
IL-6 and IL-8 Suppression by Bacteria-adhered Mesenchymal Stem
Cells Co-cultured with PBMCs under TNF-α Exposure
Agung Putra
1,2,3
a
, Iffan Alif
1
b
, Mohammad Ariq Nazar
1
c
, Ardi Prasetio
1
d
Risky Chandra
Satria Irawan
1
e
, Dina Amalina
1,4
f
, Endah Permata Sari
5
g
, Azizah Retno Kustiyah
6
h
and Iqbal Pahlevi Adeputra Nasution
7
i
1
Stem Cell and Cancer Research, Medical Faculty, Universitas Islam Sultan Agung, Semarang, Indonesia
2
Department of Pathological Anatomy, Medical Faculty, Universitas Islam Sultan Agung, Semarang, Indonesia
3
Department of Postgraduate Biomedical Science, Medical Faculty, Universitas Islam Sultan Agung, Semarang, Indonesia
4
Pharmacy Study Program, Chemistry Department, Faculty of Mathematics and Natural Sciences, Semarang State
University, Semarang, Indonesia
5
Student of Medical Faculty, Universitas Islam Sultan Agung, Semarang, Indonesia
6
Department of Pediatrics, Medical Faculty, Universitas Islam Sultan Agung, Semarang, Indonesia
7
Department of Surgery, Medical Faculty, Universitas Sumatera Utara, Medan, Indonesia
Keywords: IL-6, IL-8, MSCs type-1, MSCs type-2 PBMCs, S. aureus.
Abstract: The potential of mesenchymal stem cells (MSCs) in controlling bacterial infections is an evolving field to
investigate. In terms of response to inflammatory cytokines, MSCs can polarize into MSCs type-1 and MSCs
type-2 to reach the homeostasis process, including regulating IL-8 and IL-6. MSCs are also exhibit
antimicrobial properties and regulate immune responses. This study was designed to explore the ability of
MSCs to control the inflammation produced by Staphylococcus aureus-contaminated PBMC with TNF-α
stimulation by analyzing IL-6 and IL-8 levels. We used a post-test group design with 2 study groups, consist
of vehicle control (Veh) and a treatment (1:20 comparison of MSCs: PBMCs) in triplicate supplemented with
S. aureus under 10 ng/mL TNF-α recombinant. The medium supernatant was collected after 0, 4, 8, and 12,
the IL-6 and IL-8 were measured using ELISA assay. This study showed a significant increase in IL-6 and
IL-8 at the first hour’s incubation. Interestingly, the IL-6 and IL-8 levels were significantly decreased after
12 and 8 to 12 hours of incubation, respectively. Based on our study, we conclude that MSCs may regulate
the IL-6 and IL-8 production on bacteria-contaminated PBMC with inflammation in early incubation to late
incubation.
1 INTRODUCTION
Recently, mesenchymal stem cell (MSC) therapy has
gained more attention in controlling the massive
inflammation than other stem cells. These cells have
immunomodulatory, anti-inflammatory, and
a
https://orcid.org/0000- 0002-9822-3119
b
https://orcid.org/0000- 0003-1231-9185
c
https://orcid.org/0000- 0001-5747-6032
d
https://orcid.org/0000- 0002-1137-9170
e
https://orcid.org/0000- 0002-8866-6098
f
https://orcid.org/0000- 0002-6314-3661
g
https://orcid.org/0000- 0002-1285-7656
h
https://orcid.org/0000- 0003-1609-9829
i
https://orcid.org/0000- 0001-7919-2735
antibacterial properties in addition to differentiation
capabilities (1,2). Sepsis is a systemic inflammatory
response to infection, characterized by the excessive
release of pro-inflammatory cytokines and disordered
fibrinolysis produced by immune cells and damaged
tissue (3). Although several approaches aim to reduce
Putra, A., Alif, I., Nazar, M., Prasetio, A., Satria Irawan, R., Amalina, D., Sari, E., Kustiyah, A. and Adeputra Nasution, I.
IL-6 and IL-8 Suppression by Bacteria-adhered Mesenchymal Stem Cells Co-cultured with PBMCs under TNF- Exposure.
