
Characterization of Insoluble Fiber in Cassava Peel and Its
Hydrolyzate Potential as a Prebiotic for Lactobacillus Plantarum
Ilham Marvie
1,2
, Azis Boing Sitanggang
2
and Slamet Budijanto
2
1
Food Technology Study Program, Institute Technology of Sumatera, South Lampung, Indonesia
2
Department of Food Science and Technology, IPB University, West Java, Indonesia
Keywords: Cassava Peel, Cellobiose, Cellulase, Cellulose, Prebiotic.
Abstract: The cassava peel is an uncommon material for consumption as food for Indonesians because its use is widely
used in non-food industries. Exploration of the use of cassava peel in the food industry in this case as a
functional food ingredient such as prebiotics has the potential to be carried out. This study aims to determine
the characteristics of the insoluble fiber content of the cassava peel and testing its hydrolysate potential as a
prebiotic in growing Lactobacillus plantarum. The varieties of the cassava plant used in this study were Ratim
(RTM 22) and Ulujami (UJ 17). The method used in this study is the characterization of insoluble fiber. As
well as testing the prebiotic potency by determining the prebiotic activity score by doing Lactobacillus
plantarum. The results of this study indicate that the characteristics of insoluble fiber in the cassava peel are
hemicellulose content is more dominant than cellulose and lignin in the cassava peel. The hydrolysate of
cellulose from cassava peel showed its potential as a prebiotic in growing Lactobacillus plantarum. RTM 22
varieties had a higher prebiotic activity score than UJ 17. The prebiotic activity scores of RTM 22 and UJ 17
were 1.70 and 1.48, respectively.
1 INTRODUCTION
The Cassava peel is an uncommon material for
consumption as food for Indonesian people, its use is
more widely used as industrial raw material (Aripin
et al., 2013), animal feed (Oluwanike and Adeneye,
2014), and biodegradable packaging (Widiarto et al.,
2017). The use of the cortex of cassava peel as a
traditional Sumedang food ingredient called
kadedemes has inspired the development of cassava
peel as a food ingredient. Meanwhile, the cassava
peel has a dry weight that reaches 13% of the total
weight of cassava peel (Aripin et al., 2013). The
availability of raw material for cassava is also
predicted to continue to increase in line with the
increase in the national consumption of cassava
reaching 12.7 million tons in 2020 (BPS, 2013).
Therefore, exploration of utilization in the food
industry in this case as functional food ingredients
such as prebiotics has the potential to be carried out.
Cassava peel has a crude fiber content of up to
12.2% (Idugboe, Nwokoro and Imasuen, 2017).
Insoluble fiber is a part of crude fiber (Idris et al.,
2020). Cellulose under natural conditions is coated by
a matrix of hemicellulose and lignin (Elechi et al.
2016). The presence of lignin and hemicellulose
content is thought to affect the hydrolysis of cellulose
(Surendran et al., 2018). Therefore, it is necessary to
characterize the content and insoluble in the skin of
the cassava peel. The process to reduce the
hemicellulose and lignin content is also needed to
obtain the dominant cellulose content. Cellulose is
composed of D-anhydro glucopyranose in β-1,4-
glycoside or β-glucose bonds (Elechi et al., 2016).
The hydrolysate from the hydrolysis of cellulose with
cellulase enzymes can produce cellobiose as an
intermediate product (Razie et al., 2011).
Previous research has been carried out by
knowing the ability of cellobiose as a stimulus in the
growth of Lactobacillus acidophilus NCFM (van
Zanten et al., 2012). In-vivo test results have also
been carried out and do not give unwanted side effects
to humans (Van Zanten et al., 2014). Testing the
ability to grow other Lactobacillus strains such as
Lactobacillus plantarum also needs to be carried out
on products from the hydrolysis of cassava peel to add
information on the ability of cellobiose as a prebiotic.
This study aims to determine the characteristics of the
insoluble fiber content of the skin of cassava peel. and
testing its hydrolysate potential as a prebiotic in
Marvie, I., Sitanggang, A. and Budijanto, S.
Characterization of Insoluble Fiber in Cassava Peel and Its Hydrolyzate Potential as a Prebiotic for Lactobacillus Plantarum.
DOI: 10.5220/0010507300003108
In Proceedings of the 6th Food Ingredient Asia Conference (6th FiAC 2020) - Food Science, Nutrition and Health, pages 31-37
ISBN: 978-989-758-540-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
31

growing Lactobacillus plantarum. This research is
expected to expand the exploitation of cassava peel as
raw material for functional food as a prebiotic and
increase its added value in the food industry.
2 METHOD
2.1 Material
The main material used in this study were cassava
peel (Manihot utilissima Sp) varieties of Ratim (RTM
22) and Ulujami (UJ 17) from the experimental garden
of the Department of Agronomy and Horticulture,
Bogor Agricultural University, Cigombong, Sukabumi
Regency, West Java. The enzymes used were complex
cellulase enzymes (Wathringthon, Murni). The
bacteria used were Lactobacillus plantarum and
Escherichia coli obtained from SEAFAST IPB.
Nylon-66 membrane pore size 0.20 µm and 0.45 µm
with a diameter of 25mm (Himedia). The media used
for bacterial growth are de Man Rogosa Sharpe
(Merck) and M9 (Merck).
2.2 Preparation of Cassava Peel
Material
The process of preparing the cassava peel raw
material consists of sorting, washing, reducing the
size, and peeling the tuber skin. Samples were dried
using a cabinet drier at 50
o
C for 24 hours and crushed
using a disc mill to form flour with a size of 40 mesh.
