
Detection of Rat (Rattus Norvegicus) DNA Fragments using Specific:
Species Primer mt-DNA 12S rRNA and Cyt-b with Polymerase Chain
Reaction (PCR) Technique
Ersita Putri Aisah
1
and Joni Kusnadi
1,2
1
Department of Agricultural Product Technology, Faculty of Agricultural Technology,
University of Brawijawa Malang, Indonesia
2
Central Laboratory of Science, University of Brawijaya Malang, Jl. Veteran, Malang 65145, Indonesia
Keyword: 12S rRNA, Mitochondrial DNA, PCR, Primer.
Abstract: A pair of species-specific mt-DNA primer 12S rRNA has been designed based on rat (Rattus norvegicus)
DNA sequences. In this study, the specificity test of 12S rRNA and Cyt-b were carried out to detect rat DNA
fragments. Samples of this research were used non-halal animal meat consisted of rats, lamb, dog, pig and
halal animal meat consisted of cow, chicken, and horse. Furthermore, DNA was isolated from animal meat
using modification of chloroform isoamyl alcohol method then quantitatively tested for its concentration and
purity. Animal meat isolates were amplified using 12S rRNA and Cyt-b primers using PCR techniques.
Analysis of PCR results using agarose gel electrophoresis 1.5%. The amplification results showed that 12S
rRNA primer produced DNA bands of 228 bp length and Cyt-b primer produced DNA bands of 603 bp length.
The amplification results showed that both of 12S rRNA and Cyt-b primers were specific to detect rat DNA
fragments. Thus, both of primers are recommended to be further tested for sensitivity and applied to processed
meat products such as meatballs, sausages, and corned beef.
1 INTRODUCTION
Rats often cause health issues such as bubonic plague,
leptospirosis, murine typhus, and plague (Center for
Disease Control and Prevention, 2011). Ironically, rat
meat holds a high possibility to be used in various
food products, most of the time, meatballs. This
adulteration case rises among society in Indonesia as
it exhibits promising profit. Moreover, that such
adulteration could not be easily identified. The recent
case of meat adulteration were 63 tons. Those cases
were substituted beef into pig meat that claimed as
pure beef (Warta Ekonomi, 2012). This criminal case
complicate the Muslims society as they are prohibited
from consuming non-halal foods. The lack of food
management that related to halal, safety, and health
resulting the production process violations (Yasmin,
2013). The low awareness of the importance about
halal logo, halal certification at Slaughterhouses
(RPH) and Poultry Slaughterhouses (RPU), also the
low the protection from local governments leads the
increasing of new cases arise (Arifiani, 2019).
Indonesian Law no. 33 year 2014 regarding to Halal
Product Guarantee is regulated to eliminate the meat
adulteration cases.
The developing of an effective and efficient
method in meat contamination detection and
adulteration are essential in order to support the
guarantee of Halal. Polymerase Chain Reaction
(PCR) is a method of DNA analysis by amplifying
DNA in vitro involving several stages, it’s
denaturation, annealing, and extension (Handoyo et
al., 2000). Some constituent components including
DNA template (DNA template); primer;
deoxynucleoside triphosphates (dNTPs); DNA
polymerase enzyme; and PCR buffer, are required to
optimize the process. The primers were analyze as
primers are the success key in PCR technique. Mt-
DNA 12S rRNA gene contains high base variations
between species and low base variations in the same
species (Kitpipit, 2014). Several advantages of the
PCR technique are its specificity and high sensitivity,
short time, and its ability to detect contaminated
samples and to work on samples with a complex
mixture (Aminah et al., 2019). The PCR technique is
known as accurate, fast, affordable, and able to
20
Aisah, E. and Kusnadi, J.
Detection of Rat (Rattus Norvegicus) DNA Fragments using Specific: Species Primer mt-DNA 12S rRNA and Cyt-b with Polymerase Chain Reaction (PCR) Technique.
DOI: 10.5220/0010507100003108
In Proceedings of the 6th Food Ingredient Asia Conference (6th FiAC 2020) - Food Science, Nutrition and Health, pages 20-24
ISBN: 978-989-758-540-1
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

analyze a DNA (Deoxyribonucleic acid) sample from
high to low concentration (Arslan et al., 2006;
Aminah et al., 2019).
