
Secretion of IFN-γ and IL-17 after Stimulation of ESAT-6-CFP10
(EC610) Fusion Antigen from PBMC in Groups Active TB and
Latent TB
Nika Andriani
1a
, Nova Kurniati
2b
, Muhammad Irsan Saleh
3c
, Eddy Mart Salim
2d
,
Zen Hafy
4e
, Jusak Nugraha
5 f,
, Kemas Ya’kub Rahadiyanto
6g
,
FranciscaSrioetamiTanoerahardjo
7h
1
Master Program in Biomedical Sciences, Medical Faculty, Sriwijaya University, Palembang, Indonesia
2
Department of Internal Medicine, Medical Faculty, Sriwijaya University, Palembang, Indonesia
3
Department of Pharmacology, Medical Faculty, Sriwijaya University, Palembang, Indonesia
4
Department of Histology, Medical Faculty,Sriwijaya University, Palembang, Indonesia
5
Department of Clinical Pathology, Medical Faculty, Airlangga University, Surabaya, Indonesia
6
Department of Clinical Pathology, Medical Faculty, Sriwijaya University, Palembang, Indonesia
7
Consultant of Molecular and Microbiology Laboratory in TB Research, Center for Biomedical and Basic Health
Keywords: Tuberculosis, IL-17,PBMC, EC610 Antigen
Abstract: Tuberculosis is an infectious disease that is transmitted by the bacteria Mycobacterium tuberculosis. The
immune system has an important role in the pathogenesis of TB. The protective response to TB involves the
secretion of proinflammatory cytokines, namely Th1 cells that produce IFN-γ and Th 17 which produce IL-
17, which plays a very important role in the body's defense system, especially in dealing with intracellular
bacterial infections. The EC610 fusion antigen is a specific M.TB antigen which has antigenicity to T cells so
that T cells secrete cytokines. The study aimed to determine the immune response of proinflammatory
cytokines against Mycobacterium tuberculosis infection by looking at the secretion of IFN-γ and IL-17 levels
after stimulation of the ESAT 6-CFP 10 (EC610) fusion antigen in active and latent TB patients. This type of
research was a quasi experimental in vitro. The research was conducted at the Palembang Lung Special
Hospital. The research subjects were 21 samples of active TB and 28 samples of latent TB. PBMC blood
samples were isolated using Ficoll-Paque, induced with ESAT-6 - CFP-10 Fusion Antigen (EC610) for 24 -
72 hours at 37 ° C. IFN-γ and IL-17 were measured by ELISA Reader. Analysis used the Mann Whitney test
ρ <0.05. IFN-γ levels and IL-17 levels in active TB were higher than latent TB, but statistically there was no
significant difference between IFN-levels (ρ = 0.769) and IL-17 levels with a value of ρ = 0.000, meaning
that there was a significant difference between both groups. The cut-off points were IFN-γ (6850 pg / mL)
and IL-17 (85 pg / mL) using Receiver Operating Curve (ROC) curve analysis. As a conclusions, IFN levels
were not different and IL-17 levels were different. This shows that IL-17 levels play a role in the protective
immune response against Mycobacterium tuberculosis during the progression of TB disease.
a
https://orcid.org/0000-0002-2588-0556
b
https://orcid.org/0000-0002-1520-7421
c
https://orcid.org/0000-0003-4788-8409
d
https://orcid.org/0000-0002-5654-0757
e
https://orcid.org/0000-0001-9682-2591
f
https://orcid.org/0000-0001-6700-9921
g
https://orcid.org/0000-0001-9557-467X
h
https://orcid.org/0000-0001-6948-0645
Andriani, N., Kurniati, N., Saleh, M., Salim, E., Hafy, Z., Nugraha, J., Rahadiyanto, K. and Tanoerahardjo, F.
Secretion of IFN- and IL-17 after Stimulation of ESAT-6-CFP10 (EC610) Fusion Antigen from PBMC in Groups Active TB and Latent TB.