The raw material is again dried in the cabinet drier for
24 hours until the moisture content is <10% (Tasaso,
2015). The cooking of the cassava peel is done by
heating 50 g of cassava root flour in 1 L of water and
10% NaOH using an autoclave at a temperature of
130
o
C, a pressure of 190 kPa for 60 minutes.
Separation of the extraction results between the solid
residue and the black concentrated solution using a
filter cloth and washed with distilled water until the
solid residue reaches a pH of 7.0. The residue was
dried at 70
o
C for 24 hours. Bleaching process by
adding 30% H
2
O
2
at 70
o
C for 3 hours. Cassava peel
is separated again to get the residue and rinsed using
distilled water. The bleaching residue is dried again
at a temperature of 70
o
C (Tasaso, 2015).
2.3 Characterization of the Insoluble
Fiber Content
Characterization of cassava peel flour was carried out
using fiber content analysis consisting of Acid
Detergent Fiber (ADF), Neutral Detergent Fiber
(NDF), cellulose, lignin, and hemicellulose levels
(van Soest, 1963). All tests were carried out in two
duplicate replications.
2.3.1 Neutral Detergent Fiber (NDF)
NDF solution consisted of distilled water 1 L, Sodium
Sulfate 30 g, EDTA 18.81 g; Sodium Borate 10 H
2
O
6.81 g, 4.5 g anhydrous di-Na-HPO4 and 10 ml pure
2-ethoxy ethanol. a sample of 0.5 g (A) was put into
a 250 mL beaker. The sample was then added with
NDF solution and filtered with the help of a vacuum
pump, rinsed alternately with hot water and acetone.
The filter results were dried in an oven at 105
o
C until
stable, after that they were put in a desiccator for one
hour, then weighed (B). The measurement results are
reduced by the weight of the dry glass filter before
use (C)
NDF Content= ((B-C))/A ) x 100% (1)
2.3.2 Acid Detergent Fiber (ADF)
A sample of 1 g (A) was put into a beaker and ml of
ADF solution was added. The ADF solution consisted
of 1 L of 1 N H
2
SO
4
and 20 g of CTAB (cethyle
trimethyl ammonium bromide). The sample to which
the solution was added was heated for one hour on the
back cooler. Filtering is done with the help of a
vacuum pump using a glass filter. Washing is carried
out alternately with acetone and hot water. The filter
results were dried in an oven at 105°C until stable,
after that they were put in a desiccator for one hour,
then weighed (D). The measurement results are
reduced by the weight of the dry glass filter before
use (C)
ADF Content= ((D-C)/A) x 100% (2)
2.3.3 Cellulose, Hemicellulose, and Lignin
Content
ADF residue (E) which is in the glass filter is placed
on a tray of water about 1 cm high. Then added H
2
SO
4
as high as ¾ part of the glass filter and left for 3 hours
while stirring. Filtering with a glass filter is assisted
by a vacuum pump. Washing is done with acetone
and hot water. Do the drying and put the filter results
into the oven. After that, it is put back into the
desiccator to cool down and weigh (F). Furthermore,
the glass filter is in the furnace at 450
o
C for 3-4 hours,
then put again into the desiccator to cool down and
weigh (G)
𝐶𝑒𝑙𝑙𝑢𝑙𝑜𝑠𝑒 𝐶𝑜𝑛𝑡𝑒𝑛𝑡 = ((𝐹 − 𝐺)/𝐸) 𝑥 100% (3)
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
32

To find out the hemicellulose and lignin content,
you can use the equation below using the results of
the ADF and NDF tests.
𝐻𝑒𝑚𝑖𝑐𝑒𝑙𝑙𝑢𝑙𝑜𝑠𝑒 𝐶𝑜𝑛𝑡𝑒𝑛𝑡 = 𝑁𝐷𝐹 𝐶𝑜𝑛𝑡𝑒𝑛𝑡 − 𝐴𝐷𝐹 𝐶𝑜𝑛𝑡𝑒𝑛𝑡
(4)
𝐿𝑖𝑔𝑛𝑖𝑛 𝐶𝑜𝑛𝑡𝑒𝑛𝑡 = 𝐴𝐷𝐹 𝐶𝑜𝑛𝑡𝑒𝑛𝑡 − 𝐶𝑒𝑙𝑙𝑢𝑙𝑜𝑠𝑒 𝐶𝑜𝑛𝑡𝑒𝑛𝑡
(5)
2.4 Probiotic Growth Testing on
Cassava Peel Hydrolysates
The lactic acid bacteria used as probiotics are
Lactobacillus plantarum. Before use, Lactobacillus
plantarum was refreshed on MRS Broth for 24 hours
at 30
o
C. Growth was carried out on MRS Base media
with the addition of cellobiose as a substitute for
glucose for carbohydrate sources. The composition
for making MRS Base is peptone 10 g/L, meat extract
8 g/L, yeast extract 4 g/L, sodium acetate 5 g/L,
magnesium sulphate 0.2 g/L, manganese sulphate
0.05 g / l , dipotassium sulphate 0.05 g/L, polysorbate
80 1 g/L and prepared with a pH of 6.2±0.2 at 25
o
C
(De Man, Ragosa and Sharpe, 1960). Inoculation of
0.1 mL of Lactobacillus plantarum on MRS added
0.1 mL of cellobiose. Lactobacillus plantarum was
also grown on MRS with 0.1 glucose added at a
concentration of 250 mg/mL to compare with the
growth on MRS with cellobiose. The cellobiose to be
added to the MRS must first be filtered on a
membrane with a sterile 0.25 µm pore size to avoid
bacterial contamination. The growth of Lactobacillus
plantarum can be observed by growing it on MRS
agar. Measurements were carried out on
Lactobacillus plantarum which had been incubated
for 0, 24 and 48 hours at 30
o
C (Herawati et al., 2019).
as much as 1 mL of Lactobacillus plantarum was
inoculated on MRS agar and incubated for 48 hours.