Specific primers of mt-DNA 12S ribosomal RNA
have been developed for detecting and identifying
DNA fragments of cats (Felis catus), dogs (Canis
familiaris), and rat (Rattus norvegicus) that contained
in food and food ingredients. The results showed that
3 pairs of primers that had been designed from the
DNA of 24 animals and plants produced specific
sequences with lengths of 108 bp, 101 bp, and 96 bp
were successfully amplified in cats, dogs, and rat
(Martin et al., 2014). The comparison primer was mt-
DNA Cyt-b which had been tested for it’s specificity
on detected DNA fragments of rat (Nuraini et al.,
2012). Cyt-b primers that designed manually, had
used to amplify 6 species which are goats, chickens,
cows, lamb, pigs, and horses successfully, while the
Cyt-b primers that designed using software based on
the Cyt-b sequence on Rattus norvegicus were
success to amplify 7 animal species which indicated
that the Cyt-b primer was specific. It is possible to
detect tissue samples of cats, dogs, rats on food and
foodstuffs in high sensitivity and specificity by using
specific primers (Martin et al., 2014). In this study, a
species-specific mt-DNA 12S rRNA primer was
designed and the specificity was analyzed in detecting
DNA fragments in rats as the initial stage of testing.
The comparison genes used mt-DNA Cyt-b primers
that had been studied previously for the detection of
rat DNA fragments. It is expected that further
research will be develop in concerning primer testing
based on the primer sensitivity and its applicate on
detecting rat DNA fragments in various meat
products such as meatballs, corned beef, and
sausages.
2 RESEARCH METHODS
The samples of non-halal raw meats were collected
from rats (positive control), dogs, and pigs while the
halal meat samples were obtained from cattle,
chicken, horse, and lamb (negative control). The
DNA isolation was performed by Chloroform
Isoamyl Alcohol method modified from Sambrook
and Russel’s (1989) procedure. The reagent used in
DNA isolation were consisted of STE lysis buffer (0.1
M NaCl, 1 mM EDTA, 10 mM Tris-HCl pH 8), 10%
SDS, and 10 mg / mL Proteinase-K. C: I (Chloroform:
Isoamyl) with a ratio of 24:1 was used in the
purification stage. Absolute ethanol (EtOH) was used
in the DNA washing stage, treated on cold condition.
In the DNA precipitation stage, 70% ethanol and 5M
NaCl were used. The final stage was DNA elution
that used TE buffer pH 7.6. The modified procedures
were used Pro-K instead of phenol in the DNA
purification stage.
The results of DNA isolation were analyzed
quantitatively using (Nanodrop / ND-1000 UV / Vis)
to obtain the concentration and purity, and were
amplified using Thermal Cycler (Applied Biosystem
/ PCR System 9700) and Thermocycler (Takara /
Version 3 Model TP600). The PCR process with a
total volume of 10µl consisted of 5 µl Go Taq Green
Master Mix (PROMEGA), a pair of 12S rRNA
primers with a concentration of 5 pmol / µl as much
as 0.5 µl, DNA template 1 µl, and ddH2O 3 µl. The
PCR program for primers 12S rRNA consisted of five
stages. Firstly, the initial denaturation at 95˚C for 5
minutes. Secondly, denaturation at 95˚C for 30
seconds. Thirdly, the 12S rRNA primers annealing at
54˚C for 45 seconds. Fourth, the extension at 72˚C for
30 seconds, and the last, final extension with a
temperature of 72˚C for 5 minutes. The same program
was applied for Cyt-b primers amplification,
excluding the annealing step 54˚C for 45 seconds.
12S rRNA gene primers were specifically
designed from mt-DNA rat (Rattus norvegicus) based
on the data from GenBank NCBI (National Center for
Biotechnology Information). The primers were
designed manually by bioinformatics programs such
as clustal X and bioedit to align the 12S rRNA gene
sequences from several test animals, such as cattle
(Bos indicus), dogs (Canis lupus familiaris), pigs (Sus
scrofa domesticus), chickens (Gallus gallus), horse
(Equus caballus), and lamb (Ovis aries). This study
was performed the 12S rRNA gene with a target
length of 228 bp employing a primer sequence length
of 20 bp, GC content (50%), melting temperature of
60°C, and annealing temperature of 54 °C (Figure 1).
Table 1: Primers 12S rRNA and Cyt-b genes.
