
Preparation of Double Emulsion of Vitamin C with Two Different
Emulsifiers in the Outer Aqueous Phase
Marcellina Indah Permatasari
1
, M. Yusuf Sulaeman
1
and Bambang Nurhadi
1,2
1
Food Technology Department, Faculty of Agric. Industrial Technology, Universitas Padjadjaran,
Jl. Raya Bandung Sumedang km. 21, Jatinangor Sumedang 45363, Indonesia
2
Study Centre of Agric. Technology Development, Faculty of Agric. Industrial Technology, Universitas Padjadjaran,
Jl. Raya Bandung Sumedang km. 21, Jatinangor Sumedang 45363, Indonesia
Keywords: Double Emulsion, Tween 80, Vitamin C, W/O/W, WPC-Pectin.
Abstract: Vitamin C is one of common bioactive compound widely known and used by many people, especially in
Indonesia. The weakness of vitamin C stablity are sensitive with extreme pH, temperature, oxygen and direct
light. Because of that, the encapsulation of itamin C with W
1
/O/W
2
emulsion can be the solution for
maintaining the stability of vitamin C. This study is aimed to emulsify Vitamin C in the inner aqueous phase
of water-in-oil-water (W
1
/O/W
2
) emulsions with soybean oil as the oil phase. Two type of emulsifier Tween
80 and WPC-Pectin in the outer aqueous phase used to compare their stability during storage. The result of
this research showed that emulsion with WPC-Pectin had bigger droplet size 1.34 μm than emulsion with
tween 80 0.91 μm. Tween 80 had a better stability with 14.41% of creming index compared to WPC-Pectin
with 23.06% of creaming index. Morphology of W
1
/O/W
2
emulsion with Tween 80 can be described as
W
1
/O/W
2
when emulsion with WPC Pectin cannot be described as W
1
/O/W
2
Emulsion. Tween 80 is a better
emulsifier than WPC-Pectin to stabilized the W
1
/O/W
2
emulsion in the outer aqueous phase.
1 INTRODUCTION
Vitamin C is a water-soluble vitamin which is widely
known and popularly used in Indonesia. This is
proven by the number of food products that contain
vitamin C in it. Vitamin C has the main function as a
compound that can maintain body endurance and can
increase skin moisture (Gregory, 2017). Vitamin C
consists of ascorbic acid compounds that have a low
pH and sour taste that is obtained from citrus fruits
such as oranges, lemons, lime and berries
(strawberries, blueberries and others). However the
stability of Vitamin C is very prone and it would
affect it functionality. Vitamin C is unstable to
extreme temperatures, extreme pH, high oxygen, and
light (Levine et al., 2004). Prolonged contact can
cause damage to vitamin C and ultimately can be bad
for health. Therefore, further efforts are needed so
that the vitamin C compound remains stable during
processing and storage.
Emulsification is one method of maintaining
vitamin C compounds in food products. Emulsion is
a dispersion system which consists of two solutions
which do not dissolve each other. There are two basic
types of emulsions, namely oil-in-water (O/W)
emulsion and water-in-oil (W/O) emulsion. This
emulsion is called as single emulsion. Another
emulsion system namely water-in-oil-in-water
(W
1
/O/W
2
) is a method for emulsifying hydrophilic
bioactive components. W
1
/O/W
2
consists of an
internal water phase (W
1
) which can be filled with
bioactive components such as vitamin c, intermediate
oil phase (O) and external water phase (W
2
). The use
of water as an outer phase of the emulsion system
aims to keep vitamin C soluble in water so that it is
easily applied in various processed food products.
The emulsion system W
1
/O/W
2
uses two
emulsifiers where the first emulsifier has high
hydrophobic properties while the second emulsifier
has high hydrophilic properties. The use of these two
types of emulsifiers play an important role in the
stability of the emulsions formed (Anton,
Vandamme, Ding, Yu, & Serra, 2018). The use of
emulsifiers to be studied are high molecular weight
emulsifiers such as bio complex proteins (whey
protein concentrate, and pectin) and low molecular
weights such as tween 80 in the outer phase of the
system. The effect of both type emulsifiers on the
Permatasari, M., Sulaeman, M. and Nurhadi, B.
Preparation of Double Emulsion of Vitamin C with Two Different Emulsifiers in the Outer Aqueous Phase.
DOI: 10.5220/0010513900003108
In Proceedings of the 6th Food Ingredient Asia Conference (6th FiAC 2020) - Food Science, Nutrition and Health, pages 47-51
ISBN: 978-989-758-540-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
47
stability of the double emulsion during storage was
studied.