The growth of Lactobacillus plantarum is indicated
by the presence of white colonies.
The prebiotic activity score is a comparison
between the ability of prebiotics to grow probiotics
and inhibit the growth of Escherichia coli against
growth in glucose during 24 hours of incubation.
Lactobacillus plantarum was grown on MRS media
added by prebiotics and Escherichia coli was growth
on M9 media added by prebiotics (Moongngarm,
Trachoo and Sirigungwan, 2011). The prebiotic
activity score can be found using the equation below.
𝑃𝑟𝑒𝑏𝑖𝑜𝑡𝑖𝑐 𝐴𝑐𝑡𝑖𝑣𝑖𝑡𝑦 𝑆𝑐𝑜𝑟𝑒
=
log
𝑐𝑓𝑢
𝑚𝑙
𝑝𝑟𝑜𝑏𝑖𝑜𝑡𝑖𝑐 & 𝑝𝑟𝑒𝑏𝑖𝑜𝑡𝑖𝑐 24 ℎ𝑜𝑢𝑟𝑠 − log
𝑐𝑓𝑢
𝑚𝑙
𝑝𝑟𝑜𝑏𝑖𝑜𝑡𝑖𝑐 & 𝑝𝑟𝑒𝑏𝑖𝑜𝑡𝑖𝑐 0 ℎ𝑜𝑢𝑟𝑠
log
cfu
mL
𝑝𝑟𝑜𝑏𝑖𝑜𝑡𝑖𝑐 & 𝑔𝑙𝑢𝑐𝑜𝑠𝑒 24 ℎ𝑜𝑢𝑟𝑠 − log
𝑐𝑓𝑢
𝑚𝑙
𝑝𝑟𝑜𝑏𝑖𝑜𝑡𝑖𝑐 & 𝑔𝑙𝑢𝑐𝑜𝑠𝑒 0 ℎ𝑜𝑢𝑟𝑠
−
log
𝑐𝑓𝑢
𝑚𝑙
𝐸 𝑐𝑜𝑙𝑖 & 𝑝𝑟𝑒𝑏𝑖𝑜𝑡𝑖𝑐 24 ℎ𝑜𝑢𝑟𝑠 − log
𝑐𝑓𝑢
𝑚𝑙
𝐸 𝑐𝑜𝑙𝑖 & 𝑝𝑟𝑒𝑏𝑖𝑜𝑡𝑖𝑐 0 ℎ𝑜𝑢𝑟
log
𝑐𝑓𝑢
𝑚𝑙
𝐸 𝑐𝑜𝑙𝑖 & 𝑔𝑙𝑢𝑐𝑜𝑠𝑒 24 ℎ𝑜𝑢𝑟𝑠 − log
𝑐𝑓𝑢
𝑚𝑙
𝐸 𝑐𝑜𝑙𝑖 & 𝑔𝑙𝑢𝑐𝑜𝑠𝑒 0 ℎ𝑜𝑢𝑟
In this test, the composition of the addition of
cellobiose and glucose to M9 media followed the
concentration on MRS. Before using Escherichia
coli, its condition can be freshened by growing it on
Triptic Soy Broth (TSB) / Brain Heart Infusion Broth
(BHIB) and M9 media for 24 consecutive hours
respectively. Escherichia coli grown on M9 was
focused on Triptic Soy Agar (TSA) to count the
number of colonies that grew during 24 hours of
incubation at 35oC. The number of colonies that grew
on TSA and MRS agar media was converted to log
cfu/mL and then entered in equation (1)
2.5 Data Analysis
Data presentation was carried out using the Microsoft
Excel 2016 program and the Minitab 18 program.
Analysis of variance (ANOVA) between samples was
carried out using Tukey's honest real difference
(HSD) test at the 5% level (p <0.05).
3 RESULT AND DISCUSSION
3.1 Characterization of Cassava Peel
The skin of the Ratim variety of cassava peel (RTM
22) has physical characteristics in the form of red
colour on the inner peel (cortex) and has a bland taste.
While the Ulujami variety (UJ 17) has white peel with
a bitter taste. Based on its structure, the cassava peel
consists of two layers, namely the periderm and
cortex (Mohd-asharuddin et al., 2017). The cortex
layer used as the raw material in this study has a
slippery texture, is flexible, is lighter in colour than
the periderm. According to Idris et al., (2020) the
cortex layer has a higher crude fiber content than the
tuber content (flesh), while the cyanide compound
content is not significantly different at 0.01 mg/Kg
dry basis. The raw material preparation process can
be carried out to clean cassava peels from soil
impurities and reduce the cyanide acid content found
in cassava peels (Falade and Akingbala, 2010).