DOI: 10.5220/0010491903110317
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 311-317
ISBN: 978-989-758-499-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
311

mortality rates in severe sepsis, such as goal-oriented
treatments, appropriate antibiotic treatment, and
corticosteroid treatment, there is still no effective
treatment for the sepsis (4). The uncontrolled
activation of the immune response in sepsis leads the
macrophages and endothelial and epithelial cells to
produce the release of cytokine cascades such as
tumor necrosis factor-alpha (TNF-α), interleukin
(IL)-1, IL-6, IL-8, IL-12, IL-18, and interferon (IFN)-
γ (5). However, the pro-inflammatory cytokines IL-8,
the chemoattractant of neutrophil and IL-6, the
induction of the acute-phase response have been
studied extensively concerning its possible role in the
pathogenesis of sepsis (6). Therefore, investigating
the role of MSCs to control IL-8 and IL-6 under the
inflammation microenvironment is currently one of
the most promising options.
Mesenchymal stem cells (MSCs) are non-
hematopoietic, multipotent, and plastic adherent
fibroblast-like cells capable of differentiation into
mesenchymal and nonmesenchymal lineages (7).
They are characterized by the expressions of surface
markers CD73, CD90, CD105, and the absence of
CD45, CD34, CD14 or CD11b, CD79a or CD19, and
Human Leucocyte Antigen (HLA) class II (8,9)
MSCs can differentiate into osteocytes,
chondrocytes, and adipocytes under standard in-vitro
differentiating conditions (7). They also can be
isolated from bone marrow, mobilized peripheral
blood, cord blood, umbilical cord (UC), placenta,
adipose tissue, dental pulp, and even fetal livers and
lungs (10). MSCs can regulate immune responses in
various disease models by polarizing into MSCs type-
1 as proinflammation phenotype and MSCs type-2,
anti-inflammatory cells depend on inflammation
exposure. MSCs can also regulate immune responses
in a variety of disease models through polarizing into
type-1 (proinflammation) and type-2 (anti-
inflammation), depending on inflammation exposure
(11). MSCs originating from either bone marrow or
adipose tissue are beneficial in sepsis, indicating that
MSC may upregulate antimicrobial activity in the
presence of infection by releasing Antimicrobial
Peptides (AMPs) (12-14).
MSCs have immunoregulator properties that can
control inflammatory cells by releasing anti-
inflammatory cytokine IL-10 leading to the decrease
of pro-inflammatory cytokines including IL-8 and IL-
6 (1,15,16). Theoretically, IL-8 belongs to the class
of pro-inflammatory chemokines produced by the
active macrophages post an infectious process in
which its level follows a course of time similar to that
of IL-6 (17). In line with IL-8, increased IL-6 also
shows in response to severe infection (15). Thus, the
IL-8 and IL-6 indicated as an early marker of sepsis.
However, several studies reported that MSCs could
suppress severe inflammation, leading to improved
sepsis and decreased sepsis animal models (2,18).
Thus far, the treatment of MSCs in patients with
sepsis has not been used. Furthermore, the role of
MSCs to control the level of IL-6 and IL-8 released
by inflammatory cells following bacteria
contamination and under TNF-α stimulation in
human PBMCs culture to mimic sepsis conditions
remains unclear. Therefore, in the present study, we
explored the ability of Staphylococcus aureus-
adhered MSCs co-cultured with PBMCs at a
comparison of 1:20 (MSCs and PBMCs) under 10
ng/mL TNF-α exposure in regulating the IL-6 and IL-
8 level.
2 MATERIALS AND METHODS
2.1 Research Design and Ethical
Approval
This study conducted at the Stem Cell and Cancer
Research (SCCR) Laboratory from October to
December 2019. This study used two groups: vehicle
control (Veh) and a treatment (T) group. The
institutional review board of the Committee ethic of
Medical Faculty, Sultan Agung Islamic University of
Semarang, Indonesia, approved this study.
2.2 Isolation and Culture of Human
Umbilical Cord-MSCs
MSCs were isolated from umbilical cords and cord
blood obtained from donors with written informed
consent. The isolation and expansion of MSCs were
performed, as described previously (1). Briefly, cords
were cut into smaller pieces and transferred into a T25
culture flask (Corning, Tewksbury, MA, USA)
containing DMEM (Sigma-Aldrich, Louis St, MO)
supplemented with 10% Fetal Bovine Serum (FBS)
(Gibco™ Invitrogen, NY, USA), 1% penicillin (100
U/mL)/streptomycin (100 µg/mL) (Gibco™
Invitrogen, NY, USA). Cultures were incubated at
37°C in a humidified atmosphere containing 5% CO
2
.
