
Development of Alginate-based Antibacterial Edible Films by
Incoporating Green Betel Leaf Extract
Giyatmi Giyatmi
1
, Hari Eko Irianto
2
, Mohammad Sabariman
1
and Bintang Anggoro
1
1
Study Program of Food Technology, Sahid University, Jakarta University, Jakarta, Indonesia
2
Research and Development Center for Marine and Fisheries Product Processing and Biotechnology, Indonesia
Keywords: Antibacterial, Edible Film, Green Betel Leaf Extract, Staphylococcus aureus, Escherichia coli.
Abstract: The aim of this research was to develop an environmentally friendly packaging of alginate-based antibacterial
edible films. The antibacterial sources used was green betel leaf extract. Concentration levels of green betel
leaf extract which was studied were 0,5%, 1,0% and 1,5%. The edible film obtained was evaluated physically,
mechanically, chemically and microbiologically including parameters of Water Vapor Transmission Rate
(WVTR), water solubility, tensile strength, elongation, brightness, thickness, moisture content, and
antibacterial clear zone test. Experiment was conducted two replicates. The results showed that concentration
level of green betel leaf extract affected on the quality and antibacterial properties of alginate-based edible
films. Overall, the best alginate-based antibacterial edible film is produced by incorporating green betel leaf
extract at concentration level of 1,5%.
1 INTRODUCTION
Edible films are types of biodegradable plastic that
attract scientists around the world to explore. Edible
films have been employed to pack a variety of food
products, such as meat, sausages, fresh fruits and
vegetables. Edible films can maintain the quality of
packaged food because edible film can withstand
carbon dioxide, oxygen diffusion, water evaporation,
and flavor contamination with other products.
Another benefit of edible films is that it can extend
the shelf life and is environmentally friendly. Edible
films can be consumed together with the product.
(Junianto et al. 2012). The main components of
edible film can be grouped into three categories,
namely hydrocolloids, lipids, and composites.
Composites are a combination of hydrocolloids and
lipids. The hydrocolloids that can be used to make
edible films are proteins and carbohydrates.
Meanwhile, the lipids used are wax and fatty acids
(Donhowe and Fema, 2994). Alginate is one of
hydrocolloids polysaccharides which are available in
nature derived from seaweed and can be applied as
bio polymeric film. Film forming and binding ability
of alginate makes it suitable for the use of packaging
material (Parveen et al, 2019).
Production of edible films from alginate have
been explored by Murdinah et al. (2007), Khairunnisa
et al. (2018), Koushki et al. (2015) and others.
Alginate based edible films are found strong, but poor
water resistance due to hydrophilic nature. One way
to increase the performance of alginate based edible
film is by developing it as an active packaging. The
film can be used as a carrier of antioxidants, flavoring
and/or coloring agents and antimicrobials to improve
food safety and quality (Siracusa et al, 2018).
Incorporating antimicrobial into alginate film can
increase the resistance of the film to bacteria so that
the film has good mechanical quality and can inhibit
bacteria that potentially contaminate food.
One of the natural ingredients that contain
antimicrobials is green betel leaf. The active
antibacterial components found in green betel are
tannins, flavonoids and essential oils (Baskaran et al.,
2011). These compounds are able to inhibit the
growth of Escherichia coli and Staphylococcus
aureus. These bacteria produce enterotoxins which
can cause diarrhea. The antimicrobial activity of betel
leaf active compounds is by denaturing proteins and
damaging bacterial cell walls, as well as destructing
lipids in cell membranes by reducing the surface
tension of the cell membranes.
This study was aimed to develop an
environmentally friendly packaging of alginate-based
52
Giyatmi, G., Irianto, H., Sabariman, M. and Anggoro, B.
Development of Alginate-based Antibacterial Edible Films by Incoporating Green Betel Leaf Extract.
DOI: 10.5220/0010514000003108
In Proceedings of the 6th Food Ingredient Asia Conference (6th FiAC 2020) - Food Science, Nutrition and Health, pages 52-58
ISBN: 978-989-758-540-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

antibacterial edible films by using green betel leaf
extract as antimicrobial agent. added during the
process. Meanwhile, physical, mechanical, chemical
and antibacterial properties of the edible film were
also observed.