2 MATERIAL AND METHODS
2.1 Material
Vitamin C (Brataco, Indonesia), Aqua Deminerals
(Brataco, Indonesia), Commercial soybean oil (Salim
Ivomas, PT., Indonesia), Natrium Chloride (Brataco,
Indonesia), Propyl Paraben (Brataco, Indonesia),
Citric Acid (Weifang Ensign Industry Co. Ltd.,
China), Magnesium Sulfate (Brataco, Indonesia),
Tween 80 (Croda International, UK), Span 65 (Futura
Ingredients Pte, Ltd., Singapore), Pectin (CP Kelco,
U.S.) and Whey Protein Concentrate 80% (Avonlac,
Glanbia USA).
2.2 Methods
2.2.1 Preparation of the W₁ / O (Water in
Oil) Emulsion System
Aqueous phase in the form of a 10% vitamin C solution
was prepared by mixing 10 g (10% w / v) into 100 ml
aqua demineralized and adding 2 grams of MgSO₄.
The oil phase is made by adding 3.5 grams (5% w / v)
Span 65 to 70 ml of soybean oil at 70°C until it
dissolves at a speed of 500 rpm. W
1
/O emulsions were
prepared by adding 30 ml of the water phase to 70 ml
of the oil phase at room temperature (around 25 ℃) at
800 rpm, followed by the first stage emulsification at
4000 rpm for 2 minute using homogenizer.
2.2.2 Preparation of W
2
Biopolymer
Solution and Tween 80
Biopolymer solution was used as a second emulsifier
made from a mixture of 80% WPC (Whey Protein
Concentrate) and pectin. Biopolymer solution was
prepared by dissolving 3.1 g (3.5% w/v) WPC into
100 ml of aqua demineralized at 50˚C for 30 minutes
then adding 0.1 g of pectin, 0.7 g of citric acid, 3.9 g
of NaCl and 0.5 g of propyl parabens. After 30
minutes, the solution is lowered to room temperature
and stored for 24 hours at 4°C. Preparation of tween
80 solution refers to Gharehbeglou et al., (2019) with
a slight modification. Tween 80 of 3.6% (w/v) was
dissolved into aqua demineralized at room
temperature, also added 3.3% NaCl (w/v) to maintain
the osmotic pressure of the emulsion system that will
be formed. The solution is then stored at a chiller
temperature before use.
2.2.3 Preparation of W
1
/O/W
2
(Water in Oil
in Water) Emulsion Systems
Preparation of the W
1
/O/W
2
emulsion system was
firstly done by mixing 20 ml of W
1
/O emulsion into
80 ml of W₂ solution, then the mixture was
homogenized with Ultrasonic Homogenizer (Qsonica
Q500, US.) for 10 minutes with a pulse of 3 seconds
on 3 seconds off 70% amplitude. The homogeneity
process aims to homogenize the solution and reduce
the size of the droplet emulsion. The W
1
/O/W
2
emulsion formed is stored in a bottle at 25℃ for
stability testing for 14 days. The process of double
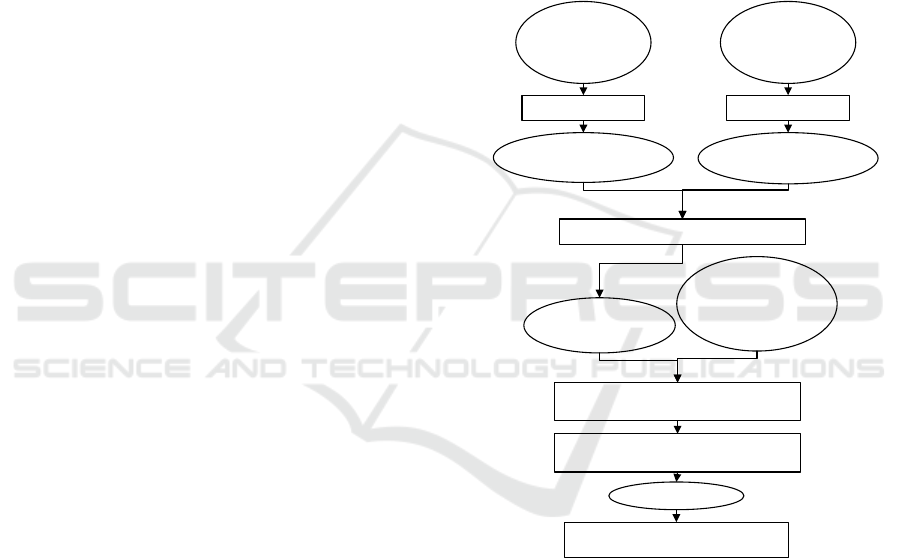
emulsion can be seen in Figure 1.