The medium was renewed every 3 days and passaged
after reaching 80% confluences (14 days). UC-
MSCs-like at passages 4–6 was employed.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
312

2.3 MSCs Characteristic
The of UC-MSCs-like cells was confirmed by
analyzing MSCs specific markers and the capability
to differentiate into mature cells. The 5th passage of
RUC-MSCs-like was stained with fluorescence-
labelled specific MSCs antibody including PE-CD44
(Clone G44-26, 555479; BD Biosciences), APC-
CD73 (Clone AD2, 560847; BD Biosciences), FITC-
CD90 (Clone 5E10, 561969 BD Biosciences), PerCP-
CD105 (Clone 266, 560819, BD Biosciences), and
PE-Lin negative (CD45/CD34/CD11b/CD19/HLA-
DR) antibodies, then incubated for 30 minutes at
room temperature, washed twice with stain buffer
(554657, BD Biosciences) and examined using a BD
C6 Plus flow cytometer (BD Biosciences) and BD
Accuri C6 Plus Software (BD Biosciences).
2.4 Differentiation of hUC-MSCs
To characterize the isolated cells, we further
performed the osteogenic differentiation assay in the
fourth passage. Osteogenesis was induced by
osteogenic induction medium containing 10 mmol/L β
glycerophosphate, 10−7 mol/L/ 0.1 μM
dexamethasone, 50μmol/L ascorbate-2-phosphate
(Sigma-Aldrich, Louis St, MO) and supported with
10% FBS (Gibco™ Invitrogen, NY, USA) in DMEM
(Sigma-Aldrich, Louis St, MO) at 37°C and 5% CO2.
Calcium deposition was shown by Alizarin Red
staining (Sigma-Aldrich, Louis St, MO) after 21 days
incubation.
2.5 Bacteria Preparation
We used S. aureus as the source of infection in this
study. The bacteria were obtained from the
Laboratory of Microbiology, Faculty of Medicine,
Unissula. S. aureus was propagated in LB medium
(BD Falcon) overnight at 37°C and used to coat mesh
implanted material during the log phase of growth.
2.6 Isolation of Human Peripheral
Blood Mononuclear Cells
(hPBMCs)
Human Peripheral Blood Mononuclear Cells
(PBMCs) were isolated by Ficoll-Paque (Axis-Shield)
density gradient centrifugation from health
volunteers’ venous blood after informed consent.
PBMCs were cultured in 2 ml of advanced Roswell
Park Memorial Institute medium (RPMI) 1640 culture
medium (Invitrogen, Grand Island, NY, USA)
supplemented with 10% FBS, 2 mM glutamine, 100
U/ml penicillin, and streptomycin, and allowed to
adhere at 37°C and 5% carbon dioxide incubator for
12 h.
2.7 Co-culturing MSCs-adhered
Bacteria with PBMCs under TNF-α
Stimulation
For the T group, S. aureus and hUC-MSCs (4 × 10
4
cells) were co-cultured in coverslip and put in a T25
culture flask in DMEM (Sigma-Aldrich, Louis St,
MO) at 37°C and 5% CO
2
for 12 h. The co-cultured
cells were then transferred to the culture flask, which
contains 8x105 PBMCs (1:10 comparison), combined
with DMEM-LG and RPMI 1640 culture medium
(Invitrogen, Grand Island, NY, USA), 10% FBS, 2
mM glutamine, and 100 U/ml penicillin and
streptomycin. The co-cultured cells were also
supplemented with TNF-α recombinant (10 ng/mL)
(BioLegend, San Diego, CA). The medium
supernatant was collected after 1, 4, 8, and 12 h
incubation for ELISA analysis. On the other hand, the
co-culture between PBMCs and S. aureus was
performed for the Veh group.
2.8 Quantification of Cytokines
The levels of both IL-6 and IL-8 were quantified in
the cell culture supernatants by enzyme-linked
immunosorbent assay (ELISA) from the various
treatment groups. IL-6 and IL-8 were calculated
according to a standard curve constructed for each
assay, and each assay performed in triplicate. The
colorimetric absorbance was recorded at a
wavelength of 450 nm. The measurement was done
entirely according to the manufacturer’s protocol
(QAYEE, Wuhan, China).