2 MATERIALS AND METHODS
2.1 Material
Alginate flour used was extracted from Sargassum sp.
by employing an extraction method developed by
Yunizal (2000). The modified cassava (mocaf) flour
used was the “Ladang Lima” brand, while sorbitol
and beeswax were purchased from PT Geochem
Globalindo, Jakarta. Green betel leaves were obtained
from the Ciracas market, East Jakarta.
2.2 Method
2.2.1 Extraction of Antibacterial
Compounds from Green Betel Leaves
Antibacterial from green betel leaves were extracted
through modifying a method previously employed by
Sitorus (2018) for extracting antibacterial from betel
leaves as well. After washing, the betel leaves were
chopped so that they were exposed to more ethanol.
The mixing was carried out in a large and closed
container so that the maceration process of the
material run perfectly. During mixing, the mixture
was stirred periodically. After that, the soaking
process was conducted for 3 days with stirring every
24 hours. Then the mixture was filtered with a 100
mesh filter cloth and the obtained filtrate was
evaporated using a rotary vacuum evaporator until a
thick extract was obtained (Sitorus, 2018).
2.2.2 Preparation of Edible Film
Edible film was prepared according to the experiment
conducted by Murdinah et al (2007) with
modification. About 97 mL of distilled water in a
beaker glass was heated to 50
o
C, then 1,5 g of alginate
powder was gradually added while stirring with a
magnetic stirrer. After that, 0,3 g of mocaf starch was
added with stirring for 30 minutes. After mixing
completely, the antibacterial green betel leaf extract
was added into the solution according to the treatment
(0,5%; 1,0%; 1,5%) and stirred for 30 minutes until
evenly mixed at 75
o
C. Furthermore, the solution is
casted by pouring into a square acrylic plate, where
one edible film solution recipe can be casted on two
acrylic plates. After that the acrylic plate was left to
stand at room temperature for approximately 24 hours
and then the dried edible film was removed.
2.2.3 Experiment
Experiment was executed in two stages, i.e.
preliminary and main experiments. Preliminary
experiment was conducted to investigate antibacterial
activity of green betel leaf extract to S. aureus and E.
coli. The results of this preliminary experiment will
be used in the main experiment. The main research
was performed to determine the optimum addition
level of green betel leaf extract as antibacterial agent
on alginate-based edible film. The concentration level
of betel leaf extract tested consisted of 3 levels (0,5%,
1,0%, 1,5%) with 2 replications.
2.2.4 Analyses
Alginate based antibacterial edible films obtained
were assessed their performance in terms of physical,
mechanical, chemical and antibacterial properties
including thickness using digital micrometer
(Mitutoyo, Japan), tensile strength and elongation
using Texture Analyzer (TAXT Plus, Stable Micro
System, UK) (Balqis et al., 2017), water vapor
transmission rate (WVTR) based on ASTM E96
using PERME® W3 / 031 Water Vapor Transmission
Rate Tester (Labthink, China), brightness using
Hunter Lab, water solubility (Murni et al, 2013),
moisture content (Farhan and Hani, 2017), as well as
antibacterial activity for S. aureus and E.coli (da Silva
et al, 2013) and surface morphological analysis using
SEM (Scanning Electron Microscopy) JEOL JSM-
6360LA, Japan (Setiani et al, 2013).
3 RESULT AND DISCUSSION
3.1 Antibacterial Activity of Green
Betel Leaf Extract
Antibacterial compounds extracted from green betel
leaves using alcohol as the extracting solvent showed
antibacterial activity on S. aureus and E. coli as
shown in Table 1. The highest antibacterial activity
of green betel extract against S. aureus based on the
largest inhibition zone shown at the extract
concentration of 1% was 14 mm, and the lowest
activity exhibited by the extract concentration of
0,5% was 12,50 mm. While, the highest inhibition
zone of green betel leaf extract against E. coli
occurred at
a concentration of 1,0% extract was 14
Development of Alginate-based Antibacterial Edible Films by Incoporating Green Betel Leaf Extract
53

mm, and the lowest zone of inhibition shown at a
concentration of 0,5% extract was 12,50 mm.
