
Oral Delivery of ACE2 Bioencapsulated in Plant Cells as Potential
Adjuvant Therapy to Reduce the COVID-19 Disease Severity
Eka B. Layadi
1a
, Robert Sinto
1b
, Friska W. Wijaya
1c
and Oemar Ichsan
1d
1
Division of Tropical Disease and Infection, Department of Internal Medicine, Faculty of Medicine, Universitas Indonesia,
Dr. Cipto Mangunkusumo National General Hospital, Jakarta, Indonesia
Keywords: SARS-CoV-2, COVID-19, ACE2, Bioencapsulated plant cell, Novel therapy
Abstract: COVID-19 has become a widespread pandemic and a devastating public health emergency. Numerous trials
have been conducted to search beneficial therapeutical interventions. ACE2 plays an essential role in the
pathogenesis of SARS-CoV-2 infection as this receptor become the entry point of virus to the cell. The
blockage of the ACE2 receptor and the delivery of ACE2 in the soluble form are some mechanisms that have
been proposed for potential therapy of COVID-19. Parenteral administration of ACE2 in soluble form has
been conducted in trials using the hrACE2 (human recombinant ACE2) and showed a favorable result.
However, the possibility of administering ACE2 through an oral route has not been extensively explored.
Bioencapsulated plant cell technique has shown resistant to digestive enzymes and gastric acid and able to
carry ACE2 to be absorbed safely into the circulation. Previous study showed promising utilization of
ACE2/Ang1-7 Bioencapsulated in Plant Cells to treat ocular inflammatory disorders in mice. Although no
clinical studies have been done yet, similar concept can be theoretically applied to hinder the development of
SARS-CoV-2 severe manifestation. The increasing soluble ACE2 may reduce the circulatory levels of
detrimental Angiotensin II effects as well as acting as a decoy to bind free virions from attaching to the target
cells.
1 INTRODUCTION
COVID-19 has become a global public health
emergency with the increasing emergence of new
cases daily in countries worldwide. There is an urgent
need for therapeutics now, more than ever, to control
SARS-CoV-2 infection (Vellingiri et al., 2020). The
biomedical community has made a massive effort to
find potential drugs by conducting many trials in
search of an effective cure for COVID-19. (The
Lancet Infectious Diseases, 2020).
ACE2 activator,
especially in the soluble form, is deemed one of the
plausible therapeutic strategies to control SARS-
CoV-2 infection, for this receptor acts as a gateway
of SARS-CoV-2 infection and the foundation of the
pathogenesis of COVID-19. This soluble form of
ACE2 can act as a competitive interceptor of SARS-
CoV-2 by binding the virus particles and limiting the
virus's attachment to the host's cell membranes
(Battle et al., 2020; Rodríguez-Puertas, 2020).
Parenteral administration of human recombinant
soluble ACE2 (hrsACE2), has been tested in patients
and has passed phase 2 clinical studies with a great
safety profile. A recent study by Zoufaly et al. (2020)
has shown that the administration of parenteral
hrsACE2 to a patient with severe COVID-19 resulted
in marked reductions in SARS-CoV-2 viral load,
serum levels of inflammatory cytokine, and serum
levels of Angiotensin II of the recipient (Abd El-Aziz
et al. 2020).
The route of administration of soluble
ACE2 through oral route has not been explored
extensively, despite successful trials of oral ACE2
bioencapsulated plant cell in animal studies with
favorable results (Shil et al., 2014). In this study, we
explore the possibility and feasibility of oral
bioencapsulated ACE2 in plant cells as a potential
adjuvant therapy to ameliorate COVID-19
manifestations in humans and how this drug would
potentially revolutionize the pharmaceutical industry,
____________________________________________________
a
https://orcid.org/0000-0001-5120-5848
b
https://orcid.org/0000-0003-3857-300X
c
https://orcid.org/0000-0001-6070-9079
d
https://orcid.org/0000-0002-8178-9335
330
Layadi, E., Sinto, R., Wijaya, F. and Ichsan, O.
Oral Delivery of ACE2 Bioencapsulated in Plant Cells as Potential Adjuvant Therapy to Reduce the COVID-19 Disease Severity.
