
The Effectiveness of Mesenchymal Stem Cell and Colostrum Bovine
Combination in Post Hepatectomy Liver Failure with Liver Fibrosis
Animal Model
Dimas Aryo Kusumo
1
a
, Agung Putra
2
b
, Albertus Ari Adrianto
3
c
, Erik Prabowo
3
d
and Ignatius Riwanto
3
e
1
Departement of Digestive Surgery Subspecialist Program, Faculty of Medicine, Diponegoro University, Semarang,
Indonesia
2
Stem Cells and Cancer Research, Faculty of Medicine, Sultan Agung Islamic University, Semarang, Indonesia
3
Department of Digestive Surgery. Faculty of Medicine, Universitas Diponegoro, Semarang, Indonesia
Keywords: Post hepatectomy liver fibrosis, mesenchymal stem cell, bovine colostrum
Abstract: Post hepatectomy liver fibrosis (PHLF) is the most common cause of morbidity and mortality after liver
surgery. Recently, mesenchymal stem cells (MSCs) and bovine colostrum (BC) have been studied to exert an
anti-fibrotic. However, the effect of MSCs, BC, and their combination of tissue regeneration after PHLF
remains unclear. This study aimed to evaluate the effect of MSCs, BC and its combination in regulating
transforming growth factor-β (TGF-β) and serum glutamic pyruvic transaminase (SGPT) associated with liver
destruction inhibition on PHLF animal models. Eighteen Sprague-Dawley were injected with CCl4 for eight
weeks and 50% liver resection (LR). Mice were divided into three groups: group NaCl 0.9 % with
parenchymal injection, group MSC with parenchymal injection, and a group of MSCs with parenchymal
injection and BC oral. After administration, the level TGF β and SGPT were collected on the 3rd and 7th days.
This study showed that the TGF-β levels nit significant decrease under combination treatment compared to
the MSCs group until 34,11pg/mL ±4,18 and 34,5 pg/mL ±2.94, respectively, on day 7
th
. Besides, SPGT
levels of combination treatment also did not significantly decrease compared to the MSCs group until 93,6
U/L ±23,8 and 163,2U/l ±73,62, respectively, on day 3
rd
. In conclusion, the combination therapy is no better
than single MSCs treatment in regenerating liver tissue on PHLF.
1 INTRODUCTION
Hepatocellular carcinoma (HCC) is the liver's
primary cancer, accounting for most liver cancers.
HCC is one of the leading causes of cancer-related
death worldwide and has high evidence in 2012. The
incidence of HCC found fourteen million patients,
and it grew to twenty-two million patients in the last
two decades. HCC causes infection of hepatitis B
virus infection, often accompanied by cholestasis. It
makes inflammatory processes that encourage liver
fibrosis. On normal liver can perform liver resection
a
https://orcid.org/0000-0003-4070-948X
b
https://orcid.org/0000-0002-9822-3119
c
https://orcid.org/0000-0002-2907-034X
d
https://orcid.org/0000-0002-2429-5419
e
https://orcid.org/0000-0003-3441-3738
up to 75% of the total liver volume by maintaining
25% of the remnant liver on large HCC. HCC cases
with fibrosis and less than 40% of liver remnants after
resection mostly progress into small for size liver
syndrome (SFSS) and liver failure (Wiliam and
Janagin, 2017; Xia, Lu, and Wang. 2008)
The progression of SFSS into liver failure is not
determined by the size of the liver remnant only but
also the hemodynamic liver circulation (
Golriz et
al.,2015)
. There was a disruption of normal hepatocyte
regeneration due to various events, including
parenchyma loss and hepatic vascular bed reduction.
The increasing portal pressure makes shear stress on
Kusumo, D., Putra, A., Adrianto, A., Prabowo, E. and Riwanto, I.
The Effectiveness of Mesenchymal Stem Cell and Colostrum Bovine Combination in Post Hepatectomy Liver Failure with Liver Fibrosis Animal Model.