Primer Sequence (5’ – 3’) Amplicon (bp) Base
12S rRNA
Forward: GGA CCT
AAG CCC AAT AAC
GA
228
20
Reverse: TTC TAC
CTT ACC CCT TCT
CG
20
Cyt-b
Forward: GAC CTC
CCA GCT CCA TCA
AAC ATC TCA TCT
TGA TGA AA
603
38
Reverse: GAA TGG
GAT TTT GTC TGC
GTT GGA GTT T
28
The comparison primers used forward SIM
primers which were designed based on the Cyt-b gene
Detection of Rat (Rattus Norvegicus) DNA Fragments using Specific: Species Primer mt-DNA 12S rRNA and Cyt-b with Polymerase Chain
Reaction (PCR) Technique
21
sequence from 6 animal species. Forward SIM
primers were selected according to sequence
missmatch within control species of 3-5 bases for 38
bp. SIM was designed longer than species-specific
primers with nucleotides measuring 26-29. DNA
fragments were formulated using software for
determining primers that were designed based on
species-specific areas. Reverse primers for detecting
the DNA fragments of rat were designed based on
primer design software
(http://www.ncbi.nlm.nih.gov/tools/primerblast/inde
xshtml). Reverse primers for rat DNA fragments
detection have a target sequence of 603 bp. (Nuraini
et al., 2012).
The amplification results were qualitative
analyzed using 1.5% agarose gel electrophoresis
(agarose, TBE buffer, EtBr, loading dye, and 1 kB
DNA ladders). The electrophoresis was performed
using Horizontal Electrophoresis (Mupid 2 Plus) with
a power of 50 V for 45 - 55 minutes and visualized
using Chemidoc Gel Imaging (Bio-Rad / BR-200).
Table 2: Concentration and Purity of Fresh Meat DNA
Isolate.
Sample Purity (λ 260/280) Concentration (ng/µl)
Rat 1.98 152.19
Mice 1.94 62.98
Chicken 1.72 90.20
Cattle 1.50 111.16
Horse 1.72 77.33
Lamb 1.96 392.78
Dog 1.84 50.31
Pig 1.96 60.30
3 RESULT AND DISCUSSION
Based on the results, DNA isolation performing
modified Chloroform Isoamyl Alcohol (PCI) method
on fresh meat samples from various non-halal animals
produced DNA with concentrations ranging from
50.30 ng / µl to 392.78 ng / µl with a purity of 1.50 to
1.98 (Table 2). The highest DNA concentration was
found in lamb meat while the lowest was in cattle.
The protein contaminants with purity values of 1.50,
1.72, and 1.72 were detected in most of the pure DNA
isolations, except in cattle, horse, and chicken. The
purity values with less than 1.8 indicate, there is a
presence of protein contamination, while the values
more than 2.0 indicate that there’s a presence of RNA
contamination (Nzilibili et al., 2018). The presence of
protein contamination in DNA isolation results
implies a shortage of effectiveness in using Pro-K to
denature all proteins in chicken, horse, and cattle
meat. In the future study, a modified with the addition
of Pro-K or phenol needs to be done in order to
maximize the protein denaturation on the purification
stage. The PCI method using proteinase on DNA
isolation was more effective comparing the used of
ammonium to remove the protein content
(Minematsu et al., 2004; Haunshi et al., 2008).
The DNA isolation was performed based on
comparing the PCI conventional method and
commercial KIT to produce a high-quality DNA
isolation. However, there are several inadequacy on
PCI method which required a longer time and toxic
reagents content: phenol, chloroform, SDS, etc.
Besides, the use of commercial KIT is relatively
expensive. Modification of the PCI method has been
used to produce good quality DNA isolates without
smear even though it was used only one purification
stage (PCI and incubation with minimal time)
(Haunshi et al., 2008).
The specificity test was used the DNA isolates
from several animals and amplify used PCR
technique. The amplification results that used 12S
rRNA primers showed DNA bands in rat meat
samples with sizes ranging from 228 bp.
