change in sweet potato starch granule by using
scanning electron microscopy.
2.3 Data Analysis
Experimental unit was repeated three times. All data
were analyzed to find the average and subjected to
polynomial trend line to find either linearly or
quadratically pattern in which the rate of the
parameter observed was determined.
3 RESULTS AND DISCUSSION
3.1 Change of Lactic Acid Bacteria,
Total Lactic Acid and Ph
During fermentation process, LAB utilized starch and
sugar in yellow sweet potato as an energy source for
cell multiplication and produced metabolites such as
lactic acid (Oloo, 2013) and exopolysaccharides
(Zubaidah et al., 2014) and resulting in a decrease in
pH (Yuliana et al., 2013). LAB activity will degrade
and modify starch granules (Liao & Wu, 2017;
Yuliana et al., 2014) and leave reducing sugars.
(Yuliana et al., 2013) The data recapitulation of
biochemical changes during fermentation is
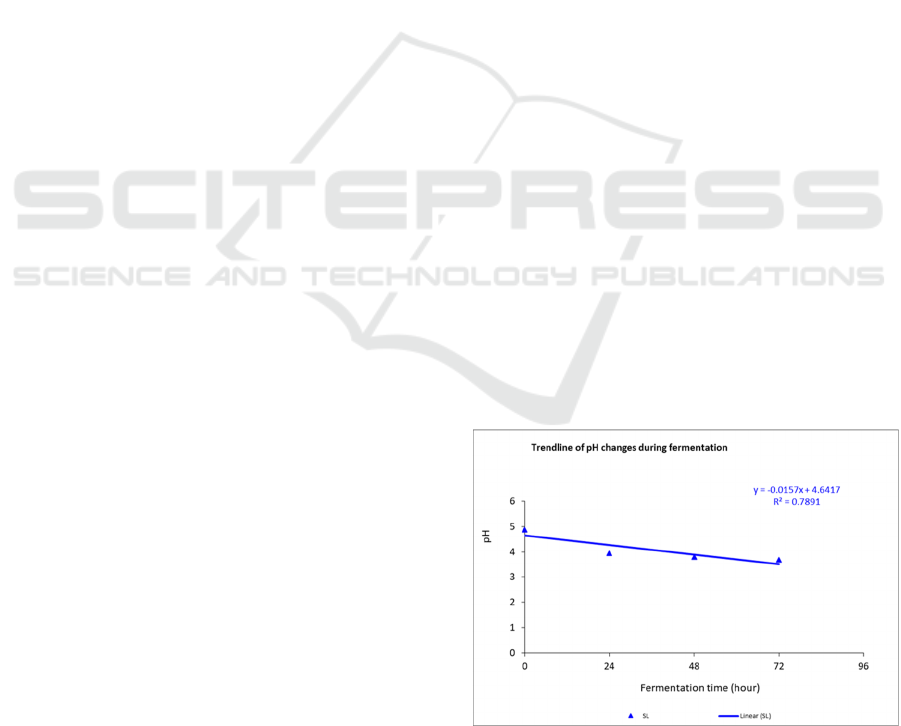
presented in Figure 1.
There was a linear increase in total lactic acid
from 0.61 to 1.802% during fermentation. On the
other hand, there was a linier decreased in pH from
point 4.9 to 3.6. An increase of total lactic acid during
fermentation occurred at a rate of 0.2%, as
Leuconostoc mesenteroides FNCC-0023 starter
activity.
Figure 1: Trend line of pH, Lactic Acid Bacteria, Total
Lactic Acid, EPS and Reducing Sugar during Fermentation
of Yellow Sweet Potato with Leuconostoc mesenteroides.