DOI: 10.5220/0010491803050310
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 305-310
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
305

the sinusoid endothelial cell. Nitric oxide (NO) is then
released by liver sinusoidal endothelial cells
(LSECs), and a continuous pro-inflammatory
mediator exposure produced by the inflammatory
cell, particularly Kupffer cell (
Golriz et al.,2015, Ray et
al.,2015). During chronic inflammation, Kupffer cells
induce the prolonged release of TGF-β1 leading to LF
formation that contributed to liver failure following
liver resection (Hoffmann et al., 2020). Considering this
complicated process, finding an effective treatment in
resolving post hepatectomy liver fibrosis (PHLF)
could be very challenging. Only a few methods to
prevent SFSS in the preoperative, perioperative, and
post-operative due to liver transplantation as the lack
of liver donors (Kim et al., 2019).
On the other hand, previous studies reported that
MSC has the robust capability to suppress the release
of TGF secreted by inflammatory cells leading to
wound healing acceleration (Yo et al.,2013; Putra et
al., 2020). Other studies revealed that BC containing
growth factor and anti-oxidant also has an essential
role in liver regeneration (Sinn et al., 2017). MSCs
are multipotent cells characterized by the high
expressions of several surface markers such as
CD105, CD73, CD29, CD90, and CD44 and lack of
expressions of CD79a, CD11b. CD14, CD34, CD45,
and CD19 (Ly et al.,2014). These cells have low
immunogenicity, self-renewal, and multidirectional
differentiation properties (Zhang et al., 2018).
Previous studies have shown that MSCs have
immunomodulatory properties by secreting soluble
cytokines to inhibit inflammatory cells and prevent
excessive inflammatory damage to the liver tissue in
drug-induced liver failure animal model (Hu, Wu, and
Li, 2020). Bone marrow-derived mesenchymal stem
cells (BM-MSCs) reduced the expression of TGF β
lead to inhibition of the TGF β signalling pathway in
liver fibrosis formation
(Jang et al., 2014). A previous
study reported that the post hepatectomy LF animal
model, which received MSC treatment is successfully
survived with lower ALT and AST levels (Ding et al.,
2019)
BC is a complex mixture of protein, lipids,
lactose, vitamin, and mineral. It includes
immunoglobulin, multiple growth factors, and total
anti-oxidants capacity affecting liver injury due to
decreased TGF-β associated with fibrogenesis
inhibition and SGPT level decreased (Sinn et
al.,2017). Although MSCs and BC could resolve liver
injury in LF, the efficacy of both modalities in
decreasing the progressivity of liver damage, in this
case, need further investigation. Therefore, in this
study, we compare the effectiveness of MSCs with
BC combination compared to MSCs alone in
decreasing the progressivity of liver destruction in
PHLF by analyzing the regulation of TGF β and
SGPT level.
2 MATERIALS AND METHODS
2.1 Isolation of UC-MSCs
The umbilical cord (UC) derived MSCs (UC-MSCs)
were obtained from pregnant single Sprague-Dawley
(SD) rats under deep anaesthesia and transplanted
into an ALF rat model. The umbilical cord was cut
into pieces after the blood vessels were removed. It
was then transferred to a T25 culture flask containing
complete Dulbecco's Modified Eagle's medium
(DMEM) (Catalog #2192773 Sigma-Aldrich, Louis
St, MO) enriched with 10% Fetal Bovine Serum
(FBS) (Calatog #42A1190K GibcoTM Invitrogen,
NY, USA) and 100 IU/mL penicillin/streptomycin
(Catalog # 15070063 Sigma-Aldrich, USA). These
cells were incubated in a 5% CO2, 37ºC incubator,
and the medium was changed every three days. After
the cells reached 80% confluency, the MSC-like cells
were passaged with trypsin. The cells from the 4
th
passage were used for experiments. The Institutional
Review Board approved this study of the Medical
Department's Ethics Committee with number
254/VIII/2020/ commission bioethics, Sultan Agung
Islamic University, in Semarang, Indonesia.