Table 1: Antibacterial activity of green betel leaf extract
(Clear Zone, mm).
Microorganism
Concentration of Green Betel Leaf
Extract
0,5% 1,0% 1,5%
Staphylococcus
aureus
14,00 13,50 14,00
Escherichia
coli
12,50 14,00 13,00
The antibacterial activity of betel leaf extract is
suspected to be produced by the chemical compounds
of the betel leaves, namely saponins, flavonoids,
polyphenols, and essential oils. Saponin compounds
can work as antimicrobials by damaging the
cytoplasmic membrane and killing cells. Flavonoid
compounds are thought to denaturate bacterial cell
proteins and irreparably damage cell membranes.
Natural phenols contained in essential oils have
antiseptic properties 5 times stronger than ordinary
phenols (bactericides and fungicides) but are not
sporacid. The mechanism of phenol as an
antibacterial agent acts as a toxin in the protoplasm,
damages and penetrates the walls and precipitates
bacterial cell proteins (Carolia and Noventi, 2016).
The main components of essential oil are betel
phenol and some of its derivatives including eugenol
allypyrocatechin 26,8 – 42,5%, cineol 2,4 – 4,8%,
mehyl eugenol 4,2 – 15,8%, caryophyllen 3,0 -9,8 %,
kavikol hydroxy, kavikol 7,2-16,7%, kabivetol 2,7
6,2%, estragol, ilypryrocatekol 9,6%, carvacol 2,2 –
5,6%, alkaloids, flavonoids, triterpenoids or steroids,
saponins, terpenes, phenylpropane, terpinen, diastase
from 0,8 - 1,8%, and tannins 1,0 – 1,3% (Damayanti
and Mulyono, 2003). Phenol at a concentration of 0,1
– 1,0% is bacteriostatic, while it is as bactericidal at a
concentration of 1 - 2% (Fuadi, 2014),
3.2 Characteristics of Alginate-based
Antibacterial Edible Film
The moisture content of the edible film increased with
the increase in the concentration of antibacterial
extract of green betel leaves in the film (Table 2).
Suryaningrum et al (2005) noted that edible films
being biodegradable with high moisture content will
be easily grown by microorganisms, due to the
presence of nutritional components in the film such
as protein. On the otherhand, edible films with low
moisture content will be more resistant to
microbiological destruction.
WVTR value of alginate edible film with different
antibacterial concentration treatment resulted in
different WVTR values with a tendency to decrease
with increasing concentration. WVTR test results for
edible film made with various concentrations of betel
leaf extract yielded WVTR values ranging from
1640,65 to 1871,79 g/m
2
.24h. The lowest WVTR
value of edible film was obtained at the addition of
1,5% antibacterial extract of betel leaves. Irianto et al
(2006) reported that the lowest WVTR was resulted
from a combination of 2,0% carrageenan, 0,7%
tapioca and 0,3% beeswax, i.e. 746,2g/m
2
/day, which
was lower than the value from this study.
The WVTR value in this study showed that the
edible film with the addition of green betel leaf
extract had a WVTR value which increased in line
with the increase in the addition level of betel leaf
extract. The higher the WVTR value, the higher the
permeability of the edible film. When edible film is
made into packaging, it will cause more moisture to
come out and enter the package. A good edible film
must not be easily passed by moisture or have a low
WVTR. The compact edible film structure can inhibit
water vapor diffusion through the edible film. Betel
leaf extract contains oil and water. Oil components
have high protective properties against water vapor
which will reduce the hydrophilic properties of the
film. Guilbert & Biquet (1996) informed that the fatty
components such as wax, emulsifier and fatty acids in
composite edible films have an effect on reducing
WVTR because fat has low polarity and a dense
crystal structure.
Table 2: Physical and Chemical properties of alginate based
antibacterial edible film.