Profile of Sweet Potato Fermentation using Leuconostoc Mesenteroides as a Starter
65
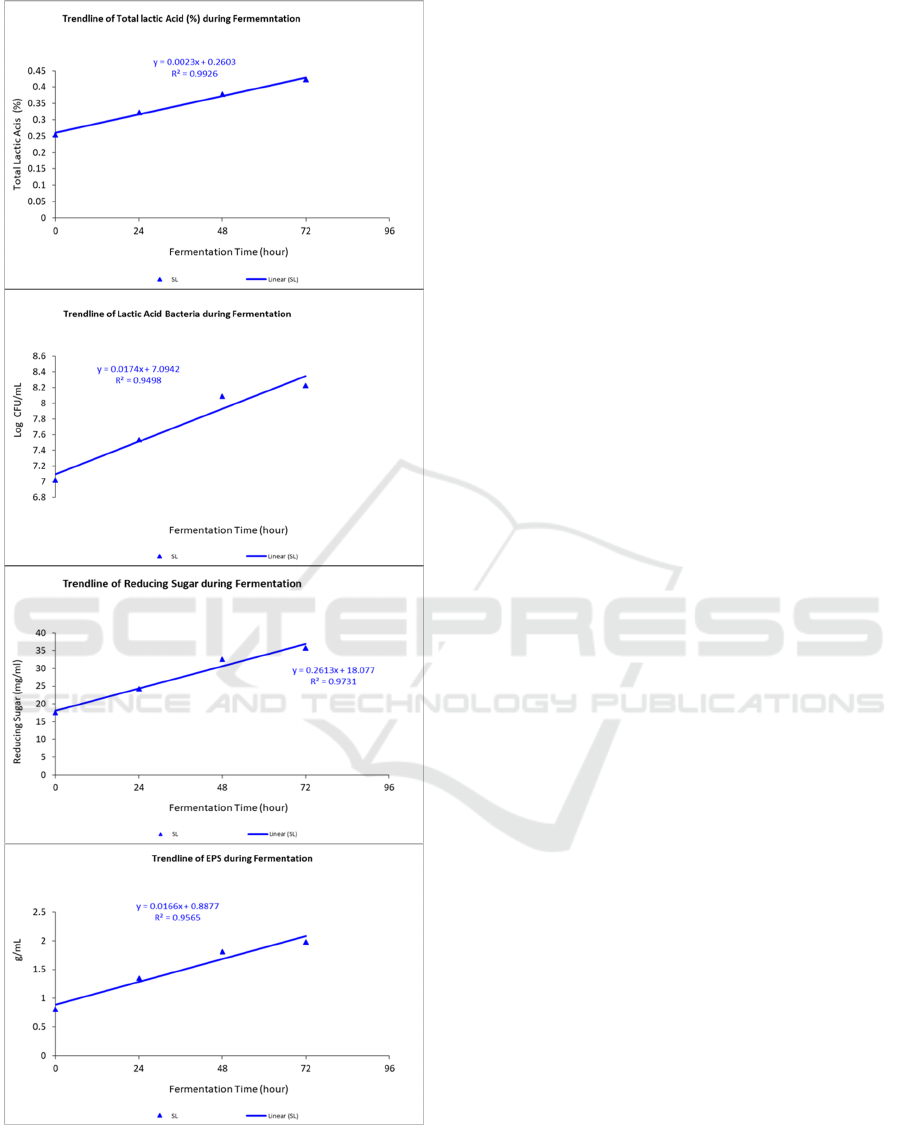
Figure 1: Trend line of pH, Lactic Acid Bacteria, Total
Lactic Acid, EPS and Reducing Sugar during Fermentation
of Yellow Sweet Potato with Leuconostoc mesenteroides
(cont.).
The lowest pH value and the highest total acid
occurred at 72 hours of fermentation. The same
pattern was reported by (Oloo, 2013). In the
fermentation of orange sweet potatoes, there was an
increase in lactic acid's total content and a decrease in
pH during fermentation. The decrease in pH during
fermentation is caused by the accumulation of organic
acids, especially lactic acid produced by Leuconostoc
mesenteroides FNCC-0023.
During fermentation, the total LAB also increased
quadratically and reduced non-lactic acid bacteria
(Table 2). Addition of Leuconostoc mesenteroides
starter treatment increased the LAB population, and
then it was stationary until 72 hours. The growth of
LAB in yellow sweet potato fermentation have time
incubation dependent. The growth pattern of
Leuconostoc mesenteroides increased from 0 to 24
hours and afterward tended to be stationary from 24
hours to 72 hours. (Yuliana et al., 2013) study
showed that the growth pattern of Leuconostoc
mesenteroides continues to increase up to 12 days of
fermentation.
At the beginning of the yellow sweet potato
fermentation (0 hours), besides LAB, non-lactic acid
bacteria colonies were also found, namely mold, with
an average of 2 log CFU / mL. The addition of starter
cultures can cause the desired microbial dominance
and suppress the growth of competing microbes.
During fermentation, LAB experiences growth by
utilizing sugar sources as energy or nutrition. The
simple sugars that are used for the development of
LAB are partially converted into organic acids such
as lactic acid (Oloo, 2013) and then LAB produces
crude exopolysaccharides which are secreted outside
the cell. In this study, there was an increase in
residual reducing sugar and an increase in lactic acid
and EPS production during fermentation. Consent
ensures that the publisher has the Author’s
authorization to publish the Contribution.