2.2 PHLF Animal Models Induction of
PHLF by Made Remnant Liver
Fibrosis Animals and Experimental
Design
Eighteen Sprague-Dawley (SD) male rats were
randomly divided into three groups (n= 18). LF
induction was performed by injecting intraperitoneal
the carbon tetrachloride (CCl4) (Catalog #56235
Sigma–Aldrich, USA) with 1 ml/kg twice per week
for eight weeks. After six weeks, three rats in the
model group were sacrificed randomly, and the liver
tissue was obtained to verify LF with Sirius red. All
of the PHLF with LF rats undergoing liver resection
50% of the liver in the median and right lateral lobes.
The surgical procedure was performed within the
sterile condition under intravenous anaesthesia using
xylazine + ketamine (5 mg/kg + 100 mg/kg
intramuscularly) (
Constandinouu et al., 2005).
The Group of NaCl 0.9 % and parenchymal
injection NaCl 0,9% 500 μL, group MSC with
parenchymal injection with doses 1 x 10
6
cells
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
306
dissolved in 500 μL of NaCl, and group combination:
MSCs parenchymal injection 1x 10
6
cells dissolved in
500 μL of NaCl and oral administration of BC doses
15μL/g per oral, daily with milk powder Good Health.
2.3 Flow Cytometry
Immunophenotyping of UC-MSCs
The immunophenotypes of MSCs were analyzed in
the fourth passage. MSCs were stained using
conjugated antibodies: fluorescein isothiocyanate
(FITC)-conjugated CD90, Allophycocyanin (APC)-
conjugated CD73, Peridinin Chlorophyll Protein
Complex (PerCP)-conjugated CD105 and
phycoerythrin (PE)-conjugated Lin monoclonal
antibodies for 30 min at 4ᴼC in the darkroom. The
cell's fluorescence intensity was evaluated through
flow cytometry (BD Bioscience, Franklin Lakes, NJ,
USA).
2.4 In Vitro Differentiation
MSCs differentiation potential was determined to
characterize the isolated cells. These cells were
cultured in DMEM medium supplemented with 10%
FBS, ten mmol/L β-glycerophosphate, 10
mol/L/ 0.1
µM dexamethasone, 50 µmol/L ascorbate-2-
phosphate (Catalog #SLBL4673V Sigma-Aldrich,
Louis St, MO), at 37ᴼC and 5% CO
2
. The fixed cells
were stained with 0.2 % Alizarin Red solution
(Catalog #MKBS9114V Sigma-Aldrich) to represent
calcium deposition (the cells were used from the
fourth passage).
2.5 Elisa TGF- β
The rat's blood was harvested via peri-orbital venous
plexus bleeding under general anaesthesia on day 3
rd
,
7
th
day after treatment, and the serum was collected
by centrifugation at 4⁰C. The TGF-β levels were
measured by ELISA kits, based on the manufacturer's
instructions (Abbkine) and according to a standard
curve constructed for each assay. The colourimetric
absorbance was recorded at a wavelength of 450 nm.
2.6 SGPT Levels
The levels of SGPT were measured on the 3
rd
, 7
th
days
after treatment to determine liver function. Blood
samples were collected from the peri-orbital vein
under anaesthesia, using xylazine + ketamine (5
mg/kg + 100 mg/kg intramuscularly) (Alfasan,
Netherlands). The serum level of SGPT was
measured using automatic analyzers (BT 3000 PLUS,
Italy).
2.7 Statistical Analysis
All data were presented as mean ± standard deviation
with differences between groups analyzed by a one-
way ANOVA and least significant difference
comparison post hoc LSD test. Analysis using a p <
0.05 significant statistical value.