DOI: 10.5220/0010492103300334
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 330-334
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

especially in developing countries all over the globe,
through its advantageous cost-efficiency.
2 BACKGROUND
2.1 SARS-CoV-2 Infection and ACE2
Molecular Pathogenesis
SARS-CoV-2 are single-stranded RNA viruses and
contain two groups of proteins, namely structural
protein such as spike (S) proteins that bind to the
receptors on the host cell, nucleocapsid (N) that
protects the genetic information of virus, matrix (M)
and envelope (E), and non-structural proteins such as
proteases (nsp3 and nsp5) and RdRp (nsp12)
(Chatterjee et al., 2020). SARS-CoV depends upon
ACE2 receptors expressed in human epithelial cells,
endothelial cells, and most abundantly in the lung
parenchyma. This receptor recognition is one of the
significant steps in viral infection of host cells and the
prelude of its pathogenesis (Chatterjee et al., 2020).
The S protein in the SARS-CoV-2 membrane
promotes virus entry into the host cell through ACE2
receptors widely spread among many cells in the
various organ system. Despite ACE2's existence in
various cells, 83% of total ACE2 in humans is
expressed in alveolar epithelium type 2, making this
virus's prominent tropism in the respiratory system.
Typically found in membrane-bound form, ACE2
comes in a smaller fraction in the soluble form
(Verdecchia et al., 2020). SARS-CoV-2 entry to the
cell will cause a depletion of ACE2 expression.
ACE2, which functions in cleaving AngII
(Angiotensin II) into Ang1-7 (Angiotensin 1-7), helps
regulate the balance of the two. An increase of AngII
due to deficiency of ACE2 will promote pro-
inflammatory, pro-oxidative, pro-fibrotic processes,
and vasoconstriction, which cumulatively contribute
to the deterioration of COVID-19 manifestations
(Zhang et al., 2020).
AngII plays a vital role in signaling cellular and
molecular events critical in the pathogenesis of
pulmonary fibrosis. The first mechanism is to
promote pro-inflammatory cytokines such as IL-6
and IL-8 by macrophages; the second is by producing
reactive oxygen species (ROS) among infected
epithelial cells and followed by its apoptosis and
lastly by proliferation, migration, and differentiation
of fibroblast to myofibroblast. Thus, the higher serum
AngII among patients with COVID-19 pneumonia,
the higher the risk of developing respiratory failure
and other adverse events (Delpino & Quarleri, 2020).