The length of fermentation has a very significant
effect on the value of reducing sugar residual
fermentation of yellow sweet potato which increases
at a rate of 26.13%. The residual reducing sugar
content increased linearly with fermentation time
(Table 1). The residual reducing sugar in yellow
sweet potato fermentation could come from the starch
and sugar contained in the yellow sweet potato tissue.
Sanoussi et al., (2016) states that yellow sweet potato
contains starch 172.87-326.73 mg/g (DW) and total
sugar 24.23-42.64 mg/g (DW). During fermentation,
yellow sweet potato starch is degraded by enzymes
both from sweet potatoes and LAB into horter chains
(simple sugar) (Guo et al., 2019). The simple sugar is
then used by Leuconostoc mesenteroides FNCC-0023
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
66
as a source of energy or nutrition for its growth. Apart
from being used for the development of LAB, some
of the simple sugars will be converted into organic
acids such as lactic acid (Oloo, 2013). During
development, LAB produces exopolysaccharides and
is secreted outside the cell. The exopolysaccharide
level analysis showed the EPS value of yellow sweet
potato fermentation increased linearly during
fermentation at a rate of 1.6%. The results of this
study are in line with previous research which states
that EPS production with lactic acid bacteria will
increase with the length of incubation time (Onilude
et al., 2013; (Zubaidah et al., 2014) .
3.2 Morphology of Starch Granula
The results of Scanning Electron Microscopy can be
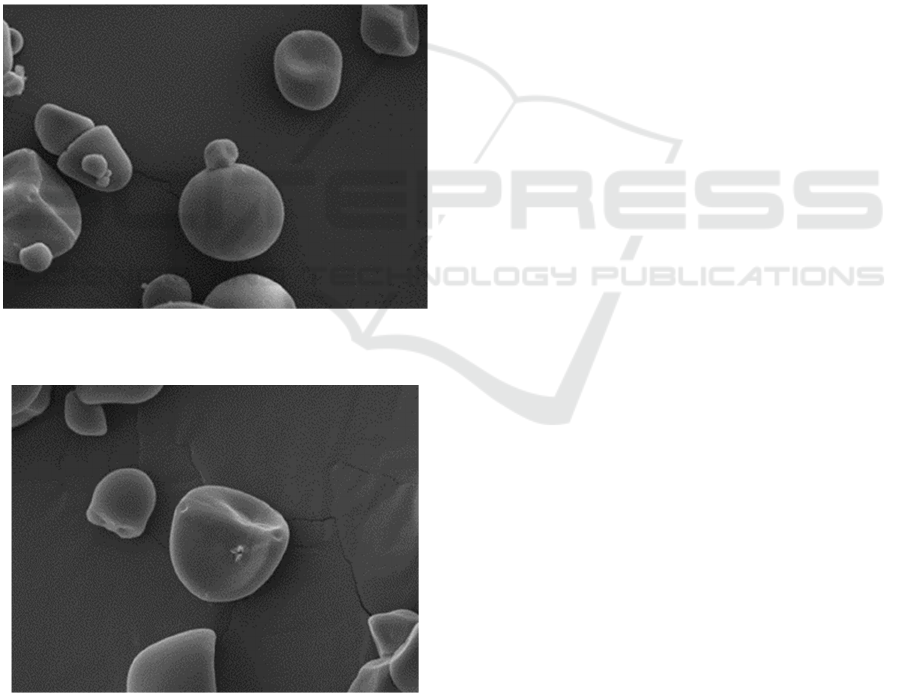
seen in Figure 2.
Figure 2a: Morphology of starch granule of yellow sweet
potatoes before fermentation (Magnification 2000 X).
Figure 2b: Morphology of starch granule of yellow sweet
potatoes after 72 hours fermentation (Magnification 2000 X).
Figure 1a shows the appearance of yellow sweet
potato starch granules (control), which do not look
hollow. Meanwhile, the yellow sweet potato starch
granules changed shape at the end of the fermentation
time (t = 72 hours), which was degraded by
Leuconostoc mesenteroides (2b).
The granule structure of control yellow sweet
potato starch and fermented starch resulted in a
significant difference in appearance and shape when
identified by Scanning Electron Microscopy. Figure
2b confirmed that there was a change in the starch
granule structure. Similar results were reported by
(Liao & Wu, 2017) on Lactobacillus plantarum
fermented yellow sweet potato starch granules.