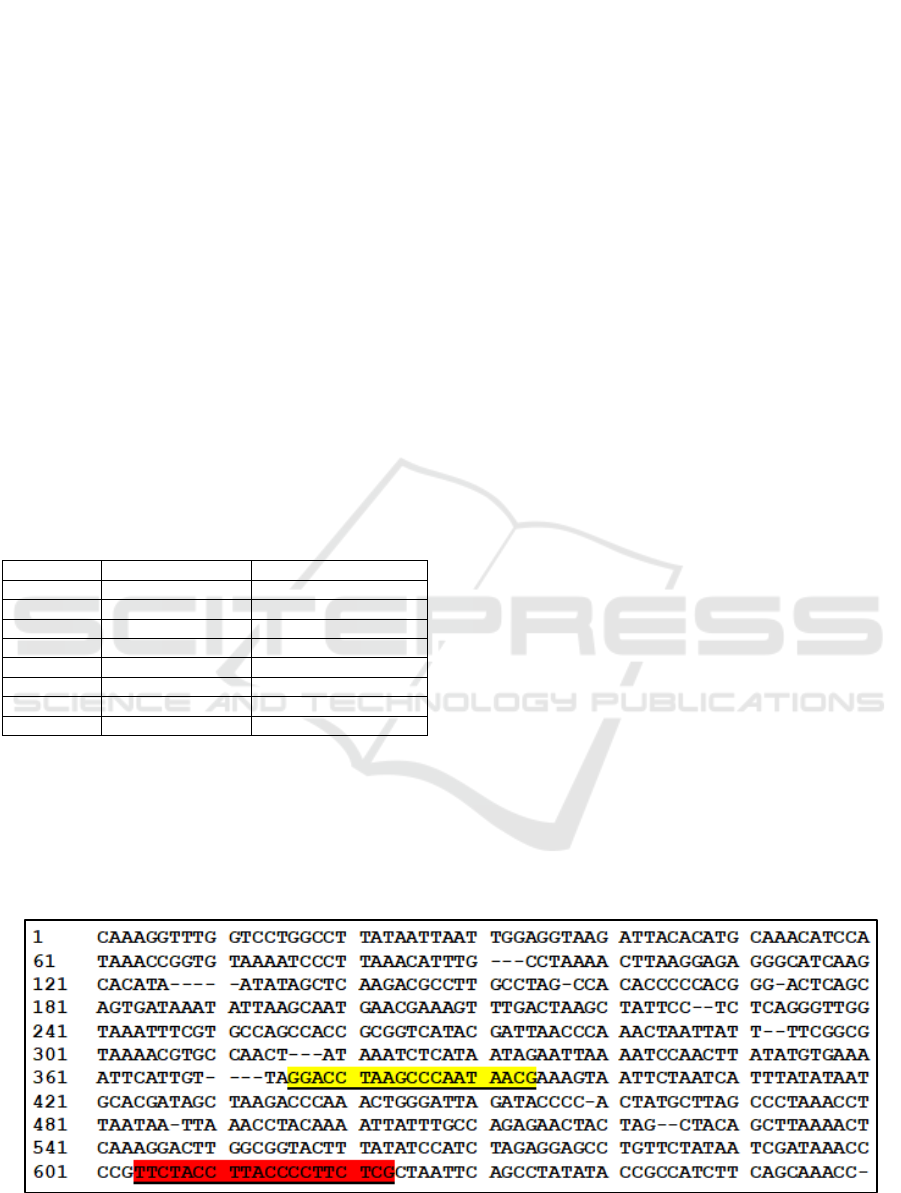
Figure 1: The site of the design of the primer attached to the 12S rRNA sequence Rattus norvegicus, note: yellow is forward
primer and red is reverse primer.
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
22

The DNA band was appeared in rat samples which
indicated the primers are specific to amplify the 12
rRNA gene sequence on rat (Figure 1). While, the
amplification result used Cyt-b primers as the
comparison gene was formed DNA bands with 603
bp in the rat meat sample. This result was verified that
both 12s rRNA and Cyt-b primers are specific to
amplify rat DNA sequences (Figure 2). Cyt-b primers
are often used to compare the phylogenetics of
species in the same genus or the same family. Several
studies of the genetic diversity of the Cyt-b gene have
been studied in cattle (Bos taurus), lamb (Ovis aries),
and goats (Capra hircus), roebuck (Capreolus
capreolus), and red deer (Cervus elaphus) (Wolf et
al., 1999; Nuraini et al., 2012). Rats (Rattus
norvegicus) have a long Cyt-b sequence of 1143 bp
(Nuraini et al., 2012).