Parameter
Concentration of Green Betel Leaf Extract
0,5% 1,0% 1,5%
Moisture
Content (%)
11,09±0,40 11,53±0,04 12,42±0,10
WVTR
(g/m
2
.24h)
1871,79±68,19 1790,28±4,15 1640,65±57,05
Water
Solubility
(%)
57,98±10,22 70,59±1,68 84,95±11,90
Brightness
(%)
14,2±1,27 12,1±0,27 11,5±1,70
Thickness
(mm)
0,114±0,014 0,104±0,013 0,108±0,008
The water solubility value of alginate edible film
with the addition of antibacterial extract of betel
leaves at different concentrations resulted in film
solubility which increased with increasing
concentration of betel leaf extract. The more betel
leaf extract is added, the more easily the edible film
will dissolve. The lowest solubility value indicates
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
54

that the edible film is the best because it plays a very
important role when the film is used for edible
products. This is also in accordance with the opinion
of Stuchell and Krochta (1994) that if the application
of a film is expected as an edible packaging, high
solubility is desired. Likewise, if the application of
edible film on foods with high water content, a film
that is not water-soluble is used. Imeson (1999) adds
that water resistance is an important property for
films applied as food protection.
The addition level of green betel leaf extract
affected the brightness of alginate edible film, in
which increasing addition level of betel leaf extract
resulted in edible film with lower brightness
percentage values. Ningsih (2015) mentioned that the
basic material used will affect the color of the edible
film. Therefore, the edible film using different
antibacterial concentrations will bring about different
brightness of edible films.
The use of betel leaf extract with different
concentrations in the production of alginate edible
film did not significantly affect the thickness of the
edible film, in which the thickness of edible film
obtained in this study was in the range of 0,104 –
0,114 mm. Those values are thicker compared to
thickness values of edible film made of composite
consisting of carrageenan, tapioca flour and beeswax,
i.e. 0,050 – 0,074 mm (Irianto et al, 2006).
Meanwhile, Saputro et al (2017) produced edible
films with a thickness of 0,03 – 0,08 mm which was
carrageenan, tapioca flour and glycerol used as raw
materials. According to Wahyu (2009) that a good
edible film has a thickness of 0,15 – 0,20 mm which
can withstand external influences and reduce WVTR.
Thickness is an important parameter that needs to be
considered in the use of edible film as a packaging.
The thickness of the film will affect the gas
permeability. The thicker the edible film, the lower
the gas permeability, which can protect the product.
The tensile strength values of alginate edible film
were insignificantly influenced by the addition levels
of green betel leaf extract. The higher addition of the
betel leaf extract did not result in a pronounced
difference in tensile strength of the edible film. The
tensile strength of the edible film in this study was in
the range of 1,31±0,84 – 2,67±0,51 MPa (Table 3).
Those tensile strength values are not in the range of
ASTM polypropylene standard values, i.e. 24,7-302
MPa. In addition, the best tensile strength of edible
films is between 10 - 100 MPa while edible films with
a tensile strength range of 1 - 10 MPa are classified as
marginal (Han and Gennadios, 2005). The increase in
the forces of attraction between the molecules making
up the edible film induces an increase in the strength
of its structure. The greater the tensile value, the
stiffness of a material will increase or be inelastic.
Sara (2015) noted that tensile strength is a mechanical
property related to the strength of the edible film to
withstand physical damage when applied as food
packaging. Edible film with high tensile strength
value is highly expected in order to minimize
packaged product damage due to mechanical
interferences.
Table 3: Mechanical properties of alginate based
antibacterial edible film.