DOI: 10.5220/0010491602890297
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 289-297
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
289

1 INTRODUCTION
Tuberculosis is an infectious disease that is
transmitted by the bacteria Mycobacterium
Tuberculosis which attacks various organs, especially
the lungs. Tuberculosis transmission occurs through
droplets of patients infected with the bacteria
Mycobacterium tuberculosis (Ministry of Health of
the Republic of Indonesia,2014).
Tuberculosis is a health problem that is of global
concern today. Indonesia is a country with the second
highest number of new cases in the world after India.
The number of new cases of TB BTA + in Indonesia
was 156,723 with the distribution of cases in several
provinces, especially in South Sumatra, the number
of new cases of TB BTA + was 5674 cases (Ministry
of Health of the Republic of Indonesia, 2017).
According to the Palembang Health Office in
2015, the number of tuberculosis cases in Palembang
was 1305 from the total population. Based on gender,
the number of cases in males is higher than in
females. Based on the age group, most tuberculosis
cases were found at the age of 25-34 years at 18.07%,
age 45-54 years at 17.25% and age 35-44 years at
16.81% (Ministry of Health of the Republic of
Indonesia, 2016).
At this time, experts suspect that there is an
immune system disorder in tuberculosis sufferers.
Helper-1 (Th1) cells play a very important role in the
body's defense system, especially in dealing with
intracellular bacterial infections. One of the cytokines
produced by Th1 cells is IFN-γ which plays an
important role in eliminating Mycobacterium
tuberculosis. IFN-γ serves to strengthen the potential
of phagocytes from macrophages infected with
Mycobacterium tuberculosis by stimulating the
formation of phagolysosomes. IFN-γ also stimulates
the formation of free radicals to destroy bacterial
components Mycobacterium tuberculosis, namely
DNA and bacterial cell walls(Widjaja J.T et al, 2010)
Recent studies have shown that IL-17 plays an
important role in the initial immune response against
Mycobacterium tuberculosis infection by forming
granulomas. Interleukin 17 (IL-17) is a pro-
inflammatory cytokine produced by Th 17 which has
an important role in the pathogenesis of TB. IL-17 is
important for modulator of inflammation and recall
memory response. The role of IL-17 as a
proinflammatory cytokine can recruit neutrophils and
induce an optimal Th1 response to stimulate IFN-γ
production and stimulate chemokines. However,
IFN-γ has the effect to suppress IL-17(Saraiva and
O’Garra, 2010; Javan et al, 2016).
Research over the last decade has resulted in the
development of Interferon-gamma realease assays
(IGRA) to detect Mycobacterium tuberculosis
infection. Based on the principle that individual T
cells that have TB infection can respond to re-
stimulation with the specific antigen Mycobacterium
tuberculosis. This test measures the production of
cytokines secreted by T lymphocytes that have been
sensitized by the specific antigen Mycobacterium
tuberculosis(CDC, 2011). The Food and Drug
Administration (FDA) has approved two IGRA
testing techniques, QuantiFERON-TB and T-
SPOT.TB to detect Mtb infection (Pai M et al., 2014).