W₁/O Emulsion
Outer Aqueous
Phase (W₂)
• WPC-Pectin
• Tween 80
W₁/O/W₂
Storage T = 25° C t = 14 Days
80%20%
Homogenizer 2 minutes
Aqua Deminerals
Vitamin C
MgSO₄
Soybean Oil
Span 65
Homogenization
70%
30%
Mixing Mixing
Inner Aqueous
Phase (W₁)
Intermediate Oil
Phase (O)
Ultrasound 10 minutes
Figure 1: Preparation of Double Emulsion.
2.2.4 Particle Size Measurement
The particle size of droplet was analyzed with particle size
analyzer (Beckman Coulter LS 13 320, Beckman Coulter,
Inc. US). Particle size was measured for the fresh emulsion
immediately after preparation (H0), Days 7 (H7) and after
14 Days (H14) storage at 25℃.
2.2.5 Creaming Index Measurment
Measurement of the creaming index by calculating the
height of the cream formed divided by the height of the
sample. The creaming index measurement was carried out
every 4 days for 14 days at 25℃ then the creaming index
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
48

percentage was calculated using the formula:
𝐶𝐼 = (
ℎ
ℎ
) × 100%
CI = Creaming Index (%)
h
0
= Cream Height
h
1
= Sample Height
2.2.6 Morphology Particle of W
1
/O/W
2
Morphology particle of W/O/W emulsions were made by
Transmission Electronic Microscope (Jeol, Japan) after 14
days of storage.
3 RESULT AND DISCUSSION
Particle size of the emulsion with tween 80 increased
after storage for 7 and 14 days. Emulsion with
biopolymer solution has a bigger droplet compare to
tween 80. Emulsion with biopolymer solution start
from 1.34 μm and tween 80 start from 0.91 μm.
According to Gharehbeglou et al., (2019) WPC and
Pectin have larger droplet sizes due to interactions
between the WPC and pectin forming a massive
complex and trapped the oil phased inside it.
Particle size was increased significantly in the
emulsion with WPC-Pectin than tween 80. Figure 2
shows droplet size of both emulsions increased on the
7th day, on the 14th day, only the droplet of emulsion
with tween 80 increased by 1.15 μm while the
emulsion with WPC Pectin decreased from 1.32 μm
to 0.87 μm. The decrease in droplet emulsion in
Figure 2 can occur due to the broken of the W
1
/O
emulsion in the W
1
/O/W
2
emulsion so it changes into
an O/W.
Figure 2: Particle Size of W
1
/O/W
2
Emulsion Vitamin C.
Increasing the size of the droplet emulsion causes
the emulsion system to become unstable. Research
from Mohammadi et al., (2016) states that the large
size of the emulsion droplet with the WPC Pectin
emulsifier because of the addition of WPC and Pectin
together causing the thickening of the biopolymer
compound around the W/O emulsion, thereby
increasing the size of the WPC and Pectin droplet
emulsion and reduce the stability of the emulsifier
proving by cream formed.
Another thing that enlarging the droplet size of the
emulsion and causes the W/OW emulsion system to
be unstable is the coalescence process of the W/O
emulsion during storage. According to Chung and
Mcclements (2018), coalescence causes the emulsion
droplet fuse into a larger droplet resulting the increase
of droplet size during storage. This is also supported
by Schuch et al., (2014) where the emulsifier
concentration also affects the stability of the emulsion
system formed. If the concentration of the emulsifier
are less, the emulsifier is not able to bind the water
completely, causing the water molecules to fuse and
coalescence occurs so the droplet enlarges. Other
studies that support coalescence as a cause of
increasing droplet size and as a sign of instability of
the emulsion system were carried out by (Vicente et
al., 2018). Coalescence occurs because the lack of
hydrocolloid compounds as a stabilizer for
emulsifiers can initiate the Brownian motion on the
droplet emulsion.