3 RESULTS
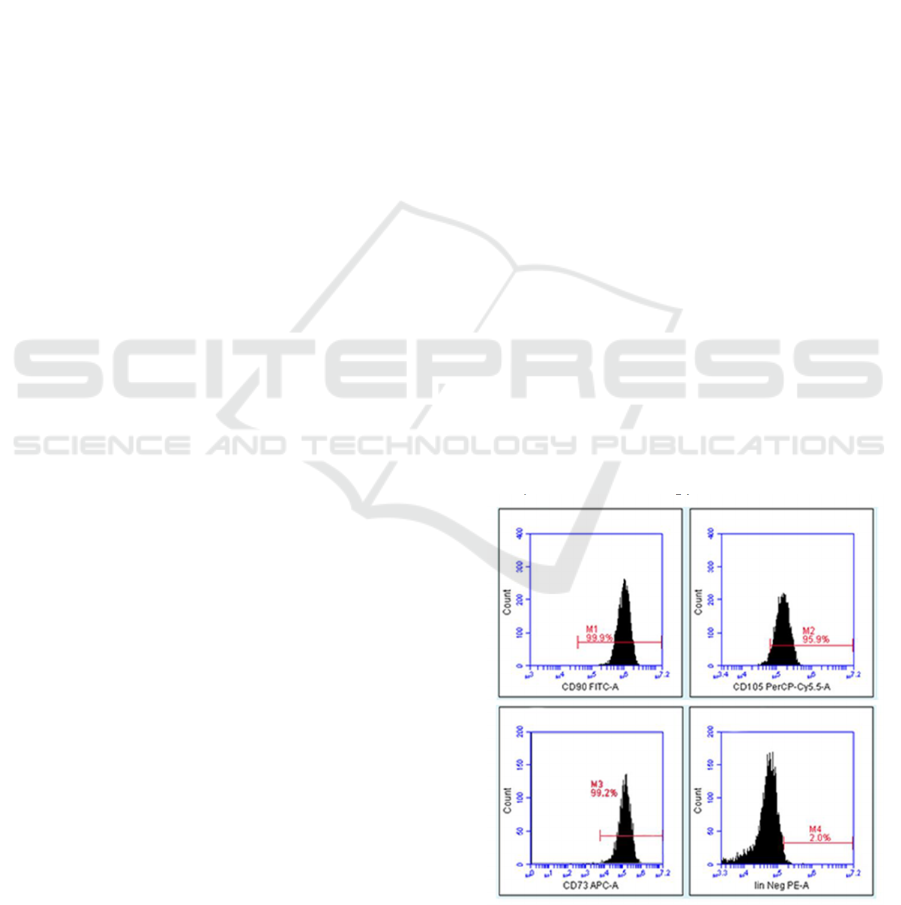
3.1 Characteristics of UC-MSCs and
Differentiation Test
The isolated cells showed peculiar fibroblast-like
(spindle shape) morphology. To determine and verify
the MSCs marker, we assessed MSC's marker
expression using flow cytometry after the fourth
passages (Figure 1). The results showed that the
isolated cells expressed an MSC-specific marker
including positive expression of CD105 (96.7%),
CD73 (99.2%), and CD90 (96.7%) and lack of Lin
(0.03%). In line with the flow cytometry analysis, we
also examined the osteogenic capabilities of MSCs.
We found the differentiation of MSC into osteogenic
has occurred, indicated by calcium deposits as red
appearance using the Alizarin red dye staining
method (Figure 2). It is according to the International
Society of Cellular Therapy (ISCT).
Figure 1: MSCs characterization. The graph displayed the
expression MSC, positive markers (CD105, CD73, and
CD90), and lack of the negative marker Lin (Lin̄)
expression.