2.9 Data Analysis
Data are presented as the means ± standard deviation.
All calculations were carried out using IBM SPSS
22.0 (IBM Corp., Armonk, NY, USA) was used for
statistical analysis. The statistical significance of the
differences between the groups was assessed using
the paired t-test. p values: *, p < 0.05.
IL-6 and IL-8 Suppression by Bacteria-adhered Mesenchymal Stem Cells Co-cultured with PBMCs under TNF- Exposure
313
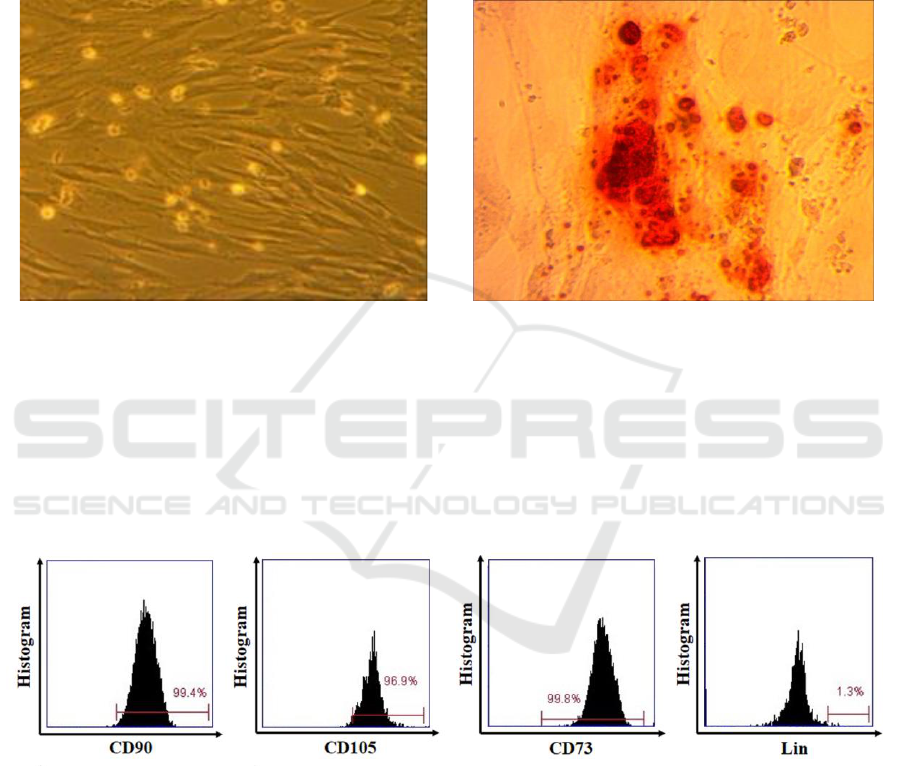
3 RESULTS
3.1 Isolation and Differentiation of
hUC-MSCs
Isolation of hUC-MSCs was performed based on the
capacity to plastic attachment under standard culture
conditions. Isolated cells were cultured for 2–3 weeks
in monolayer and used for differentiation analysis
after 4 to 5 passages. The hUC-MSCs were initially
characterized by their elongated fibroblastic cellular
phenotype (figure 1(a)); moreover, osteogenesis was
confirmed at day 21 of culture by immunodetection
with Alizarin Red staining (figure 1(b)).
Figure 1. (a) UC-MSCs characterized by their peculiar fibroblast-like (spindle shape) morphology (100x magnification). (b)
and osteogenic differentiation with Alizarin Red staining appears red color (400x magnification).
3.2 Characteristics of hUC-MSCs
According to the International Society of Cellular
Therapy (ISCT), MSCs have specific marker profiles,
such as CD73, CD105, CD90 and negative of Lin-
(CD45/CD34/CD11b/CD19/HLA-DR) and the
capability to differentiate into several mesodermal
germ layers, including osteocytes. The result showed
that UC-MSCs expressed a high level of CD90
(99.4%), CD105 (96.9%), CD73 (99.8%) and lacked
the expression of Lin- (1.3%). (Figure 2).
Figure 2. MSCs phenotypes was positive for CD73, CD90 and CD105 and negative for Lin.