Brownian motion is random motion that occurs in
droplets. When the emulsion system is stable,
brownian motion will maintain the distribution of
dissolved phase droplets evenly throughout the
solvent phase (Dickinson, 2010). If the emulsion is
not stable, there will be a tendency for droplets to
separate from each other because they have different
densities so the brownian motion can no longer move
evenly. If the density of the droplet is small, the
brownian motion will make the droplet emulsion
move towards the solution whereas if the density of
the droplet is large, then the brownian motion will
make the droplet move downward (Zhang &
Mcclements, 2018).
Based on the results in figure 3, W
1
/O/W
2
Emulsion with Tween 80 as the outer phase
emulsifier are better than with WPC Pectin as the
outer phase emulsifier. Figure 3 (a) can be described
as W
1
/O/W
2
emulsion when the dark phase are oil and
bubble phases inside the dark one are the water phase
(W
1
) and the white phase around the dark are the W
2
.
This is supported by Mainz (2006) that the dark
particle are made from mass thickness contrast
method depends on mass weight of the particle that
0,91
1,07
1,15
1,34
1,32
0,87
0,80
0,90
1,00
1,10
1,20
1,30
1,40
0714
Droplet Emulsion size (µm)
Storage Day
Tween 80 WPC Pektin
Preparation of Double Emulsion of Vitamin C with Two Different Emulsifiers in the Outer Aqueous Phase
49

used. Figure 3 (b) cannot be described as W
1
/O/W
2
emulsion because there is no dark phase between the
light phase like figure 3 (a). it means that WPC-Pectin
cannot be outer phase emulsifier. This phenomena
happens because the amplitude from sonication
process doesn’t match with WPC-Pectin as a
emulsifier, if the amplitude is high, then proteins that
contained in WPC-Pectin are denaturized (Hubinger,
2018).
Figure 3: Morphology of Double Emulsio with Tween 80
(Left) and WPC-Pectin (Right).
Based on the experimental results, the WPC
Pectin emulsifier cannot be used as an emulsifier for
the W
1
/O/W
2
vitamin C emulsion system. The
increase in the creaming index value is more
significant in the WPC Pectin emulsifier compared to
the Tween 80 emulsifier based on the curves in Figure
4. The higher creaming index value indicates that the
emulsion system formed is increasingly unstable.
Tween 80 emulsifier has better stability than the
Pectin WPC emulsifier with the same concentration.
Figure 4: Creaming Index of W/O/W Vitamin C.
The high value of the creaming index on the WPC
Pectin emulsifier can be caused by flocculation of the
formed emulsion system compo
nents. This is supported
by Khalid, Kobayashi, Neves, Uemura, & Nakajima, (2013)
who stated that the speed of creaming is influenced
by the flocculation that occurs in the W
1
/O emulsion
system components. The occurrence of flocculation
can be due to the droplet aggregation process, thereby
accelerating the process of creaming (Schmidts, Dobler,
Nissing, & Runkel, 2009). According to Dickinson (2010),
the flocculation process is the process that most influences
the creaming of an emulsion system. Flocculation has a big
role because the droplets that have undergone flocculation
move faster than individual droplets in the emulsion
solution. This occurs because the emulsion droplets that
have undergone flocculation are much larger than the intact
emulsion droplets.
Research from Schuch, Helfenritter, Funck, &
Schuchmann, (2014) stated that the instability of the
W
1
/O/W
2
emulsion can be caused by differences in the
surface tension of the emulsifier used. In addition, the
active components that become surfactants in the Pectin
WPC emulsifier can also cause instability of the W
1
/O/W
2
emulsion system that has been formed. According to
Schuch et al., (2014), the instability of an emulsifier using
a protein which is a hydrophilic emulsifier can occur due to
the lack of a stabilizer concentration in the form of a
hydrocolloid compound. Hydrocolloid compounds that can
be used as stabilizers are gum arabic, CMC and pectin.
Pectin has been added to the WPC as a stabilizer but the
small pectin concentration (0.1%) is not able to maintain
the stability of the W
1
/O/W
2
emulsion system.
Based on the results of the experiments conducted, the
tween 80 emulsifier is more stable than the WPC Pectin
emulsifier for the W
1
/O/W
2
emulsion system so that the
tween 80 emulsifier is more suitable for use as a second
emulsifier for vitamin C emulsions.
4 CONCLUSIONS
Emulsion W
1
/O/W
2
Vitamin C with WPC-Pectin as
emulsifier in the outer aqueous phase bigger particle
size 1.34 μm compared with Tween 80 with 0.91 μm
after preparation. Tween 80 as emulsifier was more
stable during storage with little cream formed in the
surface. When the droplet size become bigger, the
stability of the emulsion have been decreased. TEM
Result also showed that the morphology of emulsion
with Tween 80 remained stable as a double emulsion
after storage for 14 days. tween 80 is the best
emulsion to maintain the stability of W
1
/O/W
2
emulsion containing vitamin C.