The Effectiveness of Mesenchymal Stem Cell and Colostrum Bovine Combination in Post Hepatectomy Liver Failure with Liver Fibrosis
Animal Model
307
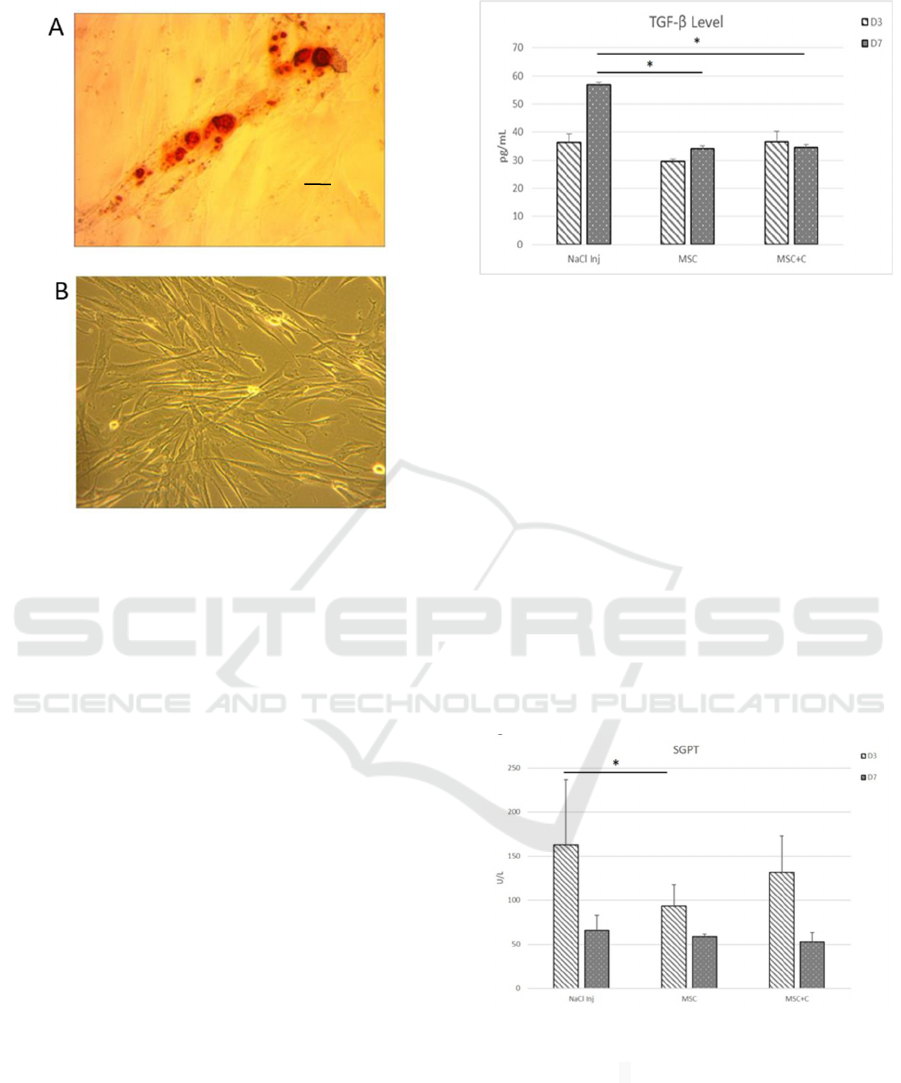
Figure 2. The morphology and differentiation of MSCs.
(A)MSC Differentiation. Alzarin red dye showed a red
colour appearance in the MSC differentiation test
(magnification x40, scale bar 50 μm). (B) Morphological
characterization. MSCs appearance; homogeneous,
spindle-shaped, fibroblast-like cells (magnification x10,
scale bar 200 μm).
3.2 MSCs Suppress TGF-β and SGPT
Levels in PHLF Animal Model
The TGF- is one of the primary growth factors with
pleiotropic capability particularly associated with
fibrosis formation due to its ability to changes
activate hepatic stellate cell (HSC) into myofibroblast
(MF) in producing collagen type III. To determine the
role of MCS in decreasing TGF- level in PHLF, we
analyzed using ELISA. In this study we found, there
is a significant decreased (p<0,05) of TGF- level on
day 7
th
in MSC treatment group (day 3
rd
29,66 pg/mL
±0,76; day 7
th
34,11 pg/mL ±4,18, respectively) and
Combination group (day 3
rd
36,54 pg/mL ±3,72; day
7
th
34,5 pg/mL ±2.94) compared with the control
group NaCl Injection (day 3
rd
36.33 pg/ml±3.04, day
7
th
56.7pg/ml ± 6.22 ) (Figure 3).