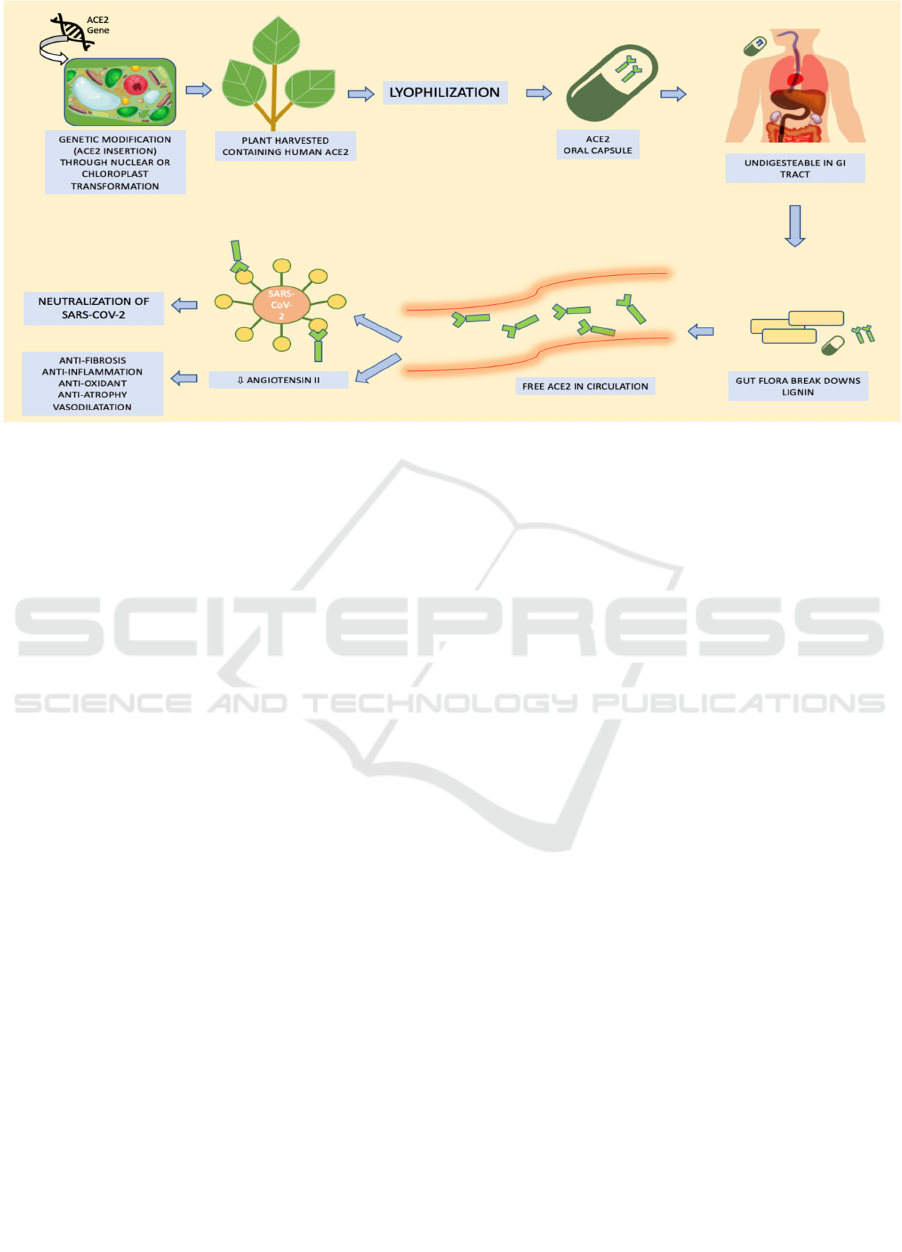
2.2 Bioencapsulated Plant Cell to
Deliver Protein-based Drug
Incorporating protein-based drugs into plant cells
has become a cutting-edge method called
bioencapsulated plant cells through complex
biotechnological engineering. There are two methods
to make the desired protein expressed in the plant cell,
through nuclear manipulation or chloroplast
manipulation. The chloroplast manipulation is
considered more superior to nuclear manipulation to
increase the levels of transgene expression. Each
plant contains about 10.000 copies of the chloroplast
genomes, and collectively they expressed up to 70%
of total leaf protein. A plethora selection of proteins,
ranging from minuscule antimicrobial peptides or
hormones to large-sized proteins encoded by
bacterial, viral, fungal, and human genes, have been
successfully expressed in chloroplasts (Kwon &
Daniell, 2015).
The genes that optimized the desired protein
expression are usually fused with CTB (cholera toxin
type B) to facilitate transepithelial transport in the gut.
After the fusion, the combined material will be cloned
into the chloroplast transformation vectors. Shoots
emerging with the modified chloroplast are further
investigated by using PCR to confirm the site-specific
integration of the chloroplast genome. After the
confirmation, the plants will be transferred to the
greenhouse for propagation and mass production. The
leaves of the plant that contain the desired protein will
go through a process called lyophilization.
Lyophilized plant cells are stable at a certain range of
temperature for many years, can further withstand the
digestive enzymes and denaturation from gastric acid,
maintaining the protein drug's structure and functions
(Park et al., 2020).
Plant cells' walls are composed of sturdy lignin
and cellulose, which cannot be broken down by
digestive enzymes. Combined with the effects of
lyophilization, layers of protection screen protect the
desired protein-based drug. After the bioencapsulated
drug arrives in the intestinal lumen, the intestinal
bacteria, especially from the Bacteroides spp. and
Firmicutes species, will break down lignin and
cellulose from the plant's cell wall cell, releasing the
drug into the intestinal lumen. In this step, the CTB
helps in absorption of the drug by translocating the
drug through the gut epithelium. After the absorption,
the drug will be released into the circulation (Kwon
& Daniell, 2016).