ACKNOWLEDGEMENTS
This work financially supported by the Directorate
General of Higher Education (Ristek Dikti) of the
Ministry of Education and Culture Republic of
Indonesia, under the grant of basic research program.
0
8,94
14,41
0
14,54
23,06
0
5
10
15
20
25
0714
Creaming Index (%)
Storage Days
Tween 80 WPC-Pectin
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
50

REFERENCES
Anton, N., Vandamme, T. F., Ding, S., Yu, W., & Serra, C.
A. (2018). Double emulsions prepared by two–step
emulsification: History, state-of-the-art and
perspective. Journal of Controlled Release, 295
(December 2018), 31–49. https://doi.org/10.1016/j.jcon
rel.2018.12.037
Chung, C., & Mcclements, D. J. (2018). Characterization
of Physicochemical Properties of Nanoemulsions :
Appearance , Stability , and Rheology. Nanoemulsions.
Elsevier Inc. https://doi.org/10.1016/B978-0-12-
811838-2.00017-5
Dickinson, E. (2010). Hydrocolloids and emulsion stability.
Handbook of hydrocolloids. Woodhead Publishing
Limited. https://doi.org/10.1533/9781845695873.23
Gharehbeglou, P., Mahdi, S., Hamishekar, H., &
Homayouni, A. (2019). Pectin-whey protein complexes
vs . small molecule surfactants for stabilization of
double nano-emulsions as novel bioactive delivery
systems. Journal of Food Engineering, 245(October
2018), 139–148. https://doi.org/10.1016/j.jfoodeng.20
18.10.016
Gregory, J. F. (2017). Vitamins. Fennema’s Food
Chemistry. Elsevier Inc. https://doi.org/10.1201/9781
315372914
Khalid, N., Kobayashi, I., Neves, M. A., Uemura, K., &
Nakajima, M. (2013). Preparation and Characterization
of Water-in-Oil-in-Water Emulsions Containing a High
Concentration of L-Ascorbic Acid. Bioscience,
Biotechnology and Biochemistry, 77(6), 1171–1178.
https://doi.org/10.1271/bbb.120870
Levine, M., Katz, A., Padayatty, S. J., Wang, Y., Eck, P.,
Kwon, O., Lee, J. H. (2004). Vitamin C. Encyclopedia
of Dietary Supplements, 745–755. https://doi.org/
10.1081/E-EDS-120022052
Mohammadi, A., Jafari, S. M., Esfanjani, A. F., &
Akhavan, S. (2016). Application of nano-encapsulated
olive leaf extract in controlling the oxidative stability of
soybean oil. FOOD CHEMISTRY, 190, 513–519.
https://doi.org/10.1016/j.foodchem.2015.05.115
Schmidts, T., Dobler, D., Nissing, C., & Runkel, F. (2009).
Journal of Colloid and Interface Science Influence of
hydrophilic surfactants on the properties of multiple W
/ O / W emulsions. Journal of Colloid And Interface
Science, 338(1), 184–192. https://doi.org/10.1016/
j.jcis.2009.06.033
Schuch, A., Helfenritter, C., Funck, M., & Schuchmann, H.
P. (2014). emulsions. Colloids and Surfaces A:
Physicochemical and Engineering Aspects.
https://doi.org/10.1016/j.colsurfa.2014.06.012
Vicente, J., José, L., Pereira, B., Pinto, L., Bastos, H.,
Carvalho, M. G. De, & Garcia-rojas, E. E. (2018).
Effect of xanthan gum or pectin addition on Sacha Inchi
oil-in-water emulsions stabilized by ovalbumin or
tween 80: Droplet size distribution, rheological
behavior and stability. International Journal of
Biological Macromolecules, #pagerange#.
https://doi.org/10.1016/j.ijbiomac.2018.08.041
Zhang, Z., & Mcclements, D. J. (2018).
Overview of
Nanoemulsion Properties : Stability , Rheology , and
Appearance. Nanoemulsions. Elsevier Inc.
https://doi.org/10.1016/B978-0-12-811838-2.00002-3
Preparation of Double Emulsion of Vitamin C with Two Different Emulsifiers in the Outer Aqueous Phase
51