Parameter
Concentration of Green Betel Leaf
Extract
0,5% 1,0% 1,5%
Tensile
Strength
(Mpa)
2,52±0,75 2,67±0,51 1,31±0,84
Elongation
(
%
)
47,05±18,88 38,30±4,95 24,40±13,58
The elongation test results showed that the more
the betel leaf extract on the alginate edible film, the
lower the elongation value. The elongation
percentage of the film is categorized as poor if the
value is less than 10% and good if the elongation
percentage exceeds 50%. Elongation percentage of
alginate based edible film added with various
concentrations of betel leaf extract in this study was
in the range of 24,40±13,5 – 47,05±18,88 %. The
elongation value of the edible film from this study
was lower than the elongation value of edible film
made of bligon goat skin gelatin, namely 70,97 –
95,33% (Said et al., 2013). Edible films from whey
and agar have comparable elongation, i.e. 26,06 –
34,52% (Hakim, 2015). Elongation of edible films
made from avocado seed starch and agar is smaller,
i.e. 0,17 – 0,45% (Coniwanti et al, 2016).
The antibacterial test showed that the alginate-
based edible film incorporated with green betel leaf
extract had antibacterial activity against S. aureus and
E. coli (Table 4) as indicated by the formation of a
clear zone on the test medium (Figure 1). The
antibacterial activity was getting more significant
with the higher concentration level of antibacterial
betel leaf extract, both for S. aureus and E. coli which
showed the same trend. Thus, if greater antibacterial
activity is expected, the addition level of betel leaf
extract must be increased. The development of
antibacterial edible films based on various types of
starch has also been carried out using several
antibacterial sources from the extracts of beluntas
leaves (Mulyadi et al, 2016), garlic (Anggraini et al,
2018), red galangal/Alpinia purpurata (Sholehah et
al, 2016) and areca nut (Ningsih, 2018).
Development of Alginate-based Antibacterial Edible Films by Incoporating Green Betel Leaf Extract
55
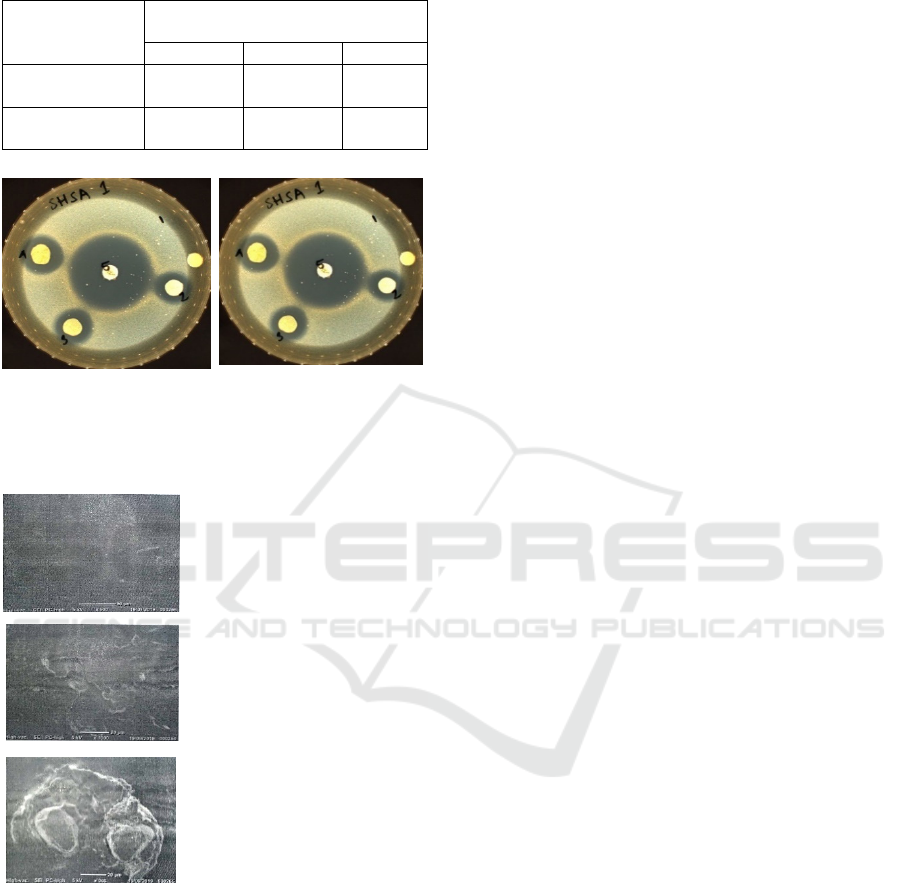
Table 4: Antibacterial properties (Clear Zone, mm) of
alginate based edible film added with green betel leaf
extract.