The drug administration technique through
bioencapsulated drug in plant cells is considered cost-
efficient because it does not need complex cold-chain
Oral Delivery of ACE2 Bioencapsulated in Plant Cells as Potential Adjuvant Therapy to Reduce the COVID-19 Disease Severity
331
storage and the drug contained within the plant cells
through lyophilization can still be viable for years.
Clinical advancement of this concept would
revolutionize protein drug production and delivery
for many metabolic and genetic disorders (Hu et al.,
2020).
2.3 The Potential Capacity of
Bioencapsulated ACE-2 As
COVID-19 Adjuvant Therapy
This Bioencapsulated technique has been used to
treat various diseases from Gaucher's disease,
diabetes mellitus, hypertension to Alzheimer's
disease. In Gaucher's disease, there is a trial using
carrot cells expressing human glucocerebrosidase
administered orally to rats, although it was found that
the concentration of glucocerebrosidase post-
intervention in the serum was 10-fold lower than the
control group with the IV formulation. In diabetes
mellitus, design for ideal oral insulin through
bioencapsulated plant cell is still in the path of
development (Kwon & Daniell, 2016). There is also
a study by Park et al. (2020) using IGF-1
bioencapsulated in lettuce cells to promote fracture
healing that showed promising results. There is still
an endless potential of this novel drug delivery
system that needs to be explored, and COVID-19 can
be one of them.
SARS-CoV-2 enters the cell through the ACE2
receptor facilitated by the S-protein spike of the virus
(Li et al. 2020). ACE2 is mostly bound to cell
membranes and only scarcely present in the
circulation in a soluble form (Verdecchia et al., 2020).
The entry of the virus is followed by downregulation
of ACE2 (Gheblawi et al., 2020). The
downregulation of ACE2 causes the levels of Ang II
to rise. The increased levels of AngII create a
detrimental chain of events that support
vasoconstriction, pro-inflammatory, pro-oxidative,
and pro-fibrotic conditions, leading to acute lung
injury in COVID-19 patients. (Lugito et al., 2020;
Bourgonje et al., 2019; Kuba et al., 2005). It explains