(Yuliana et al., 2014) also reported spontaneous
fermentation of white sweet potato, causing starch
granules changes. According to (Liao & Wu, 2017),
the prolonged treatment of fermentation destroys the
crystal structure of yellow sweet potato starch and
significantly affects the crystalline and amorphous
parts. This change is thought to be caused by the
activity of lactic acid bacteria. Yuliana et al., (2014).
stated that the size of the starch granules in the
fermentation process of white sweet potato changes
after the fermentation process, which causes changes
in the amorphous structure of starch granules, size of
starch granules, chemical composition, and also
modifies the physical and rheological characteristics
of white sweet potato starch.
The form should be completed and signed by one
author on behalf of all the other authors. Figure 2.
Morphology of starch granule of yellow sweet
potatoes (magnification 2000 X).
4 CONCLUSIONS
The fermentation profile of yellow sweet potato with
starter Leuconostoc mesenteroides is as follows:
during fermentation, there was a linear increase in
total lactic acid (at a rate of 0.1%), residual reducing
sugar (at a rate of 26.13%), crude EPS (at a rate of
1.6%), and quadratically lowering the pH (with the
lowest point at pH 3.80) with total LAB (optimum at
8.63 log CFU / mL) and a decrease in non-LAB. The
morphology of yellow sweet potato starch granules
fermented with Leuconostoc mesenteroides starter
during 72 hours of fermentation caused starch
granules changes.
REFERENCES
Razack, A. S., Velayutham, V., & Thangavelu, V. (2013).
Medium optimization for the production of
exopolysaccharide by Bacillus subtilis using synthetic
Profile of Sweet Potato Fermentation using Leuconostoc Mesenteroides as a Starter
67

sources and agro wastes. Turkish Journal of Biology,
37(3), 280–288. https://doi.org/10.3906/biy-1206-50
Ajayi, O. I., Ehiwuogu-Onyibe, J., Oluwole, O. B., Jegede,
A. A., Salami, T. A., Asieba, G. O., Chiedu, I. E.,
Suberu, Y. L., Aba, E. M., Dike, E. N., Ajuebor, F. N.,
& Elemo, G. N. (2016). Production of fermented sweet
potato flour using indigenous starter cultures. African
Journal of Microbiology Research, 10(41), 1746–1758.
https://doi.org/10.5897/AJMR2016.8016
Ajayi, O. I., Onyemali, C. P., Akinwale, T. E., Okedina, T.
A., Bamidele, M. J., Ehiwuogu-Onyibe, J., Lawal, A.
K., & Elemo, G. N. (2018). Evaluation of Functional
Properties of Spontaneous and Starter Culture
Fermented Sweet Potato Flour. Microbiology Research
Journal International, 26(4), 1–8. https://doi.org/
10.9734/mrji/2018/46910
Anggraeni, Y. P., & Yuwono, S. S. (2014). Pengaruh
Fermentasi Alami pada Chips Ubi Jalar (Ipomoea
batatas) terhadap Sifat Fisik Tepung Ubi Jalar
Terfermentasi. Pangan Dan Agroindustri, 2(2), 59–69.
El Sheikha, A. F., & Ray, R. C. (2017). Potential impacts
of bioprocessing of sweet potato: Review. Critical
Reviews in Food Science and Nutrition, 57(3), 455–
471. https://doi.org/10.1080/10408398.2014.960909
Guo, L., Tao, H., Cui, B., & Janaswamy, S. (2019). The
effects of sequential enzyme modifications on
structural and physicochemical properties of sweet
potato starch granules. Food Chemistry, 277, 504–514.
https://doi.org/10.1016/j.foodchem.2018.11.014
Kammona, S., Othman, R., Jaswir, I., & Jamal, P. (2015).
Characterisation of carotenoid content in diverse local
sweet potato (ipomoea batatas) flesh tubers.
International Journal of Pharmacy and
Pharmaceutical Sciences, 7(2), 347–351.