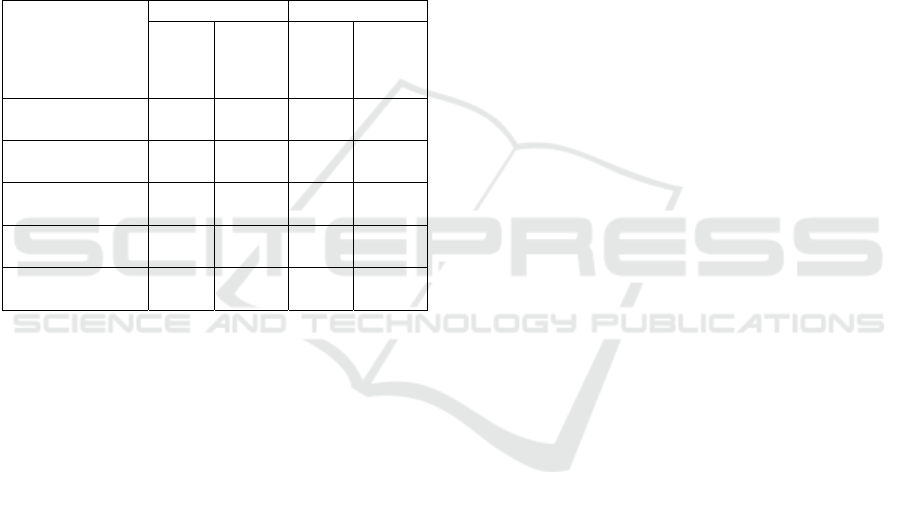
Based on Table 1, the NDF content of cassava
peel of UJ 17 variety was 13.35% and RTM 22
variety was 48.64%. Meanwhile, the ADF content of
the cassava peel of UJ 17 variety was 6.36% and
RTM 22 variety was 7.63%. Neutral Detergent Fiber
(NDF) is an insoluble fiber content in neutral
detergents consisting of cellulose and lignin, while
Acid Detergent Fiber (ADF) is an insoluble fiber
content in acidic detergents consisting of lignin,
cellulose, and hemicellulose. The difference between
ADF and NDF indicates the amount of hemicellulose
Characterization of Insoluble Fiber in Cassava Peel and Its Hydrolyzate Potential as a Prebiotic for Lactobacillus Plantarum
33
content (Oluwanike and Adeneye, 2014). The total
content of cellulose, hemicellulose, and lignin which
does not reach 100% both before cooking and after
blanching indicates other compounds that do not
include insoluble fiber such as starch, protein, fat, and
so on. The NDF and ADF content of the two varieties
of cassava bark had a significant difference. After
cooking with NaOH and blanching with H
2
O
2
, the
NDF and ADF content were not significantly
different. The NDF content of cassava peel of UJ 17
variety increased to 68.51% and RTM 22 variety to
67.60%, while the ADF content of cassava peel of UJ
17 variety increased to 60.83% while RTM 22 variety
increased to 60.27%.
Table 1: The cassava peel insoluble fiber content.
Insoluble Fiber
Before Cooking Afte
r
Bleaching
UJ17 RTM 22 UJ17 RTM 22
NDF (%)
13,35
±0,55
b
48,64
±0,09
a
68,51
±0,07
a
67,60
±0,64
a
ADF (%)
6,36
±0,10
b
7,63
±0,07
a
60,83
±1,21
a
60,27
±0,45
a
Cellulose (%)
4,99
±0,09
a
5,74
±0,05
a
55,50
±2,20
a
55,79
±0,44
a
Hemicellulose (%)
6,97
±0,45
b
41,02
±0,02
a
7,68
±1,14
a
7,32
±0,19
a
Lignin (%)
1,39
±0,01
a
1,88
±0,02
a
5,34
±0,99
a
4,49
±0,01
a
Note: Dry base with 8.98% moisture content. Tests were carried
out in 2 replications. a / b: Tukey's real difference test (ANOVA)
and honest real difference test (HSD) at the 5% level
Hemicellulose content in cassava peel flour of
varieties UJ 17 and RT 22 was 6.97% and 41.02%,
respectively. This significant difference shows that
different varieties can indicate different
characteristics of crude fiber. The lignin content of
varieties UJ 17 and RTM 22 were 1.39% and 1.88%,
respectively. A study by Barati, Latif and Müller,
(2019) states that cassava contains 30.4%
hemicellulose. The lignin content in cassava skin
reaches 7.50% (Aripin et al., 2013). After the cooking
and bleaching process, the hemicellulose content
changed to 7.32%, while the lignin content became
4.49%. Changes in hemicellulose and lignin content
are caused by dissolving insoluble non-fiber
compounds during the cooking process with NaOH
and bleaching with H
2
O
2
. The process of dissolving
lignin and hemicellulose also occurs during cooking
with NaOH at high temperatures. The reaction
between NaOH and lignin in hot conditions results in
the formation of a thick black and sticky solution that
can be separated from the solvent. The bleaching
process carried out after extraction through the
addition of H
2
O
2
aims to remove the remaining lignin
by oxidizing the chromophore molecules in the lignin
so that it becomes polar and water-soluble. This
process is important because naturally lignin is water-
insoluble and binds to hemicellulose and lignin (Allen
et al., 2016).
The cellulose content of the RTM 22 variety of
cassava peel after cooking with NaOH and blanching
with H
2
O
2
showed an increase from 5.74% to
55.70%. The increase in cellulose content in the
cassava peel varieties UJ 17 from 4.99% to 55.50%
The increase in cellulose content is due to the nature
of cellulose which has good resistance to alkaline and
heat compounds up to 280
o
C used in the extraction
process (Suryanto, 2015). Meanwhile, according to
Widiarto et al., (2017) the use of acids in extraction
makes cellulose hydrolysed into a simpler form. In
addition to the extraction method used in this study,
the alternative use of 4% NaOH and 4% NaOCl in
extraction resulted in 40.5% cellulose, 11.7% lignin,
and 21.4% hemicellulose (Widiarto et al., 2019).