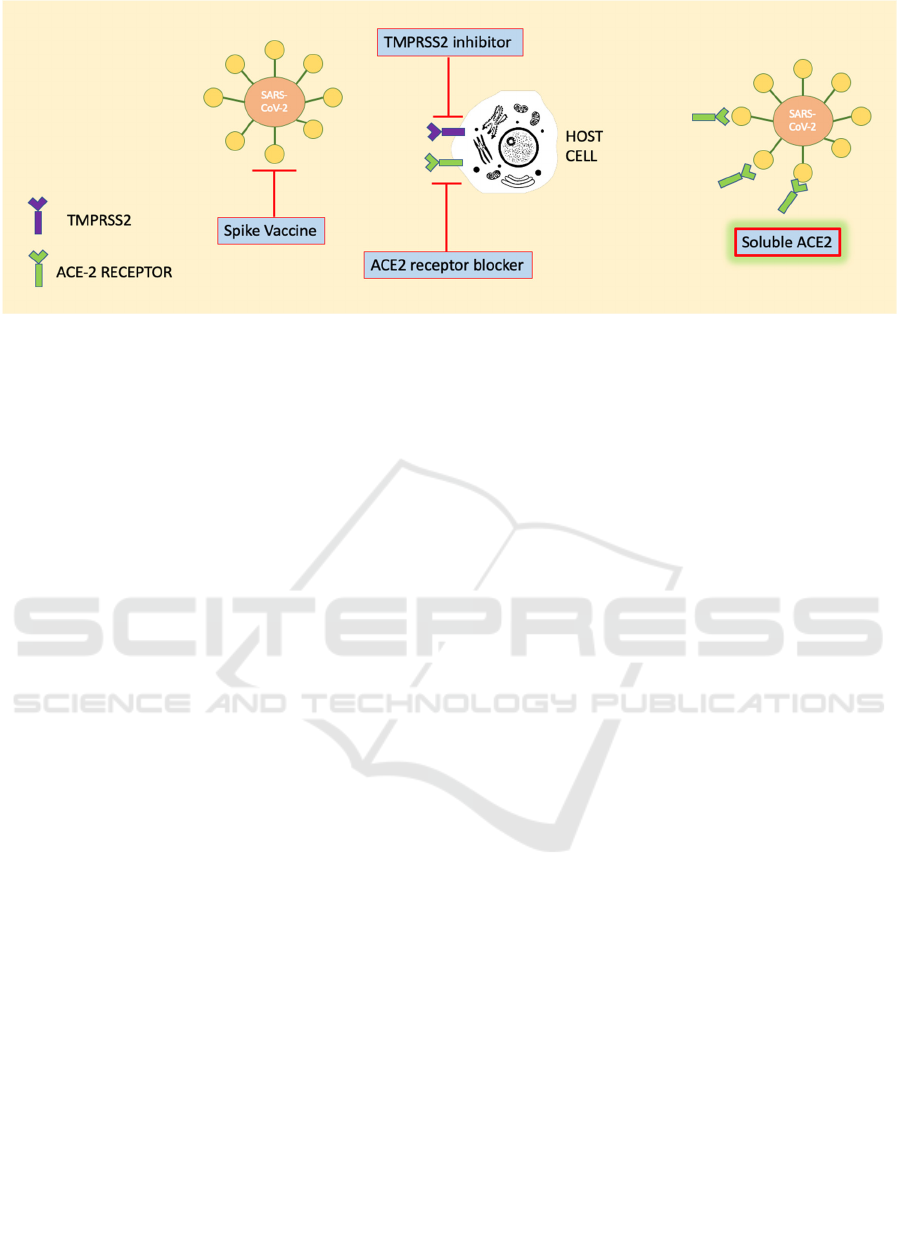
current potential therapeutic strategies to manage
SARS-CoV-2 infection, which are portrayed in figure
1, could be achieved by making spike protein-based
vaccine, inhibition of transmembrane protease serine
2 (TMPRSS2) activity, blocking ACE2 receptor, and
delivering an excessive soluble form of ACE2 (Zhang
et al., 2020).
There have been numerous trials concerning the
management of COVID-19 through the involvement
of the ACE2 receptor. One of them is a trial using
rhACE2 (recombinant human ACE2), which was
administered parenterally. This drug has completed
clinical trials and efficiently lowered plasma
angiotensin II and increased angiotensin 1-7 levels,
respectively (Gheblawi et al., 2020; Zoufaly et al.
2020). There has not been any study or trials
mentioning a possible ACE2 administration through
the oral route, but a proposed model uses
bioengineered probiotic, Lactobacillus paracasei,
that secretes soluble ACE2 to help ameliorate
COVID-19 manifestations (Senapati et al., 2020).
The administration through oral route with
bioencapsulated plant cells has never been
highlighted as a proposed COVID-19 therapy model.
A study conducted by Shil et al. (2014) used
ACE2/Ang-(1–7) Bioencapsulated in Plant Cells,
administered orally, as a cost-effective therapeutic
strategy for ocular inflammatory diseases in mice.
They succeeded in creating ACE-2 fused with CTB
in lyophilized bioencapsulated plant cell. The ACE2
activity assay through ELISA (enzyme-linked
immunosorbent assay), using protein extracts isolated
from plant leaves showed that the plant cells
successfully expressed human ACE2 which is
Figure 1: ACE2 as SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic targets.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
332
enzymatically active, and they found that the ACE2
protein can be detected in both serum and retina of the
mice subjects 5 hours after oral gavage (Shil et al.,
2014). There was also a similar study conducted by
Shenoy et al. (2014) using oral ACE2/Ang-(1–7)
Bioencapsulated in Plant Cells that successfully
prevented the progression of monocrotaline-induced
pulmonary hypertension in rats.