3.3 The Appearance of MSC-Adhered
Bacteria and PBMCs
We firstly co-cultured the MSCs with S. aureus
(figure 3(a)) in 12 hours incubation to determine the
IL-6 and IL-8 concentration at first-hour incubation,
with and without PBMCs. Next, we co-cultured the
MSCs-adhered bacteria with PBMCs and observed its
appearance at 12 hours incubation (figure 3(b)).
A
B
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
314
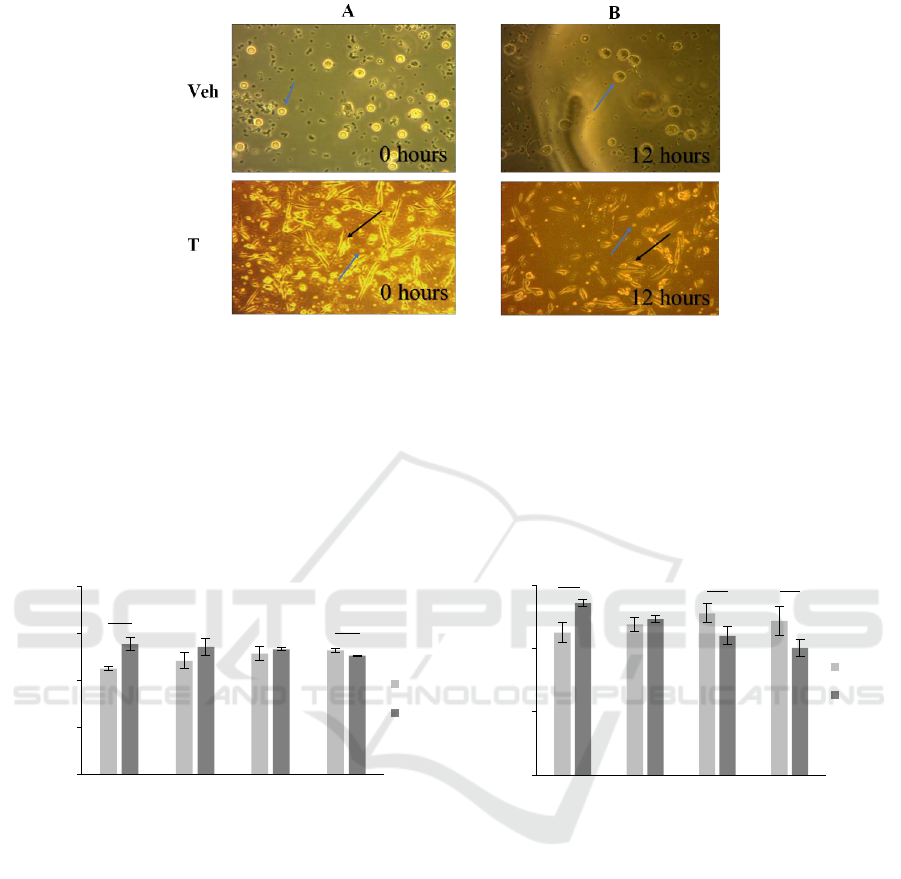
Figure 3. (a) MSCs adhered bacteria (black arrow) and PBMCs (blue arrow) appearance at 0 and (b) 12 hours incubation.
(Magnification: Veh = 40x; T = 10x).
3.4 IL-6 and IL-8 Levels
We subsequently quantify the level of IL-6 and IL-8
(Figure 3) by ELISA in triplicate. The IL-6 and IL-8
level analysis showed a significant increase in the T
group at the first-hour incubation (p < 0.05).
Interestingly, the level of IL-6 gradually decreased,
depending on time, which became significant after
12-hour incubation (p < 0.05). In line with this, the
level of IL-8 was also gradually decreased in time,
which reached significant after 8- and 12-hours
incubation (p < 0.05).
Figure 4. ELISA assays for the T group in 0,4,8 and 12 hours post-co-culture showed the gradual decrease of IL-6 and IL-8
depend on time. (a) The level of IL-6 was significantly after 12 hours of co-culture incubation. (b) In line with this, the level
of IL-8 was also significantly decreased after 8- and 12-hours incubation. *, p < 0.05.