Figure 3. The level of TGF-β at 3
rd
, and 7
th
day in the NaCl,
MSC, and combination group. *The level of TGF- day
MSC (34,11pg/mL ±4,18) and combination group,
(34,5
pg/mL ±2.94) on day 7
th
was decreased significantly
compared to NaCl group (56.7pg/ml± 6.22) p<0,05. C:
Colostrum
The increase of SGPT level indicated that there
was a hepatocellular injury, including on PHLF. We
examined the level of SGPT after MSC treatment
compared to a combination of both treatments to
regenerate the damaged liver tissue in PHLF. In this
study, we found a significant decrease (p:0,024) of
SGPT level in the MSC group only on day 3rd (day 3
rd 93,6 U/L ±23,8, day 7th 58,8 U/L±2.68). We did
not found a significant decrease in the combination
group on day 3rd and 7th (day 3 rd 131,4 U/L±41,2,
day 7th 52,8 U/l±10,57) compared with NaCl group
(day 3 rd 163,2U/l ±73,62, day 7th 66U/l±17.01)
(Figure 4).
Figure 4. SGPT level on 3
rd
, and 7
th
day in NaCl, MSC
and combine group. Data are presented as the mean ±
standard deviation. *The level of SGPT decreased
significantly in MSCs group only on day 3
rd
(93,6 U/L
±23,8) compared to NaCl group (163,2U/l ±73,62) p < 0,05.
C: Colostrum
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
308

4 DISCUSSION
The aims of this study were evaluated the
effectiveness of MSCs and combination of MSCs and
BC in the regeneration of liver tissues on PHLF by
analyzing the regulation of TGF β and SGPT level.
The ability of MSCs decrease fibrosis formation by
controlling the prolonged release of TGF-β produced
by MF as associate cell product fibrosis deposits. BC
containing anti-oxidant also can improve LF and
restore the damaged structure and function of liver
tissue. As the potent mediators, the TGF-β initiates
consistently with the activation and differentiation of
HSCs to be active MFs resulting in collagen type III
deposition. We injected CCl4 as a hepatotoxic
chemical to induce LF and performed liver resection
50 % to induce PHLF by major resection liver to
make a small liver remnant (Golriz et al., 2015)
We present here that MSCs and combination group
can suppress the release of TGF-β in PHLF model
animals. We believe this is a novel discovery to
demonstrate the mechanism of how the PHLF with
fully developed TGF-β-dependent fibrosis can be
disrupted by a combination of BC and MSCs
application. We found that MSC has two mechanisms
of action. First, MSCs can decrease TGF-β levels by
depolarizing macrophage type I (M1) into
macrophage type II (M2) (Darlan et al., 2020).
Second, we suggest that MSCs affect
immunomodulatory mechanisms by releasing IL-10.
The receptor of Kupffer-IL-10 binding might activate
Janus tyrosine kinase 1 (JAK1) and tyrosine kinase-2,
leading to activation of signal transducer and
transcription 3 (STAT3), then translocated into the
nucleus to binds the promoters of target genes, the
suppressor of cytokine signalling 3 (SOCS3)
correlated with the decreased expression of tumour
necrosis factor (TNF)-α, IL-1β, and TGF-β (
Sziksz et
al., 2015). Our findings were in line with a previous
study that showed MSCs could prevent peritoneal
fibrosis by releasing IL-10 ( Muhar et al., 2018).
Our
finding MF cell produced TGFβ for autocrine to stay
active production collagen type III. MSC Group and
combine group has released immunomodulatory
properties, leading to the decreasing of TFGβ longer
day 7
th
in PHLF
In line with the decrease of TGFβ level, we also
found SGPT level decreasing just in the MSCs group
only on day 3
rd
. Phase inflammation on PHLF
disrupts hepatocyte cells because injury from liver
resection and stagnant portal venous blood stimulates
inflammation with collagen deposition on the portal
venous release by MF (
Golriz et al.,2015, Ray et
al.,2015). MSC has an effect immunomodulator and
enhances regeneration hepatocyte by release
secretome. Our previous study releases that
intraparenchymal of MSCs administration in the
damaged liver tissue leads to the MSC migration to
the injured areas for repairing and restoring the
damaged liver structure and its function. BC previous
study can decrease SGPT level by release anti-
oxidants in acute liver injury (Sinn et al., 2017).