Therefore, the extraction method using 11% NaOH
and 30% H
2
O
2
has a better result to increase the
percentage of cellulose. In this bleaching process, it
is also possible to decrease cellulose in the
amorphous form and increase the percentage of
cellulose in the crystalline form (Leite, Zanon and
Menegalli, 2017).
3.2 Probiotic Growth Testing on
Cassava Peel Hydrolysates
The hydrolysate of the cassava peel was obtained
based on the results of the hydrolysis of the cassava
peel flour which had been cooked with NaOH and
blanched with H2O2. Hydrolysis was carried out
using cellulase enzymes with a concentration of 4.03
U/mL on UJ 17 and RTM 22 varieties. The enzyme
concentration was obtained from the results of
preliminary research using a concentration of 18.91
U/mL and 22.06 U/mL with the results of the first 1
hour of hydrolysis. has reached the degree of
polymerization 1,00. Hydrolysis using a substrate of
200 mg, 0.1 mL, 200 mL of citrate buffer pH 4.8, and
10% sodium azide as antimicrobial. The cellulase
enzyme activity used was 115 U/mL (Worthington,
2020). Hydrolysis was performed using a shaker
incubator at 37
o
C with an agitation speed of 150 rpm
for 24 hours (Selig, Weiss and Ji, 2008). Hydrolysate
is obtained when the hydrolysis process has been
going on for 12 hours. The results of hydrolysis of
cassava peels for 12 hours were used as a
carbohydrate source substitute in MRS media to
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
34
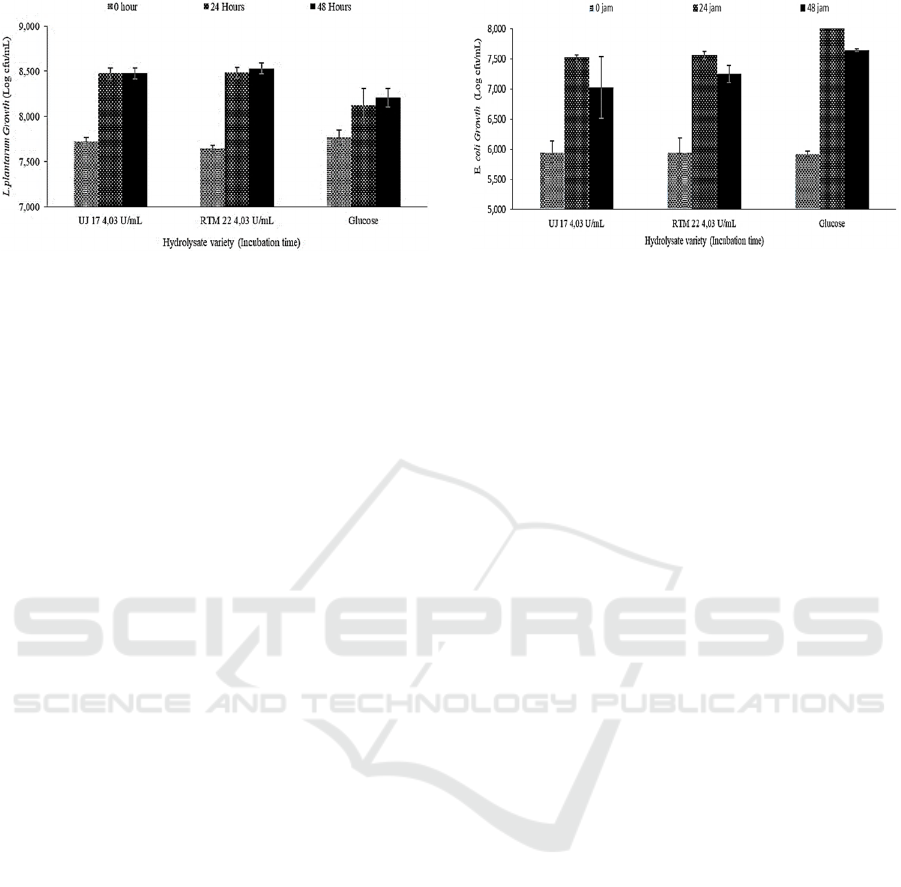
Figure 1: Lactobacillus plantarum growth on cassava peel
hydrolysate and MRS.
growth Lactobacillus plantarum and as a
carbohydrate source substitute in M9 medium to grow
Escherichia coli. Based on the preliminary research
that has been carried out, the hydrolysate has a degree
of polymerization below 2.00 which indicates that the
content of cellobiose and cello-oligosaccharide has
been dominant.
The growth of Lactobacillus plantarum growth
with UJ 17 hydrolysate at 0 hour was 7,719 log
cfu/mL and increased significantly at 24 hours of
growth with 8,475 cfu/mL, but after 48 hours there
was no significant difference, namely 8,479 cfu/mL.
The growth of Lactobacillus plantarum on RTM 22
variety hydrolysate was at 7,643 log cfu/mL at 0
hours and increased significantly to 8,482 log cfu/mL.