4 DISCUSSION
Inflammation serves as a systemic or localized
protective response caused by injury, infection, or
tissue destruction and attends to eliminate pathogens
and preserve host integrity, particularly in sepsis
model infection. MSCs may exhibit antimicrobial
properties and regulate both the innate and the
adaptive immune responses, resulting in both the pro-
inflammatory and anti-inflammatory effects when
those MSCs interact with an immune system or
exposed by various cytokines (19). Several studies
have shown that MSCs respond to the inflammatory
milieu by polarizing either into MSCs type-1 with the
pro-inflammatory phenotype or MSCs type-2 with
anti-inflammatory properties depending on Toll-like
Receptors (TLRs) type activation (1,20). Another
study also reported that MSCs under bacterial
exposure might increase phagocytosis of neutrophil
and monocyte cells, suggesting that MSCs may
increase pro-inflammatory molecules (21). Although
MSCs have been widely demonstrated
experimentally on their immune properties in
suppressing inflammation, the study of MSCs in
0
100
200
300
0 hour 4 hours 8 hours 12 hours
IL-8 level (pg/mL)
Veh
T1
0
20
40
60
80
0 hour 4 hours 8 hours 12 hours
IL-6 level (pg/mL)
Veh
T1
*
*
*
*
*
A
B
IL-6 and IL-8 Suppression by Bacteria-adhered Mesenchymal Stem Cells Co-cultured with PBMCs under TNF- Exposure
315

treating severe infections by exploring the IL8 and
IL-6 is comparatively less performed (18). Therefore,
in the present study, we demonstrate the utility of
activated MSC in treating the S. aureus-contaminated
PBMC under TNF stimulation by examining the IL-8
and IL-6 levels as the one marker of severe infections.
This study found a significant increase of IL-8
level in treatment groups starting at first to 4 hours
incubation on S. aureus-contaminated PBMC with
TNF stimulation. Our finding suggested that MSCs
exposed by bacteria could enhance neutrophils to
recognise and kill bacteria S. aureus due to expressed
IL-8 following MSCs treatment is the robust
chemoattractant for neutrophils. This study
confirmed and extended our understanding of the
direct antibacterial activity of MSC as previously
reported when neutrophils incubated with the
activated MSCs can induce significantly greater
Neutrophil Extracellular Trap (NET) area formation
(22). The increased IL-8 in the first hours after
exposure indicated MSCs could stimulate
neutrophils, the innate immune system, to
phagocytize bacteria preventing the bacteria from
spreading into tissues (23). Interestingly, we also
found that the IL-8 level gradually decreased in time
and reached significant after 8- and 12-hours
incubation. We suggest the polarization of MSCs
caused the gradual decrease of IL-8 into MSCs type-
2 following exposed bacteria as described in our
previous study (1).
In line with the increase of IL-8, there was also a
significant increase of IL-6 in treatment groups
starting at first to 4 hours of incubation on S. aureus-
contaminated PBMC with TNFα stimulation.
Likewise, with 8 to 12 hours of incubation, we also
found the decreased IL-6 decreased and reached
significant after 12 hours incubation, similar to an
episode of IL-8. We supposed that there were the
indirect mechanisms of MSC in regulating IL-8 and
IL-6, in which MSCs initially increase those
cytokines, subsequently became decreased gradually
in time. This is due to the activation of MSCs with
TLRs ligand, known as MSCs polarization. In terms
of bacteria stimulation and TNF exposure, the TLR-4
of MSCs type-1 was activated initially to induce the
activation of MyD88-dependent pathway, then to NF-
kb pathway resulting in the release of pro-
inflammatory cytokines including IL-8 and IL-6.
These released cytokines trigger the inflammation
states crucial for innate cells to eliminate the bacteria
and antigen (24). However, along with time, the
increased IL-8 and IL-6 induce the upregulation of
COX2, which increases PGE2 secretion. Binding
PGE2 to EP2 and EP4 receptor leads the shift of
MSCs from MyD88-dependent pro-inflammatory to
TRIF-TRAM mediated anti-inflammatory signal,
known as MSCs type-2 by P110δ isoform of PI3K
kinase. This MSCs type-2 can secrete anti-
inflammatory cytokines such as IL-10 (1). This
condition leads to decreased IL-8 and IL-6 levels.
Our findings indicate that the regulation of IL-6
and IL-8 by MSCs type-1 is needed for the
neutrophils to migrate and attach to the inflammatory
niche. However, the excessive release of IL-6 and IL-
8 is also needed to suppress MSCs type-2 to reach the
homeostasis process. Unfortunately, we did not
measure the concentration of NET and a direct
bacterial killing assay of MSCs and the anti-
inflammatory cytokines. Therefore, the exact
mechanism of MSCs polarization regarding pro-
inflammatory cytokines and anti-inflammatory
cytokine remains unclear.