Despite the successful creation of ACE2 drug
bioencapsulated in plant cell for animal studies and
how the bioencapsulated protein drug technique has
been successfully developed to treat some human
diseases, ACE2 drug bioencapsulated in plant cell has
never been tested to human subjects. This new drug
administration, theoretically speaking, could be one
of the therapeutical strategies to deliver a soluble
form of ACE2. This ACE2 bioencapsulated plant cell
will be released into the circulation right after the
cellulose on the plant carrier's outer cell wall is
digested by intestinal bacteria. The fusion of ACE-2
with CTB helps to translocate ACE2 into the gut
epithelium cells. The ACE2 drug that circulates in
plasma can act as a decoy for SARS-CoV-2 binding
so that some active viruses might not attach to the
ACE2 receptor in various cells in the body. If SARS-
CoV-2 already infects the patient, this drug's
administration can ameliorate the severity of the
infection. Soluble ACE2 has protective effects and
can cleave AngII (which brings pro-inflammatory,
pro-oxidant, and pro-fibrosis) into Ang1-7 (which has
beneficial effects). Thus, through these chains of
events, ACE2 that is administered bioencapsulated in
plant cells would be able to decrease the severity of
COVID-19 infection and lower the incidence of
cytokine storm by regulating the RAS system and
keeping the AngII and Ang1-7 in the right balance.
3 CONCLUSIONS
Bioencapsulated ACE2 in plant cells could be a
therapeutical strategy to deliver the soluble form of
ACE2 in COVID-19 patients. By acting as a
competitive interceptor that limits the attachment of
SARS-CoV-2 to membrane cells, this soluble ACE2
could prevent SARS-CoV-2 entry and replication in
the target cells. Aside from this effect, ACE2 also acts
by cleaving AngII, which exerts detrimental
properties that aggravate the severity of COVID-19
manifestations. In addition to those benefits,
bioencapsulated drug in plant cells is considered cost-
efficient because it does not need complicated cold-
chain storage, and the ACE2 contained within the
plant cells through lyophilization could still be viable
for years. Advancement of this bioencapsulated
protein drugs in plant cell technique could be just
what Indonesia, or other developing countries, need
as a potential cost-efficient strategy to ameliorate
SARS-CoV-2 infection.
REFERENCES
Vellingiri, B., Jayaramayya, K., Iyer, M., Narayanasamy,
A., Govindasamy, V., Giridharan, B., et al. (2020).
COVID-19: A promising cure for the global panic. Sci
Figure 2: The process of making bioencapsulated ACE2 and its desired effects on SARS-CoV2.
Oral Delivery of ACE2 Bioencapsulated in Plant Cells as Potential Adjuvant Therapy to Reduce the COVID-19 Disease Severity
333

Total Environ, 725;138277. doi:
10.1016/j.scitotenv.2020.138277.
The Lancet Infectious Diseases (2020). Curing COVID-19.
Lancet Infect Dis, 20(10):1101.
https://doi.org/10.1016/S1473-3099(20)30706-4
Batlle, D., Wysocki, J., Satchell, K. (2020). Soluble
angiotensin-converting enzyme 2: A potential approach
for coronavirus infection therapy? Clin Sci,134(5):543–
545.
Rodríguez-Puertas, R (2020). ACE2 activators for the
treatment of COVID 19 patients. J Med Virol,
92(10):1701-1702. https://doi.org/10.1002/jmv.25992
Zoufaly, A., Poglitsch, M., Aberle, J.H., Hoepler, W., Seitz
T., Traugott M., et al (2020). Human recombinant
soluble ACE2 in severe COVID-19. Lancet Respir
Med, 8(11):1554-1558. doi: 10.1016/S2213-
2600(20)30418-5
Abd El-Aziz, T.M., Al-Sabi, A., Stockand, J.D. (2020).
Human recombinant soluble ACE2 (hrsACE2) shows
promise for treating severe COVID-19. Signal
Transduct Target Ther,
5(1).https://doi.org/10/1038/s41392-020-00374-6
Shil P.K., Kwon K.C., Zhu P., Verma A., Daniell H., Li Q.
(2014). Oral delivery of ACE2/Ang-(1-7)
bioencapsulated in plant cells protects against
experimental uveitis and autoimmune uveoretinitis.
Mol Ther, 22(12):2069–2082. doi:
10.1038/mt.2014.179.
Chatterjee S.K., Saha S., Munoz M.N.M. (2020). Molecular
pathogenesis, immunopathogenesis and novel
therapeutic strategy against COVID-19. Front Mol
Biosci, 7:196. doi: 10.3389/fmolb.2020.00196
Verdecchia P., Cavallini C., Spanevello A., Angeli F.