Growth at 24 hours and 48 hours had no significant
difference with 8,535 log cfu/mL. In the two varieties
of cassava peel at 24 and 48 hours, the growth was
not significantly different. This shows that the
microbes have been in a stationary phase because
they are still in the same log colony number with
growth at 24 hours. (Karnaouri, Matsakas, Bühler, et
al., 2019) reported that the growth of Lactobacillus
plantarum in media added with cellobiose also
showed a stationary phase at the incubation time of
48 hours to 72 hours. The amount of cellobiose
consumed would correlate with the increase in the
amount of lactic acid, acetic acid, and propionic acid
formed. The growth of Lactobacillus plantarum is
suspected not because there are still peptides from the
inactivation of the cellulase enzyme by heating at
85oC for five minutes. Apart from the percentage use
of the enzyme which is 1% of the total hydrolysis
volume, the stratified filtration process with a
membrane measuring 0.45µm and 0.20µm is
expected to minimize the contamination of
macronutrients and microorganisms that bias the
growth of Lactobacillus plantarum.
The growth of Escherichia coli on M9 medium
grown on the hydrolysate of cassava peels of UJ 17
Figure 2: Escherichia coli growth on cassava peel
hydrolysate and M9.
variety in 0 hours was 5.953 log cfu/mL and increased
significantly to 7.524 log cfu/mL at 24 hours of
growth. While the growth of Escherichia coli on M9
media added to the hydrolysate of cassava varieties
RTM 22 in 0 hours was 5.952 log cfu / mL. The
increase occurred in growth within 24 hours to 7.566
log cfu / ml. The growth of Escherichia coli at 48
hours experienced a decrease in both the hydrolysates
of UJ 17 and RTM 22 varieties, respectively 7,026 log
cfu / mL and 7,253 log cfu / mL. The growth of
Escherichia coli on M9 media with added glucose
had a higher growth than in M9 media which was
added by hydrolysis of cassava peel. The growth of
Escherichia coli by two logs shows that the products
of cellulose hydrolysis of the three varieties still
contain β glucose which is easily digested by
Escherichia coli, but after 24 hours the incubation of
β glucose contained in M9 is reduced and the growth
of Escherichia coli is lower because cannot digest
cellobiose and cello-oligosaccharides found in M9.
Growth conditions in the hydrolysate that are
expected to occur in cellobiose as a prebiotic in
providing the ability to grow Lactobacillus plantarum
as a probiotic and not a source of carbohydrates for
Escherichia coli in the human digestive tract.
Cellobiose which is thought to be dominant in the
product of cellulase enzyme hydrolysis has the ability
to grow Lactobacillus plantarum which is better with
an optical density (OD) value of 600 more than 5
compared to other Lactobacillus strains and
Bifidobacterial strains as probiotics, Lactobacillus
plantarum has low growth. on media with added
glucose for 24 hours (Karnaouri, Matsakas,
Krikigianni, et al., 2019).
The ability of hydrolysis products needs to be
measured as a prebiotic potency using prebiotic
activity score analysis. The hydrolysate of RTM 22
cassava peel with 2.88 U / mL cellulase had a higher
prebiotic activity score than the UJ 17 variety. The
difference in prebiotic activity scores between
Characterization of Insoluble Fiber in Cassava Peel and Its Hydrolyzate Potential as a Prebiotic for Lactobacillus Plantarum
35
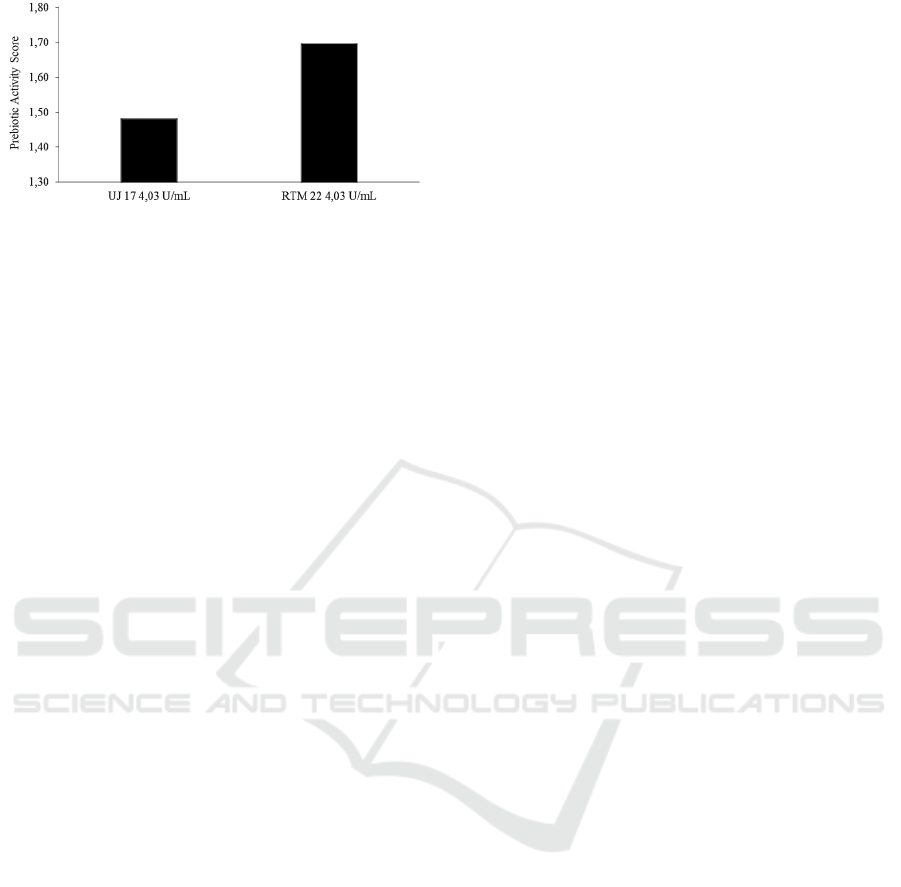
Figure 3: Prebiotic Activity Score on cassava peel
hydrolysate.