Parameter
Concentration of Green Betel Leaf
Extract
0,5% 1,0% 1,5%
Staphylococcus
aureus
14,25 17,85 18,15
Escherichia
coli
12,40 14,00 13,40
(1) Staphylococcus aureus (2) Escherichia coli
Figure 1: Clear zone as antibacterial effects of green betel
leaf extract of alginate based edible film on S. aureus and E
coli.
Figure 2: SEM images of alginate edible film added with
1.5% betel leaf extract with three times magnification.
Surface morphological analysis using SEM was
carried out on alginate based edible film added with
green betel leaf extract at a concentration of 1.5%
(Figure 2), generally showing the best physical,
mechanical and chemical properties. Analyses
revealed that the molecular structure surface of the
alginate edible film at 500 and 1000 times
magnification was dense. The higher the
magnification used, the more obvious the surface
morphology of the edible film, and it turned out that
it showed the lack of molecular density in the
resulting edible film. The less dense structure of these
molecules brought about more water to be absorbed.
The image also showed a less smooth and porous
surface. The non-smooth surface indicated that the
film was less homogeneous. This occurrence was
probably due to a too short stirring period, so that it
resulted in inhomogeneous edible film solution
inducing a non-dense molecular structure surface of
the alginate edible film. Therefore, further study to
explore this phenomenon are highly recommended.
4 CONCLUSION
1) Green betel leaf extract has antibacterial activity
against S. aureus and E. coli
2) Alginate-based edible film added with green betel
leaf extract has relatively good physical and
mechanical characteristics, especially in terms of
thickness, tensile strength, elongation and
WVTR.
3) Edible film added with betel leaf extract as
antibacterial showed antibacterial activity against
S. aureus and E, coli; so it can be called as an
antibacterial edible film
4)
Antibacterial edible film is recommended for
performance testing by being used as a packaging
for food products
ACKNOWLEDGEMENT
Authors would like to thank to the Ministry of
Research, Technology and Higher Education of the
Republic of Indonesia, who has provided funding for
executing this study through PTUPT program 2019
No: 45.3/USJ-11/H.54/2019.
AUTHORS’ CONTRIBUTIONS
Giyatmi Giyatmi and Hari Eko Irianto are the main
contributors in conducting experiment and preparing
the draft of manuscript, while Mohammad Sabariman
and Bintang Anggoro are as supporting contributors.
All authors read and approved the final manuscript.
The molecular structure
surface of the alginate
edible film is dense at 500
times magnification.
The molecular structure
surface of the alginate
edible film is dense at 1000
times magnification.
The molecular structure
surface of the alginate
edible film is not dense at
2000 times magnification
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
56

REFERENCES
Anggraini, T.N., Agustini, T.W. and Rianingsih, L. 2018.
Karakteristik Edible Film Karaginan Dengan
Penambahan Ekstrak Bawang Putih (Allium sativum)
Sebagai Antibakteri. Saintek Perikanan 14 (1) : 70-76
Baskaran, C., Rathabai, V., & Kanimozhi, D., 2011.
Screening for antimicrobial activity and phytochemical
analysis of various leaf extract of Murraya koenigii. Int
J. Res Ayurveda Pharm, 2, 1807-10
Balqis, A. I., Khaizura, M. N., Russly, A. R., and Hanani,
Z. N., 2017. Effects of plasticizers on the
physicochemical properties of kappa-carrageenan films
extracted from Eucheuma cottonii. International
Journal of Biological Macromolecules, 103, 721-732.
https://doi.org/ 10.1016/j.ijbiomac.2017.05.105
Carolia, N. and Noventi, W., 2016. Potensi Ekstrak Daun
Sirih Hijau (Piper betle L.) sebagai Alternatif Terapi
Acne vulgaris. Majority 5 (1): 140 – 145
Coniwanti, P., Sari, D.M., and Ferbriana, R., 2016.