5 CONCLUSION
Based on our study, we conclude that MSCs may
regulate the IL-6 and IL-8 production on bacteria-
contaminated PBMC with inflammation in early
incubation to late incubation.
ACKNOWLEDGMENTS
The authors gratefully acknowledge that the present
research is fully supported and carried out by Stem
Cell and Cancer Research (SCCR) Laboratory,
Medical Faculty, Sultan Agung Islamic University
(Unissula), Semarang for all Facility to finish this
research.
REFERENCES
Putra, A., Ridwan, F. B., Putridewi, A. I., Kustiyah, A. R.,
Wirastuti, K., Sadyah, N. A. C., Rosdiana, I., & Munir,
D., 2018. The Role of TNF-α induced MSCs on
Suppressive Inflammation by Increasing TGF-β and IL-
10. Macedonian journal of medical sciences, [online]
6(10), pp. 1779–1783.
Laroye, C., Boufenzer, A., Jolly, L., Cunat, L., Alauzet, C.,
Merlin, J. L., Yguel, C., Bensoussan, D., Reppel, L., &
Gibot, S., 2019. Bone marrow vs Wharton’s jelly
mesenchymal stem cells in experimental sepsis: a
comparative study. Stem cell research & therapy,
[online] 10(1), 192.
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-
Hari, M., Annane, D., Bauer, M., Bellomo, R., Bernard,
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
316

G. R., Chiche, J. D., Coopersmith, C. M., Hotchkiss, R.
S., Levy, M. M., Marshall, J. C., Martin, G. S., Opal, S.
M., Rubenfeld, G. D., van der Poll, T., Vincent, J. L., &
Angus, D. C., 2016. The third international nonsensus
definitions for sepsis and septic Shock (Sepsis-3).
JAMA, [online] 315(8), pp. 801–810.
Rello, J., Valenzuela-Sánchez, F., Ruiz-Rodriguez, M., &
Moyano, S., 2017. Sepsis: a review of advances in
management. Advances in therapy, 34(11), pp. 2393–
2411.
Cheng, B., Hoeft, A. H., Book, M., Shu, Q., & Pastores, S.
M., 2015. Sepsis: pathogenesis, biomarkers, and
treatment. BioMed research international, [online],
2015, pp. 846935.
Chaudhry, H., Zhou, J., Zhong, Y., Ali, M. M., McGuire,
F., Nagarkatti, P. S., & Nagarkatti, M., 2013. Role of
cytokines as a double-edged sword in sepsis. In vivo,
[online] 27(6), pp. 669–684.
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach,
I., Marini, F., Krause, D., Deans, R., Keating, A.,
Prockop, D. j., & Horwitz, E., 2006. Minimal criteria
for defining multipotent mesenchymal stromal cells.
The International Society for Cellular Therapy position
statement. Cytotherapy, [online] 8(4), pp. 315–317.
Mafi, P., S. Hindocha, R. Mafi, M. Griffin, and Khan W. S.,
2011. Adult mesenchymal stem cells and cell surface
characterization - a systematic review of the literature.
The open orthopaedics journal, [online], 5(Suppl 2),
pp. 253–260.
Okolicsanyi, R. K., Camilleri, E. T., Oikari, L. E., Yu, C.,
Cool, S. M., van Wijnen, A. J., Griffiths, L. R., &
Haupt, L. M., 2015. Human mesenchymal stem cells
retain multilineage differentiation capacity including
neural marker expression after extended in vitro
expansion. PloS one, [online], 10(9), pp. e0137255.
Nagamura-Inoue, T., & He, H., 2014. Umbilical cord-
derived mesenchymal stem cells: their advantages and
potential clinical utility. World journal of stem cells,
[online], 6(2), pp. 195–202.
Uccelli, A., Moretta, L., & Pistoia, V., 2008. Mesenchymal
stem cells in health and disease. Nature reviews,
[online], 8(9), pp. 726–736.
Németh, K., Leelahavanichkul, A., Yuen, P. S., Mayer, B.,
Parmelee, A., Doi, K., Robey, P. G., Leelahavanichkul,
K., Koller, B. H., Brown, J. M., Hu, X., Jelinek, I., Star,
R. A., & Mezey, E., 2009. Bone marrow stromal cells
attenuate sepsis via prostaglandin E(2)-dependent
reprogramming of host macrophages to increase their
interleukin-10 production. Nature medicine, [online],
15(1), pp. 42–49.