cassava varieties was thought to be due to differences
in the amount of cellobiose and β glucose found in
hydrolysis products. The high percentage of
cellobiose content will increase the growth of
Lactobacillus plantarum, while the higher percentage
of β glucose content will increase the growth of
Escherichia coli. The percentage of growth of the two
bacteria affects the prebiotic activity score. The
difference in the content of cellulose, lignin, and
hemicellulose contained in the substrate was thought
to affect, however, the cellulose extraction process
made the composition of the lignocellulose
compounds not significantly different between
cassava varieties. The prebiotic activity scores of the
two-cassava peel hydrolysates of UJ 17 and RTM 22
varieties were 1.48 and 1.70, respectively. The
prebiotic activity score was higher than the score on
inulin from the hydrolysis of red fruit grown on
Lactobacillus casei of 0.88 (Murtiningrum et al.,
2019). The prebiotic activity score was also higher
than the prebiotic activity score of
gallactooligosaccharide (GOS) grown on
Lactobacillus plantarum 12006 (Huebner, Wehling
and Hutkins, 2007). The prebiotic activity score on
the RTM 22 hydrolysate was also higher than the
growth of Lactobacillus acidophilus on
fructooligosaccharides (FOS), but lower than the
growth of Lactobacillus acidophilus on inulin
(Moongngarm, Trachoo and Sirigungwan, 2011).
4 CONCLUSION
The characteristics of insoluble fiber in the cassava
peel consist of cellulose, hemicellulose, and lignin.
Hemicellulose content is more dominant than
cellulose and lignin in the cassava peel. The
hemicellulose content of UJ 17 and RTM 22 varieties
had a significant difference. After cooking with
NaOH and blanching with H
2
O
2
, cellulose content
became dominant compared to hemicellulose and
lignin. This process causes changes in the
characteristics of insoluble fiber, such as the
dissolution of non-soluble fiber compounds and
hemicellulose and lignin. The cassava peel varieties
UJ 17 and RTM 22 contained no significant
difference in cellulose. The hydrolysate of cellulose
from the cassava peel showed its potential as a
prebiotic in growing Lactobacillus plantarum. The
hydrolysates from cassava peels of RTM 22 varieties
had a higher prebiotic activity score than UJ 17. The
prebiotic activity scores of the cassava peel
hydrolysates of RTM 22 and UJ 17 varieties were
1.70 and 1.48, respectively.
ACKNOWLEDGEMENTS
Thank you to the Directorate of Higher Education,
Ministry of Education and Culture of the Republic of
Indonesia for funding this research through the
master's thesis research scheme. Thanks to Prof. Dr.
Ir. Lilis Nuraida, M.Sc. who has allowed the use of
lactobacillus plantarum 026 culture that is owned and
thanks to Dr. Ir. Nurul Khumaida, M.Sc. which has
permitted the use of cassava peel of a Ratim variety
(RTM 22) dan Ulujami variety (UJ 17) that were
owned for this study.
REFERENCES
Allen, S. A. et al. (2016) ‘Lignocelluloses : An Economical
and Ecological Resource for Bio-Ethanol Production –
A Review’, International Journal of Natural Resource
Ecology and Management, 1(3), pp. 128–144. doi:
10.11648/j.ijnrem.20160103.18.
Aripin, M. et al. (2013) ‘Cassava Peels for Alternative Fibre
in Pulp and Paper Industry : Chemical Properties and
Morphology Characterization’, International Journal
of Integrated engineering, 5(1), pp. 30–33.
Barati, Z., Latif, S. and Müller, J. (2019) ‘Enzymatic
hydrolysis of cassava peels as potential pre-treatment
for peeling of cassava tubers’, Biocatalysis and
Agricultural Biotechnology. Elsevier Ltd, 20
(December 2018), p. 101247. doi: 10.1016/j.bcab.20
19.101247.
BPS, B. P. S. (2013) Pengeluaran dan Konsumsi Penduduk
Indonesia, Survey Sosial Ekonomi Nasional.
Elechi, O. O. et al. (2016) ‘Acid Hydrolysis Of Cassava
Peel’, International Journal of Scientific & Technology
Research, 5(01), pp. 184–187.
Falade, K. O. and Akingbala, J. O. (2010) ‘Utilization of
Cassava for Food’, Food Review International,
9129(27), pp. 51–83. doi: 10.1080/87559129.2010.518
296.
Herawati, E. R. N. et al. (2019) ‘Oligosaccharides Profile
and Prebiotic Potential of Gembolo Tuber ( Dioscorea
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
36

bulbifera ) Oligosaccharides Profile’, in IOP Conf.
Series: Earth and Enviromental Science. IOP
Publishing. doi: 10.1088/1755-1315/251/1/012048.
Huebner, J., Wehling, R. L. and Hutkins, R. W. (2007)
‘Functional activity of commercial prebiotics’,
International Dairy Journal, 17(7), pp. 770–775.
doi: 10.1016/j.idairyj.2006.10.006.
Idris, S. et al. (2020) ‘Physicochemical composition of
different parts of cassava (Manihot esculenta crantz)
plant’, Food Research, 4, pp. 78–84. doi:
10.26656/fr.2017.4(S1).S33.