Pengaruh Rasio Massa Pati Biji Alpukat dan Agar -
Agar Terhadap Pembuatan Edible Film. Jurnal Teknik
Kimia. 22(2) : 51-59
Damayanti R. and Mulyono, 2003. Khasiat & manfaat daun
sirih: obat mujarab dari masa ke masa. Agromedia
Pustaka. Jakarta.
da Silva, M., Lamanaka, B., Taniwaki, M. and Kieckbusch,
T. 2013. Evaluation of the Antimicrobial Potential of
Alginate and Alginate/ Chitosan Films Containing
Potassium Sorbate and Natamycin. Packaging
Technology and Science 26 (8): 479-492
Donhowe, G. and Fennema, O., 1994. Edible film and
coating: Characteristic, formation, definitions and
testing methods. In Krochta, J.M., Baldwin, E.A. and
Nisperos-Carriedo, M.O. (eds.). Edible Coating and
Film to Improve Food Quality. Technomic Publ. Co.
Inc. Lancaster, Pennsylvania. 378 pp
Farhan, A. & Hani, N. M., 2017. Characterization of edible
packaging films based on semi-refined kappa
carrageenan plasticized with glycerol and sorbitol.
Food Hydrocolloids, 64, 48-58. https://doi.org/
10.1016/j.foodhyd.2016.10.034
Fuadi S., 2014. Efektivitas ekstrak daun sirih hijau (Piper
betle L.) terhadap pertumbuhan bakteri Streptococcus
pyogenes in vitro. Skripsi. Universitas Islam Negeri
Syarif Hidayatullah. Jakarta.
Guilbert, S. and Biquet, B., 1990. Edible Films and
Coatings. In Food Packaging Technology Vol. 1. (Eds.
Bureau, G. and Multon, J.L.). VCH Publisher, Inc. New
York.
Hakim, M.Q., 2015. Karakteristik Edible Film dari Whey
Dangke yang Ditambahkan Level Agar yang Berbeda.
Skripsi. Fakultas Peternakan. Universitas Hasanuddin.
Makassar
Han, J.H. and Gennadios, A., 2005. Edible Film and
Coatings: A Review. Innovations in Food Packaging.
Academic Press.
Irianto, H. E., Darmawan, M. and Mindarwati, E., 2006.
Pembuatan Edible Film dari Komposit Karaginan,
Tepung Tapioka dan Lilin Lebah (Beeswax). J.
Pascapanen Dan Bioteknologi Kelautan Dan Perikanan
1 (2): 93-101.
Imeson, A., 1999. Thickening and Gelling Agents for Food.
Aspen Publishers Inc. Maryland.
Junianto., N., Kurniawati, O.S., Djunaidi, and Khan,
A.M.A., 2012. Physical and Mechanical Study on
Tilapia’s Skin Gelatine Edible Films with Addition of
Plasticizer Sorbitol. African Journal of Food Science
6(5):142-146.
Khairunnisa, S., Junianto, Zahidah, and Rostini, I., 2018.
The effect of glycerol concentration as a plasticizer on
edible films made from alginate towards its physical
characteristic. World Scientific News 112: 130-141
Koushki, M.R., Azizi, M.H., Azizkhani, M., and Koohy-
Kamaly, P., 2015. Effect of Different Formulations on
Mechanical and Physical Properties of Calcium
Alginate Edible Films. Journal of Food Quality and
Hazards Control 2: 45-50
Mulyadi, A.F., Maimunah Hindun Pulungan, M.H. and
Qayyum, N., 2016. Pembuatan Edible Film Maizena
dan Uji Aktifitas Antibakteri. Industria: Jurnal
Teknologi dan Manajemen Agroindustri 5(3): 149-158
Murdinah, Darmawan, M. and Fransiska, D., 2007.