QuantiFERON (QFT) is a measurement of IFN-γ
secreted from T cells previously exposed to
Mycobacterium tuberculosis when stimulated in vitro
with specific antigen Mycobacterium tuberculosis
ESAT-6, CFP-10 and TB 7.7 using the enzyme-
linked immunosorbent assay (ELISA) method. This
test is used to detect the amount of IFN-γ against a
specific antigen produced from the subject's T cells
exposed to M. tuberculosis with using a peptide
cocktail that simulates the proteins ESAT-6, CFP-10
and TB7.7. Antigen exposure generates an immune
response to aid screening for Latent TB (Pratomo and
Setyanto, 2013)
The T-SPOT.TB test is an in vitro diagnostic test
based on the enzyme-linked immunospot (ELISPOT)
method. This test is used to count the number of
effector T cells that respond to stimuli with a
combination of peptides that stimulate ESAT-6 and
CFP10 antigens. The immune response to
Mycobacterium tuberculosis infection is mainly
mediated through T cell activation. Activation of T
cells will fight Mycobacterium tuberculosis both CD4
+ and CD8 + which produce several cytokines
including IFN-γ and IL-17 after stimulated by antigen
ESAT-6 and CFP10. Peripheral blood mononuclear
cells (PBMC) were separated from whole blood,
washed and counted before adding to assay. Isolated
PBMCs (white blood cells) are placed into microtiter
orifices where they are exposed to
phytohemagglutinin (PHA) control (a mitogenic
stimulator that demonstrates cell function), nil
control, and tuberculosis-specific antigen. PBMCs
are incubated with antigens to allow stimulation of
sensitized T cells to produce cytokines(Oxford
Imunotec,2017).
A study conducted by Eunkyoung et al, 2016.On
patients with active TB and latent TB infection by
detecting whole blood levels of IFN-γ with IL-17 in
study subjects with TB before receiving treatment
using the QuantiFERON method found that IFN-γ
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
290

and IL-17 were lower in active TB patients rather than
latent TB.
Another study using T-SPOT (ELISPOT) in vitro
conducted by Cowan J et al with research subjects TB
patients with new cases before receiving OAT
treatment found that there was a significant increase
in IFN-γ and IL-17 levels in active TB patients and
latent in PBMC. Research conducted by Marin ND
using TB patients who were given OAT in the first 2
weeks stated that the levels of IFN-γ and IL 17 in
active TB patients were higher than latent TB by
ELISPOT (enzyme-linked immunospot) method in
PBMC.
Based on previous research regarding the
measurement of cytokine secretion, the researchers
were interested in measuring the secretion of
proinflammatory cytokines in vitro, namely IFN-γ
and IL-17 after stimulation of the ESAT 6 - CFP 10
(EC610) fusion antigen in PBMC groups of active TB
and latent TB. Measurement of cytokines using the T-
SPOT and QuantiFERON methods in this study was
used for screening latent TB. Research using PBMC
is still limited, especially in Indonesia, so this
research is expected to have a novelty in evaluating
the pathogenesis of tuberculosis, especially to see the
immune response to Mycobacterium tuberculosis
infection.
2 MATERIAL AND METHODS
This type of research is a quasi experimental study or
quasi experimental study in vitro with a non-
equivalent post test only design. The research was
conducted at the Palembang Lung Special Hospital in
the period August 2018 - January 2019 This research
has received a certificate of ethical approval from
MoehammadHoesin Hospital and Sriwijaya
University Medical Faculty.
The number of research samples was divided into
two groups, namely the first group of latent TB came
from nurses in Palembang Paru Hospital without
clinical symptoms of TB who served more than 6
months in the outpatient and inpatient unit of
Palembang City Lung Hospital who had direct
contact with pulmonary TB patients, TST
examination. positive with induration> 10 mm and
negative smear examination and or radiological
examination did not show lung abnormalities
(normal). The second group of active TB was
diagnosed by pulmonary specialist doctors at the
Palembang City Lung Hospital as new TB cases,
there were BTA examination results, radiological
examinations / chest X-rays showing a picture of
active TB and anti-tuberculosis drug therapy (OAT)
for less than 1 month. The research subjects were
selected by purposive sampling. Pulmonary TB
patients receiving corticosteroid therapy or
immunosuppressant drugs with complaints of
respiratory infections such as bronchitis and allergies,
suffering from liver disorders, kidney disorders,
diabetes mellitus, hepatitis B and HIV infection were
not included in the study (exclusion criteria).