Idugboe, Nwokoro, O. and Imasuen, S. (2017) ‘Chemical
Composition of Cassava Peels Collected from Four
Locations (Koko , Warri , Okada and Benin City),
Brewers` Spent Yeast and Three Grades of
“Caspeyeast”, International Journal of Science and
Research, 6(4), pp. 2015–2018. doi: 10.21275/ART
20172389.
Karnaouri, A., Matsakas, L., Bühler, S., et al. (2019)
‘catalysts Tailoring Celluclast ® Cocktail ’ s
Performance towards the Production of Prebiotic Cello-
Oligosaccharides from Waste Forest Biomass’,
Catalysts, 9, p. 897.
Karnaouri, A., Matsakas, L., Krikigianni, E., et al. (2019)
‘Valorization of waste forest biomass toward the
production of cello-oligosaccharides with potential
prebiotic activity by utilizing customized enzyme
cocktails’, Biotechnology for Biofuels. BioMed Central,
12(1), pp. 1–19. doi: 10.1186/s13068-019-1628-z.
Leite, A. L. M. P., Zanon, C. D. and Menegalli, F. C. (2017)
‘Isolation and characterization of cellulose nanofibers
from cassava root bagasse and peelings’, Carbohydrate
Polymers. Elsevier Ltd., 157, pp. 962–970. doi:
10.1016/j.carbpol.2016.10.048.
De Man, J. ., Ragosa, M. and Sharpe, M. E. (1960) ‘A
Medium For Cultivation of Lactobacilli’, J Appl Bact,
1, pp. 130–135.
Mohd-asharuddin, S. et al. (2017) ‘A Chemical and
Morphological Study of Cassava Peel : A Potential
Waste as Coagulant Aid’, in MATEC Web of
Conferences. EDP Sciences, pp. 1–8. doi: 10.1051/
matecconf/201710306012.
Moongngarm, A., Trachoo, N. and Sirigungwan, N. (2011)
‘Low Molecular Weight Carbohydrates, prebiotic
content, and prebiotic activity of selected food plants in
Thailand’, Advance Journal of Food Science and
Technology, 3(4), pp. 269–274.
Murtiningrum et al. (2019) ‘Identification of Inulin Profile
From Red Fruit (Pandanus Conoideus L) Pedicel
Extract Using LC-MS And Its In Vitro Prebiotic
Activity Test’, International Journal of Advance
Reasearch, 7(11), pp. 344–351. doi: 10.21474/IJAR01/
10014.
Oluwanike, A. and Adeneye, M. A. (2014) ‘Evaluation of
chemical composition and nutritive potential of oil
palm slurry fermented with cassava peel as feed for
livestock’, African Journal of Agricultural Research,
9(26), pp. 2062–2067. doi: 10.5897/AJAR2013.7565.
Razie, F. et al. (2011) ‘Aktivitas Enzim Selulase Mikroba
Yang Diisolasi Dari Jerami Padi Di Persawahan Pasang
Surut di Kalimantan Selatan’, J. Tanah Linkungan,
13(2), pp. 43–48.
Selig, M., Weiss, N. and Ji, Y. (2008) Enzymatic
Saccharification of Lignocellulosic Biomass
Laboratory Analytical Procedure. Colorado.
van Soest, P. J. (1963) ‘Use of Detergents in the Analysis
of Fibrous Feeds. II. A Rapid Method for the
Determination of Fiber and Lignin’, Journal of AOAC,
46, pp. 829–835.
Surendran, A. et al. (2018) ‘Inhibition and Kinetic Studies
of Cellulosa and Hemicellulose Degrading Enzymes of
Ganoderma Boniense by NaturallyOccurring Phenolic
Compounds’, Journal of Applied Microbiology, 124(6),
pp. 1544–1555. doi: 10.1111/jam.13717.
Suryanto, H. (2015) ‘Thermal degradation of mendong
fiber’, in The 6th Green Techonlogy. Malang.
Tasaso, P. (2015) ‘ptimiz tio of Re ctio o ditio s for Sy thesis
of rboxymethy e u ose from i P m Fro ds’, International
Journal of Chemical Engineering and Applications,
6(2), pp. 3–6. doi: 10.7763/IJCEA.2015.V6.460.
Widiarto, S. et al. (2017) ‘Preparation and Characterization
of Cellulose and Nanocellulose from Agro-industrial
Waste - Cassava Peel’, in IOP Conference Series:
Materials Science and engineering. IOP Publishing, p.
176. doi: 10.1088/1742-6596/755/1/011001.
Widiarto, S. et al. (2019) ‘Cellulose Nanofibers Preparation
from Cassava Peelsvia Mechanical Disruption’, Fibers,
7(44), pp. 1–11. doi: 10.3390/fib7050044.
Worthington (2020) Worthington Enzyme Manual
Cellulase, Worthington Biochemical Corporation.
Available at: http://www.worthington-biochem.com/
CEL/default.html.
van Zanten, G. C. et al. (2012) ‘The Effect of Selected
Synbiotics on Microbial Composition and Short-Chain
Fatty Acid Production in a Model System of the Human
Colon’, PloS ONE, 7(10). doi: 10.1371/journal.pone.0
047212.
Van Zanten, G. et al. (2014) ‘Synbiotic Lactobacillus
acidophilus NCFM and cellobiose does nt affect humat
gut bacterial diversity but increases abudance f
lactobacili, bifidobacteria and branched-chain fatty
acids: a randomizes, double-blinded cross-over trial.’
doi: 10.1111/1574-6941.12397.
Characterization of Insoluble Fiber in Cassava Peel and Its Hydrolyzate Potential as a Prebiotic for Lactobacillus Plantarum
37