Figure 2: Result of 12S rRNA Primer. Note: E1: (-), E2:
Rat, E3: Mice, E4: Chichken, E5: Cattle, E6: Horses, E7:
Lamb, E8: Dog, E9: Pig, M: DNA Marker 100 bp.
The success amplifying uses PCR technique was
demonstrated by the 12S rRNA and Cyt-b primers
tested on various animal meat isolates producing
target DNA fragments with different lengths
according to the length of the targeted DNA. Cyt-b
primer has been used previously to detect rat DNA
fragments resulting in 603 bp of target DNA (Nuraini
et al., 2012) which conforms to the results obtained in
this study.
The result of the 12S rRNA primer specificity test
showed that the amplified band was formed in the
meat sample of rats with a length of 228 bp. It shows
that the mitochondrial DNA of the 12S rRNA gene
can be used as a marker or primer for specific species
to identify species as it has a large variation among
the species (Springer and Douzery, 1996), and 12S
rRNA is mostly used in intra and inter-species
phylogenetic studies (Tougard et al., 2001). In the
other hand, the examined of specificity of Cyt-b
primer were represent the quite clear bands with 603
bp of rat meat sample. The Cyt-B primer used in this
study had quite specific properties (Nuraini, 2012).
The Cyt-b primer was further examined on various
samples contained some mixtures beef and pork or
pork and rats with varying concentrations (1%, 5%,
10%, 15%, 20%, and 25%). As a result, the specificity
of the Cyt-b primer was shown by a specific primer
detecting samples of rat DNA fragments contained in
a mixture of beef and rat. Meatballs which are
composed of 15% rat meat would be more clearly
detected compared to 1% that could not be detected
due to the very small value (Nuraini et al., 2012). The
succession of primers to amplify the DNA target was
supported by the primers sequence contain of
nucleotide bases that might specifically hybridized to
DNA template (Yuwono, 2006) and a nucleotides
sequence originating from the DNA target. A good
primer was consisted of nucleotide bases that
conserved on the template, thus not exist in any other
location on its template (Pelt-verkuil et al., 2008). The
succession of specifically designed of 12S rRNA
primers are need further analyzed to detect the
sensitivity on rat DNA fragments (Rattus
norvegicus). In addition, the 12S rRNA primer needs
to be applied in detecting rat DNA fragments in
various processed food products. Thus, conventional
PCR techniques using species-specific primers are
qualifiable to detect DNA fragments of non-halal
animals (Rattus norvegicus) with specifically,
rapidly, and conveniently.
4 CONCLUSION
Based on the results in this study, the 12S rRNA
primer specificity test on rat meat samples produced
a sequence with 228 bp. Therefore, both 12S rRNA
and Cyt-b are specific primers which able detect rat
DNA fragments (Rattus norvegicus). Nevertheless,
there is still need to undertake further examine on
specific 12S rRNA regarding to the sensitivity of the
primers in detecting rat DNA fragments to obtain the
LOD (limit of detection) sample concentration and its
application in various meat products.
Figure 3: Result of Cyt-b primer amplification. Note: G1: (-
), G2: Rat, G3: Mice, G4: Chicken, G5: Cattle, G6: Horses,
G7: Lamb, G8: Dog, G9: Pig, M: DNA Marker 100 bp.
Detection of Rat (Rattus Norvegicus) DNA Fragments using Specific: Species Primer mt-DNA 12S rRNA and Cyt-b with Polymerase Chain
Reaction (PCR) Technique
23

ACKNOWLEDGEMENTS
The author would like to thank Brawijaya University
for funding this research through the 2020 Doctoral
Grants Program. I would also like to express my
gratitude to the LSIH Laboratory for facilitating
various equipment to support the continuity of the
research.
REFERENCES
Arslan, A., Ilhak, O. I., & Calicioglu, M, 2006. Effect of
method of cooking on identification of heat processed
beef using polymerase chain reaction (PCR) technique.
Meat science, 72(2), 326-330.
Center for Disease Control and Prevention, 2011. Diseases
Directly Transmitted by Rodents, http://www.cdc.gov/
rodents/diseases/direct.html, 05 Juli 2019.
Handoyo D, & Rudiretna A, 2000. Prinsip Umum dan
Pelaksanaan PCR [general principles and
implementation of PCR]. Unitas 9(1), 17-29
Kitpipit T, Sittichan K, & Thanakiatkrai, P, 2014. Direct-
multiplex PCR assay for meat species identification in
food products. Food Chemistry, 163, 77-82. DOI:
10.1016/j.foodchem.2014.04.062
Nuraini H, Primasari A, Andreas E, & Sumantri C, 2012.