We assume the combination group can not affect
inflammatory phase PHLF because BC has a product
growth factor pro-inflammation and disrupts
immunomodulator from MSC (
Quiles et al., 2006). In
repairing and restoring damaged tissue, MSCs should
previously control inflammation by releasing IL-10 to
inhibit the prolonged release of TGF, leading to
healing process acceleration (Putra et al., 2019).
Under prolonged controlled inflammation, MSC
induces decreased remnant liver destruction and
restored liver function (
Fiore, 2018). Taken together,
the single MSCs treatment more effective than the
combination of MSCs and BC to regenerating liver
tissues on PHLF.
5 CONCLUSIONS
We conclude that MSC and BC's combination is not
better than MSCs alone in decreasing TGFβ and
SGPT. We suggest further research about the
regeneration of the PHLF by examining marker
regeneration and portal flow using MSC to explain
the liver's healing process.
Conflict of Interest
The authors declare that they have no conflict of
interests.
ACKNOWLEDGMENTS
We acknowledge the Digestive Surgery Subspecialist
Program of Diponegoro University, and thank the
Stem Cell and Cancer Research (SCCR) Laboratory,
the medical faculty at Sultan Agung Islamic
University (UNISSULA), Semarang, and who
contributed to this research.
REFERENCES
Constandinouu C., Henderson N., Iredale JP. Modeling
liver fibrosis in rodent In: Varga J., Brenner DA., Phan
The Effectiveness of Mesenchymal Stem Cell and Colostrum Bovine Combination in Post Hepatectomy Liver Failure with Liver Fibrosis
Animal Model
309

SH. Fibrosis reseach method and protocols. Humana
press 2005: pp 237-49.
Darlan, D. M., Munir D., Putra A. Jusuf NV.' MSCs-
released TGFβ1 generate CD4+CD25+Foxp3+ in T-reg
cells of human SLE PBMC', Journal of the Formosan
Medical Association.2020, DOI:
10.1016/j.jfma.2020.06.028
Ding, H. R. Wang JL., Tang Z. Zhou WG., Liu Y., Ren HZ.,
Shi XL. 'Mesenchymal stem cells improve
glycometabolism and liver regeneration in the
treatment of post-hepatectomy liver failure', Frontiers
in Physiology,2019: 10(APR), pp. 1–13. doi:
10.3389/fphys.2019.00412.
Fiore, E. J.' Taking advantage of the potential of
mesenchymal stromal cells in liver regeneration: Cells
and extracellular vesicles as therapeutic strategies',
World Journal of Gastroenterology.2018 DOI:
10.3748/wjg.v24.i23.2427
Golriz M.,Majlesara A., Sakka SE., Asrafi M., Arwin j.,
Fard N., et al. Small for size and flow syndrome (
SFSS): An alternative description for hepatic liver
failure. Clinical research in hepatology and
gastroenterology. 2015. Available http :// dx
.doi.org/10.1016/ j. clinre.2015.06.024
Hoffmann, K. Nagel AJ., Tanabe K., Fuchs J., Dehlke K.,
Ghamarneja O., Lemekhova A., Mehrabi A. Markers of
liver regeneration - The role of growth factors and
cytokines: A systematic review'. 2020 BMC Surgery,
20(1), pp. 1–15. DOI: 10.1186/s12893-019-0664-8.
Hu, C., Wu, Z, and Li, L.‘Mesenchymal stromal cells
promote liver regeneration through regulation of
immune cells', International Journal of Biological
Sciences,2020: 16(5), pp. 893–903. DOI:
10.7150/ijbs.39725
Jang, Y. O. et al. (2014) 'Effect of bone marrow-derived
mesenchymal stem cells on hepatic fibrosis in a
thioacetamide-induced cirrhotic rat model', BMC
Gastroenterology, 14(1), pp. 1–12. DOI:
10.1186/s12876-014-0198-6.