The study sample was a supernatant after
stimulation of the ESAT-6-CFP 10 antigen (EC610)
with the following research stages as a 16 ml venous
blood sample and put into five heparin anticoagulant
tubes. Then one tube of the sample was treated with
stimulation of the ESAT-6-CFP 10 antigen contained
in TB 1 and TB 2 tubes which were incubated for 16-
24 hours using the QuantiFERON method. Four
sample tubes were isolated by Peripheral Blood
Mononuclear Cells (PBMC) using Ficoll-Paque and
induced with ESAT-6 - CFP-10 Fusion Antigen for
24 - 72 hours using the T-SPOT method. Then the
levels of IFN-γ and IL-17 were checked with an
ELISA reader. The difference in mean levels of IFN-
γ and IL-17 was analyzed statistically by the Mann-
Whitney test with a significance level (ρ <0.05). To
get the cut-off-point, the Receiver Operating Curve
(ROC) curve analysis will be used.
3 RESULTS
The research subjects were 49 people who met the
inclusion criteria of the researcher. The research
subjects were divided into 2 patients, namely active
TB and latent TB. Active TB was 21 new TB patients
collected since January 2018, while 28 people with
latent TB had contact history of TB patients
consisting of 23 nurses and 5 family of patients. who
has TB.
The characteristics of the research subjects
included gender, age, education, BCG status, BMI,
chest X-ray, QFT results, and laboratory experiment
results were depicted inTable 1 and Table 2.
The latent TB group, as many as 28 people were
tested for TST and IGRA which were used to screen
for the LatentTB group. Obtained TST induration
varied. The results of TST induration in the Latent TB
group can be seen in the Table 3 and IGRA using
QuanTIFERON in the Latent TB Group can be seen
in the Table 4.
The value of ρ <0.05 was 0.000, which means that
there was a significant difference between IL-17
levels between the two groups after stimulation with