(2020). The pivotal link between ACE2 deficiency and
SARS-CoV-2 infection. Eur J Intern Med, 76:14-20.
doi: 10.1016/j.ejim.2020.04.037
Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S.
(2020). Angiotensin-converting enzyme 2 (ACE2) as a
SARS-CoV-2 receptor: molecular mechanisms and
potential therapeutic target. Intensive Care Med,
46(4):586–590. https://doi.org/10.1007/s00134-020-
05985-9
Delpino M.V., Quarleri J. (2020). SARS-CoV-2
Pathogenesis: Imbalance in the Renin-Angiotensin
System Favors Lung Fibrosis. Front Cell Infect
Microbiol, 10:340. doi: 10.3389/fcimb.2020.00340
Kwon K. C., Daniell H. (2015). Low-cost oral delivery of
protein drugs bioencapsulated in plant cells. Plant
Biotechnol J, 13(8):1017–
1022.http://doi.wiley.com/10.1111/pbi.12462
Park J., Yan G., Kwon K.C., Liu M., Gonnella P.A., Yang
S., et al. (2020). Oral delivery of novel human IGF-1
bioencapsulated in lettuce cells promotes
musculoskeletal cell proliferation, differentiation and
diabetic fracture healing. Biomaterials, 1;233:119591.
https://doi.org/10.1016/j.biomaterials.2019.119591
Kwon K.C., Daniell H. (2016). Oral delivery of protein
drugs bioencapsulated in plant cells. Mol Ther.
24(8):1342–1350. doi: 10.1038/mt.2016.115
Hu X., Yang G., Chen S., Luo S., Zhang J. (2020).
Biomimetic and bioinspired strategies for oral drug
delivery. Biomaterials Science, 8(4):1020–1044.
Available from:
https://pubs.rsc.org/en/content/articlehtml/2020/bm/c9
bm01378d
Li X., Geng M., Peng Y., Meng L., Lu S. (2020). Molecular
immune pathogenesis and diagnosis of COVID-19.
Journal of Pharmaceutical Analysis, 10(2):102-108.
https://doi.org/10.1016/j.jpha.2020.03.001
Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong
J.C., Turner A.J., et al. (2020). Angiotensin-Converting
Enzyme 2: SARS-CoV-2 Receptor and Regulator of the
Renin-Angiotensin System: Celebrating the 20th
Anniversary of the Discovery of ACE2. Circ Res,
126(10):1456-1474. doi:
10.1161/CIRCRESAHA.120.317015
Lugito N.H., Kurniawan A., Damay V., Chyntya H.,
Sugianto N. (2020). The role of gut microbiota in
SARS-CoV-2 infection: Focus on angiotensin-
converting enzyme 2. Curr Med Issues, 18(3):261.
Available from:
http://www.cmijournal.org/text.asp?2020/18/3/261/28
9426
Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.,
Navis G.J., Gordijn S.J., et al. (2020). Angiotensin‐
converting enzyme 2 (ACE2), SARS‐CoV‐2 and the
pathophysiology of coronavirus disease 2019 (COVID‐
19). J Pathol, 251(3):228–48.
https://doi.org/10.1002/path.5471
Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al.
(2005). A crucial role of angiotensin converting
enzyme 2 (ACE2) in SARS coronavirus–induced lung
injury. Nat Med, 11(8):875–879. doi: 10.1038/nm1267
Senapati S., Dash J., Sethi M., Chakraborty S. (2020).
Bioengineered probiotics to control SARS-CoV-2
infection. Res Ideas Outcomes,
6:54802.https://doi.org/10.3897/rio.6.e54802
Shenoy V., Kwon K.C., Rathinasabapathy A., Lin S., Jin
G., Song C., et al (2014). Oral delivery of angiotensin-
converting enzyme 2 and angiotensin-(1-7)
bioencapsulated in plant cells attenuates pulmonary
hypertension. Hypertension, 64(6):1248-1259. doi:
10.1161/HYPERTENSIONAHA.114.03871.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
334