Mei, S. H., Haitsma, J. J., Dos Santos, C. C., Deng, Y., Lai,
P. F., Slutsky, A. S., Liles, W. C., & Stewart, D. J.,
2010. Mesenchymal stem cells reduce inflammation
while enhancing bacterial clearance and improving
survival in sepsis. American journal of respiratory and
critical care medicine, [online], 182(8), pp. 1047–1057.
Gonzalez-Rey, E., Anderson, P., González, M. A., Rico, L.,
Büscher, D., & Delgado, M., 2009. adult stem cells
derived from adipose tissue protect against
experimental colitis and sepsis. Gut, [online], 58(7), pp.
929–939.
Gu, Y., He, M., Zhou, X., Liu, J., Hou, N., Bin, T., Zhang,
Y., Li, T., & Chen, J., 2016. Endogenous IL-6 of
mesenchymal stem cell improves behavioral outcome
of hypoxic-ischemic brain damage neonatal rats by
supressing apoptosis in astrocyte. Scientific reports,
[online], 6, pp. 18587.
Wang, J., Wang, Y., Wang, S., Cai, J., Shi, J., Sui, X., Cao,
Y., Huang, W., Chen, X., Cai, Z., Li, H., Bardeesi, A.
S., Zhang, B., Liu, M., Song, W., Wang, M., & Xiang,
A. P., 2015. Bone marrow-derived mesenchymal stem
cell-secreted IL-8 promotes the angiogenesis and
growth of colorectal cancer. Oncotarget, [online] 6(40),
pp. 42825–42837.
Bernardo, M. E., & Fibbe, W. E., 2013). Mesenchymal
stromal cells: sensors and switchers of inflammation.
Cell stem cell, [online], 13(4), pp. 392–402.
Johnson, V., Webb, T., Norman, A., Coy, J., Kurihara, J.,
Regan, D., & Dow, S., 2017. Activated mesenchymal
stem cells interact with antibiotics and host innate
immune responses to control chronic bacterial
infections. Scientific reports, [online], 7(1), pp. 1–18.
Kim, D. S., Lee, W. H., Lee, M. W., Park, H. J., Jang, I. K.,
Lee, J. W., Sung, K. W., Koo, H. H., & Yoo, K. H.,
2018. Involvement of TLR3-dependent PGES
expression in immunosuppression by human bone
marrow mesenchymal stem cells. Stem cell reviews and
reports, [online],14(2), pp. 286–293.
Krasnodembskaya, A., Song, Y., Fang, X., Gupta, N.,
Serikov, V., Lee, J. W., & Matthay, M. A., 2010.
Antibacterial effect of human mesenchymal stem cells
is mediated in part from secretion of the antimicrobial
peptide LL-37. Stem cells, [online], 28(12), pp.2229–
2238.
Chow, L., Johnson, V., Impastato, R., Coy, J., Strumpf, A.,
& Dow, S., 2020. Antibacterial activity of human
mesenchymal stem cells mediated directly by
constitutively secreted factors and indirectly by
activation of innate immune effector cells. Stem cells
translational medicine, [online], 9(2), pp. 235–249.
Brandau, S., Jakob, M., Bruderek, K., Bootz, F., Giebel, B.,
Radtke, S., Mauel, K., Jäger, M., Flohé, S. B., & Lang,
S., 2014. Mesenchymal stem cells augment the anti-
bacterial activity of neutrophil granulocytes. PloS one,
[online] 9(9), pp. e106903.
Aksoy, E., Taboubi, S., Torres, D., Delbauve, S., Hachani,
A., Whitehead, M. A., Pearce, W. P., Berenjeno, I. M.,
Nock, G., Filloux, A., Beyaert, R., Flamand, V., &
Vanhaesebroeck, B., 2012. The p110δ isoform of the
kinase PI(3)K controls the subcellular
compartmentalization of TLR4 signaling and protects
from endotoxic shock. Nature immunology, [online],
13(11), pp. 1045–1054.
IL-6 and IL-8 Suppression by Bacteria-adhered Mesenchymal Stem Cells Co-cultured with PBMCs under TNF- Exposure
317