The use of cytochrome b gene as a specific marker of
the rat meat (Rattus norvegicus) on meat and meat
products. Media Peternakan, 35(1), 15.
Nzilibili SMM, Ekodiyanto MKH, Hardjanto P, &
Yudianto A, 2018. Concentration and Purity DNA
Spectrophotometer: Sodium Monofluorophosphate
forensic impended effect. Journal of Forensic Sciences
8-34. DOI: 10.1186/s41935-018-0065-7
Pelt-Verkuil EV, Belkum AV, & Hays JP, 2008. Principals
and Technical Aspects of PCR Amplification. Springer,
Netherland. 30 p.
Sambrook, J.E.F., Fritsch, T., dan Miniatis, 1989.
Molecular Cloning : A LaboratoryManual. Cold Spring
Harbor Laboratory Press. New York
Springer MS, & Douzery E, 1996. Secondary Structure and
Patterns of Evolution among Mammalian
Mitochondrial 12S rRNA Molecules. Journal Mol. E
vol. 43:357-373. DOI: 10.1007/BF02339010
Tougard C, Delefosse T, Hänni C & Montgelard C, 2001.
Phylogenetic Relationships of the Five Extant
Rhinoceros Species (Rhinocerotidae, Perissodactyla)
Based on Mitochondrial Cytochrome b and 12S rRNA
Genes. Mol. Phylogenet. E vol. 19:34-44.
https://doi.org/10.1006/mpev.2000.0903
Yasmin A, 2013. Bakso Tikus #Jurnalistik Investigasi.
http://yasminainun.blogspot.com/2013/11/bakso-tikus-
jurnalistik-investigasi.html. 05 Juli 2019
Yuwono T, 2006. Teori dan Aplikasi Polymerase Chain
Reaction. Penerbit Andi, Yogyakarta. 246 hlm.
Warta Ekonomi, 2012. Penjualan Daging Babi Bak Daging
Sapi Terjadi Lagi, MUI Minta Tindakan Tegas Dari
Pemerintah. https://www.wartaekonomi.co.id/read285
313/penjualan-daging-babi-bak-daging-sapi-terjadi-lag
i-mui-minta-tindakan-tegas-pemerintah. Diakses pada
Agustus 2020.
Aminah, A., Ramadini, R., & Naid, T, 2019. Analisis
Cemaran DNA Tikus pada Bakso Daging Sapi yang
Beredar di Makassar dengan Metode Polymerase Chain
Reaction (PCR). Jurnal Farmasi Galenika (Galenika
Journal of Pharmacy)(e-Journal), 5(1), 93-100.
Martín, I., García, T., Fajardo, V., Rojas, M., Hernández, P.
E., González, I., & Martín, R, 2007. Detection of cat,
dog, and rat or mouse tissues in food and animal feed
using species-specific polymerase chain reaction.
Journal of animal science, 85(10), 2734-2739.
Arifiani, S, 2019. Mulai Oktober 2019, Semua Produk
Wajib Bersertifikat Halal. https://www.solopos.com/
mulai-oktober-2019-semua-produk-wajib-bersertifikat
-halal-983941. Diakses pada Agustus 2020.
NCBI, 2000. Rattus norvegicus Mitochondrial Gene for
Cytochrome b, Partial cds. http://www.ncbi.nlm.ni.gov.
Wolf, C., Rentsch, J., & Hübner, P, 1999. PCR− RFLP
analysis of mitochondrial DNA: A reliable method for
species identification. Journal of agricultural and food
chemistry, 47(4), 1350-1355.
Haunshi, S., Pattanayak, A., Bandyopadhaya, S., Saxena, S.
C., & Bujarbaruah, K. M, 2008. A simple and quick
DNA extraction procedure for rapid diagnosis of sex of
chicken and chicken embryos. The Journal of Poultry
Science, 45(1), 75-81.
Minematsu, T, Sugiayama, M., Tohama, Y., Tajima, A. &
Kanai, Y, 2004. Simplifed DNA Extraction Methods
for sexing chicken embryos. Journal of Poultry Sience,
41:147-154.
6th FiAC 2020 - The Food Ingredient Asia Conference (FiAC)
24