Kim, W. R.Lake J R., Smith JM., Schladt DP., Skeans MA.,
Nooren SM. et al. 'OPTN/SRTR 2017 Annual Data
Report: Liver', American journal of transplantation :
official journal of the American Society of
Transplantation and the American Society of
Transplant Surgeons.2019: 19, pp. 184–283. DOI:
10.1111/ajt.15276.
Lv, F. J. Tuan RS., Cheung KM., Leung V.' Concise review:
The surface markers and identity of human
mesenchymal stem cells', Stem Cells. 2014 , DOI:
10.1002/stem.1681.
Muhar, A. M. Putra A., Warli SM., Munir D.' Hypoxia-
mesenchymal stem cells inhibit intra-peritoneal
adhesions formation by upregulation of the il-10
expression', Open Access Macedonian Journal of
Medical Sciences. DOI: 10.3889/oamjms.2019.713.
Putra, A.Rosdiana I., Darlan DM., Alif I., Hayungningtyas
F., Wijaya I., Aryanti R.et al. 'Mesenchymal stem cells
accelerate liver regeneration in acute liver failure
animal model', Biomedical Research and Therapy,
2020: 5(11), pp. 2802–2810. DOI:
10.15419/bmrat.v5i11.498.
Putra, A Pertiwi D. Milla MN., Indaryani UD, Jannah D.,
Sahariyani M., Trisnadi S., Wibowo JW. 'Hypoxia-
preconditioned MSCs have a superior effect in
ameliorating renal function on acute renal failure
animal model', Open Access Macedonian Journal of
Medical Sciences.2019 DOI:
10.3889/oamjms.2019.049
Quiles, J. L.Ochoa JJ., Tortosa M., Linde J., Bompadre S.,
Battino M., et al. 'Coenzyme Q concentration and total
anti-oxidant capacity of human milk at different stages
of lactation in mothers of preterm and full-term infants',
Free Radical Research.2006 DOI:
10.1080/10715760500404805
Ray, S. Mehta NN. Golhar A. Nundy S.' Post hepatectomy
liver failure –. A comprehensive review of current
concepts and controversies', Annals of Medicine and
Surgery. 2018: 34(2222), pp. 4–10. DOI:
10.1016/j.amsu.2018.08.012.
Sinn, D. H.Gwak GY., Kwon YJ., Paik SW. 'Anti-fibrotic
effect of bovine colostrum in carbon tetrachloride-
induced hepatic fibrosis', Precision and Future
Medicine, 2017 1(2), pp. 88–94. DOI:
10.23838/pfm.2017.00121
Sziksz, E. Pap D., Lippai R., Beres NR., Fekete A. Szabo
AJ., Vannay A.' Fibrosis Related Inflammatory
Mediators: Role of the IL-10 Cytokine Family',
Mediators of Inflammation. 2015 DOI:
10.1155/2015/764641.
Wiliam R., Janagin. Hepatic resection: general
consideration.In: Jarnagin W., Allen W., D'angelica
M., Matteo R. Gian R. Vauthey editors. Surgery Liver,
Biliary tract, and Pancreas 6 th edition Vol 1
Philadelpia Elsevier 2017 ;1520-21
Xia S Y., Lu L., Wang HL. Fibrosis cholestasis hepatitis:
clinicopathologic spectrum, diagnosis, and
pathogenesis. Int J Clin Exp Pathol. 2008 (1) pp. 396-
402
Yoo, S. W. Chang DY., Lee HS., Kim GH, Park JS., Ryu
BY. et al. 'Immune following suppression
mesenchymal stem cell transplantation in the ischemic
brain is mediated by TGF-β', Neurobiology of Disease.
2013, DOI: 10.1016/j.nbd.2013.06.001.
Zhang, Y.Guan SB., Li XM., Gu W., Xu Wei. 'Bone
Marrow Mesenchymal Stem Cells Inhibit the Function
of Dendritic Cells by Secreting Galectin-1', BioMed
Research International, 2017. DOI:
10.1155/2017/3248605.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
310