Karakteristik Edible Film dari Komposit Alginat,
Gluten dan Lilin Lebah (
Beeswax). Jurnal Pascapanen
dan Bioteknologi Kelautan dan Perikanan 2(1): 19-25
Murni, S. W., Pawignyo, H., Widyawati, D., & Sari, N.,
2013. Pembuatan Edible Film dari Tepung Jagung (Zea
mays L.) dan Kitosan. Prosiding Seminar Nasional
Teknik Kimia Kejuangan (p. B17 1-9).
Ningsih, W., 2018. Formulasi Dan Uji Efektivitas
Antibakteri Edible Film Ekstrak Biji Pinang (Areca
catechu Linn). Jurnal Ilmu Farmasi dan Farmasi Klinik
(JIFFK) 15 (2): 71-76
Parveen, I., Maraz, K.M., Mahmud, M. and Khan, R.A.,
2019. Seaweed Based Bio Polymeric Film and Their
Application: A Review on Hydrocolloid
Polysaccharides. Scientific Review 5 (5): 93-102
Said, M.I., Triatmojo, S., Erwanto, Y., and Fudholi, A.,
2013. Evaluasi Karakteristik Fisik Edible Film dari
Gelatin Kulit Kambing Bligon yang Menggunakan
Gliserol Sebagai Plasticizer. Jurnal Ilmu dan Teknologi
Hasil Ternak. 8(2) : 32
Saputro, B.W., Eko Nurcahya Dewi, E.N. and Susanto, E.,
2017. Karakteristik Edible Film Dari Campuran
Tepung Semirefined Karaginan Dengan Penambahan
Tepung Tapioka Dan Gliserol. J. Peng. & Biotek. Hasil
Pi. 6 (2): 1-6
Sara, N.E.M., 2015. Karakteristik edible film berbahan
dasar whey dangke dan agar dengan penambahan
konsentrasi sorbitol. Skripsi. Universitas Hasanuddin.
Makassar
Setiani, W., Sudiarti, T. and Rahmidar, L. 2013. Preparasi
Dan Karakterisasi Edible Film Dari Poliblend Pati
Sukun-Kitosan. Valensi 3 (2): 100 - 109
Sholehah, M.M., rid Ma’ruf, W.F. and Romadhon., 2016.
Karakteristik Dan Aktivitas Antibakteri Edible Film
Dari Refined Carageenan Dengan Penambahan Minyak
Atsiri Lengkuas Merah (Alpinia purpurata). J. Peng. &
Biotek. Hasil Pi. 5 (3): 1-8
Development of Alginate-based Antibacterial Edible Films by Incoporating Green Betel Leaf Extract
57

Siracusa, V., Romani, S., Gigli, M., Mannozzi, C.,
Cecchini, J.P., Tylewicz, U. and Lotti, N., 2018.
Characterization of Active Edible Films based on Citral
Essential Oil, Alginate and Pectin. Materials 11(10):
1980
Sitorus, P. 2018., Uji Efek Kombinasi Amoksisilin Dengan
Ekstrak Etanol Daun Sirih (Piper betle L) Terhadap
Pertumbuhan Bakteri Escherichia Coli Dan
Staphylococcus Aureus. TM Conference Series 01:
313–319
Stuchell, Y. M. and J. M. Krochta., 1994. Enzymatic
treatments and thermal effects on edible soy protein
films. J. Food Sci. 59(6): 1332-1337.
Suryaningrum, Th. D., Basmal, J. and Nurrochmawati,
2005. Studi pembuatan Edibel Film dari Karagenan.
Jurnal Penelitian Perikanan Indonesia. Edisi Pasca
Panen. Badan Riset Perikanan dan Kelautan
Departemen Kelautan dan Perikanan 2(4): 1 – 13.
Wahyu, M. K., 2009. Pemanfaatan Pati Singkong sebagai
Bahan Baku Edibel film. Jurusan Teknologi Industri
Pangan, Fakultas Teknologi Industri Pertanian,
Universitas Padjajaran. Bandung
Yunizal, 2000. Penelitian Teknologi Ekstraksi Alginat dari
Rumput Laut Coklat (Phaeophyceae). Balai Penelitian
Perikanan Laut. Jakarta. 100 pp.
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
58