Li, Y., Liu, Y., Cao, C., Zhu, X. Y., Wang, C., Wu, R., &
Wu, J. (2020). Extraction and biological activity of
exopolysaccharide produced by Leuconostoc
mesenteroides SN-8. International Journal of
Biological Macromolecules, 157, 36–44.
https://doi.org/10.1016/j.ijbiomac.2020.04.150
Liao, L., & Wu, W. (2017). Fermentation Effect on the
Properties of Sweet Potato Starch and its Noodle’s
Quality by Lactobacillus plantarum. Journal of Food
Process Engineering, 40(3), e12460. https://doi.org/
10.1111/jfpe.12460
Mora-Villalobos, J. A., Montero-Zamora, J., Barboza, N.,
Rojas-Garbanzo, C., Usaga, J., Redondo-Solano, M.,
Schroedter, L., Olszewska-Widdrat, A., & Lopez-
Gomez, J. P. (2020). Multi-Product Lactic Acid
Bacteria Fermentations : Fermentation, 1–21.
Oke, M., & Workneh, T. S. (2013). A review on sweet
potato postharvest processing and preservation
technology. African Journal of Agricultural Research,
8(40), 4990–5003. https://doi.org/10.5897/AJAR20
13.6841
Oloo, B. O. (2013). Effects of Lactic Acid Fermentation on
Sensory Profile of Orange Fleshed Sweet Potato.
Journal of Food and Nutrition Sciences, 1(1), 13.
https://doi.org/10.11648/j.jfns.20130101.13
Sanoussi, A. F., Dansi, A., Ahissou, H., Adebowale, A.,
Sanni, L. O., Orobiyi, A., Dansi, M., Azokpota, P., &
Sanni, A. (2016). Possibilities of sweet potato [Ipomoea
batatas (L.) Lam] value chain upgrading as revealed by
physico-chemical composition of ten elites landraces of
Benin. African Journal of Biotechnology, 15(13), 481–
489. https://doi.org/10.5897/ajb2015.15107
Sebben, J. A., Trierweiler, L. F., & Trierweiler, J. O.
(2017). Orange-Fleshed Sweet Potato Flour Obtained
by Drying in Microwave and Hot Air. Journal of Food
Processing and Preservation, 41(1), e12744.
https://doi.org/10.1111/jfpp.12744
Taylan, O., Yilmaz, M. T., & Dertli, E. (2019). Partial
characterization of a levan type exopolysaccharide
(EPS) produced by Leuconostoc mesenteroides
showing immunostimulatory and antioxidant activities.
International Journal of Biological Macromolecules,
136, 436–444. https://doi.org/10.1016/j.ijbiomac.2019.
06.078
Utami, R., Widowati, E., & Kamil, A. (2012). Kajian
kinetika fermentasi asam laktat oleh. V(2).
Yang, E., Fan, L., Yan, J., Jiang, Y., Doucette, C., Fillmore,
S., & Walker, B. (2018). Influence of culture media, pH
and temperature on growth and bacteriocin production
of bacteriocinogenic lactic acid bacteria. AMB Express,
8, 10. https://doi.org/10.1186/s13568-018-0536-0
Yuliana, N., Nurdjanah, S., & Dewi, Y. R. (2018).
Physicochemical properties of fermented sweet potato
flour in wheat composite flour and its use in white
bread. International Food Research Journal, 25(3),
1051–1059.
Yuliana, N., Nurdjanah, S., Setyani, S., & Novianti, D.
(2017). Improving properties of sweet potato composite
flour: Influence of lactic fermentation. AIP Conference
Proceedings, 1854. https://doi.org/10.1063/1.4985431
Yuliana, N., Nurdjanah, S., & Margareta, M. (2013). The
Effect of a Mixed-Starter Culture of Lactic Acid
Bacteria on the Characteristics of Pickled Orange-
Fleshed Sweet Potato L.) (Ipomoea batatas.
Microbiology Indonesia, 7(1), 1–8. https://doi.org/
10.5454/mi.7.1.1
Yuliana, N., Nurdjanah, S., Sugiharto, R., & Amethy, D.
(2014). Effect of Spontaneous Lactic Acid
Fermentation on Physico-Chemical Properties of Sweet
Potato Flour. Microbiology Indonesia, 8(1), 1–8.
https://doi.org/10.5454/mi.8.1.1
Yuliana, N., Sumardi, S., Jatmika, E., Rosaline, M., &
Iqbal, M. (2020). Potentially lactic acid bacteria as an
EPS producing starter from yellow sweet potato
fermentation. Biodiversitas Journal of Biological
Diversity,
21(9). https://doi.org/10.13057/biodiv/d210
944
Zubaidah, E., Suryawira, Y. M., & Saparianti, E. (2014).
Comparative Study Production of Exopolysaccharide (
EPS ) by Lactic Acid Bacteria ( L . casei and L .
plantarum ) in Different Media ( Dates and Mulberry
juice ). 3(1), 107–111.
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
68