EC610 Fusion Antigen.
Secretion of IFN- and IL-17 after Stimulation of ESAT-6-CFP10 (EC610) Fusion Antigen from PBMC in Groups Active TB and Latent TB
291
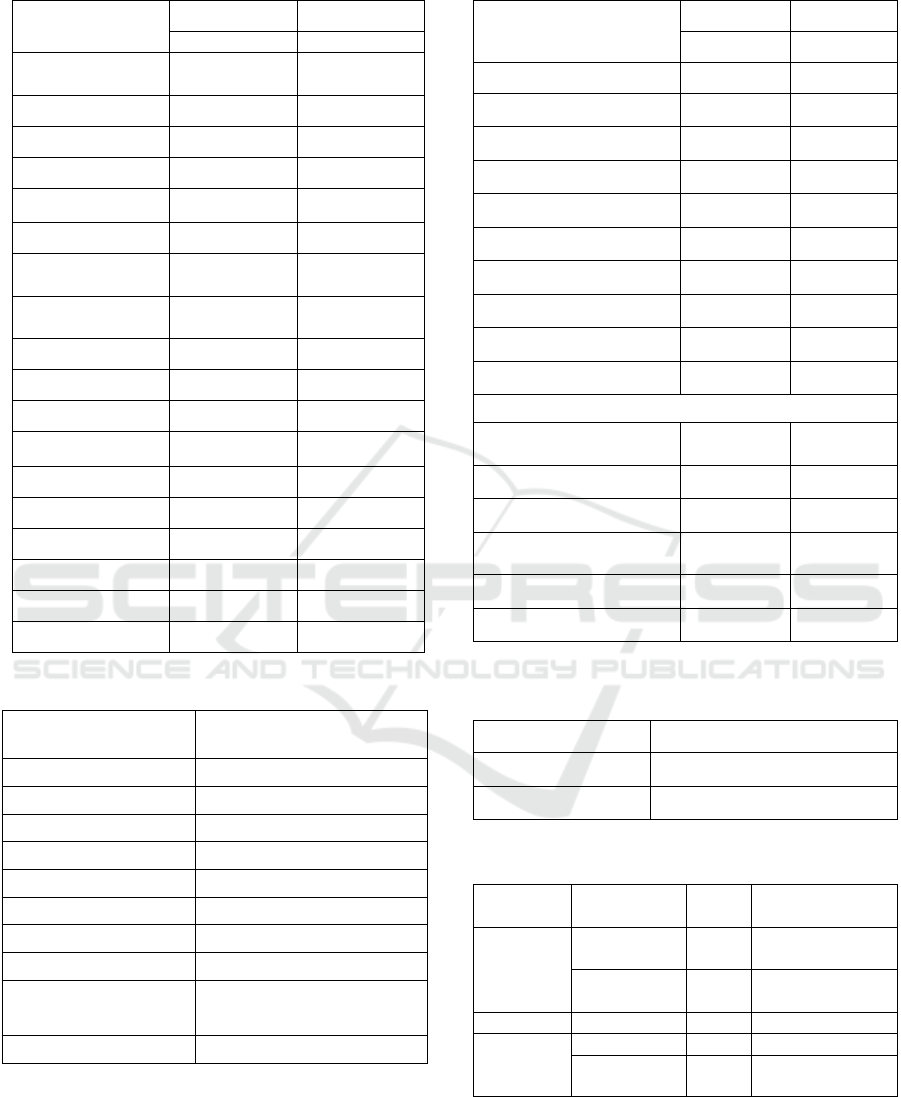
Table 1. Demographic Data of Research Subjects
Characteristics Active TB Latent TB
n (%) n (%)
Number of
Sub
j
ects
21 (100) 28 (100)
Gender
Women
13 (61,90) 21 (75,0)
Male
8 (38,10) 7 (25,0)
39,10±10,4 36,32±8,773
Junior High
School
3 (14,3) 0 (0,0)
Senior High
School
12 (57,1) 7 (25,0)
Diploma 2 (9,5) 12 (42,9)
S1 4 (19,0) 9 (32,1)
BCG status
Yes 14 (66,7) 20 (71,4)
No 1 (4,8) 0 (0,0)
Unknown 6 (28,6) 8 (28,6)
IMT
Normal 11 (52,4) 19 (67,9)
Fat 1 (4,8) 4 (14,3)
Obesity 0 (0,0) 3 (10,7)
Table 3. Results of TST Induration in the Latent TB Group
Diameter of
Induration TST
(
mm
)
n (%)
10 9
(
32.1
)
11 1(3.6)
12 3(10.7)
15 3(10.7)
16 3
(
10.7
)
18 2
(
7.1
)
20 3
(
10.7
)
21 1(3.6)
22 2(7.1)
27 1(3.6)
Total 28
(
100.0
)
Table2. Laboratory Data
Characteristics
TB Active TB Laten
n (%) n (%)
Thoracic Photo
Normal
0 (0,0) 28 (100)
Minimal lesions
15 (71,4) 0 (0,0)
Moderate lesions
6 (28,6) 0 (0,0)
BTA examination
Ne
g
ative
6 (28,6) 28 (100)
Positive
15 (71,4) 0
BTA +1
13 (61,9)
BTA +2
1 (4,8)
BTA +3
1 (4,8)
Qualitative Examination Results
IFN-γ results
T-SPOT
Ne
g
ative
4 (19.0) 22 (78.6)
Positive
17 (81.0) 6 (21.4)
IL-17 results
T-SPOT
Ne
g
ative
3 (14.3) 24 (85.7)
Positive
18 (85.7) 4 (14.3)
Table 4. IGRA (QuantiFERON) examination results in the
Latent TB Group
Result of QFT
n (%)
Negative
15 (53,6)
Positive
13 (46,4)
Table 5. IFN-γ and IL-17 levels in patients with Active TB
and Latent TB
IFN-γ ClinicalStatus N Median
(
Min-Max
)
EC610
Fusion
Antigen
Active TB 21 6700
(2000-9100)
Latent TB 28 6000
(2500-9000)
IL-17
EC610
Fusion
Antigen
Active TB 21 160 (80-210)
Latent TB 28 60 (40-90)
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
292
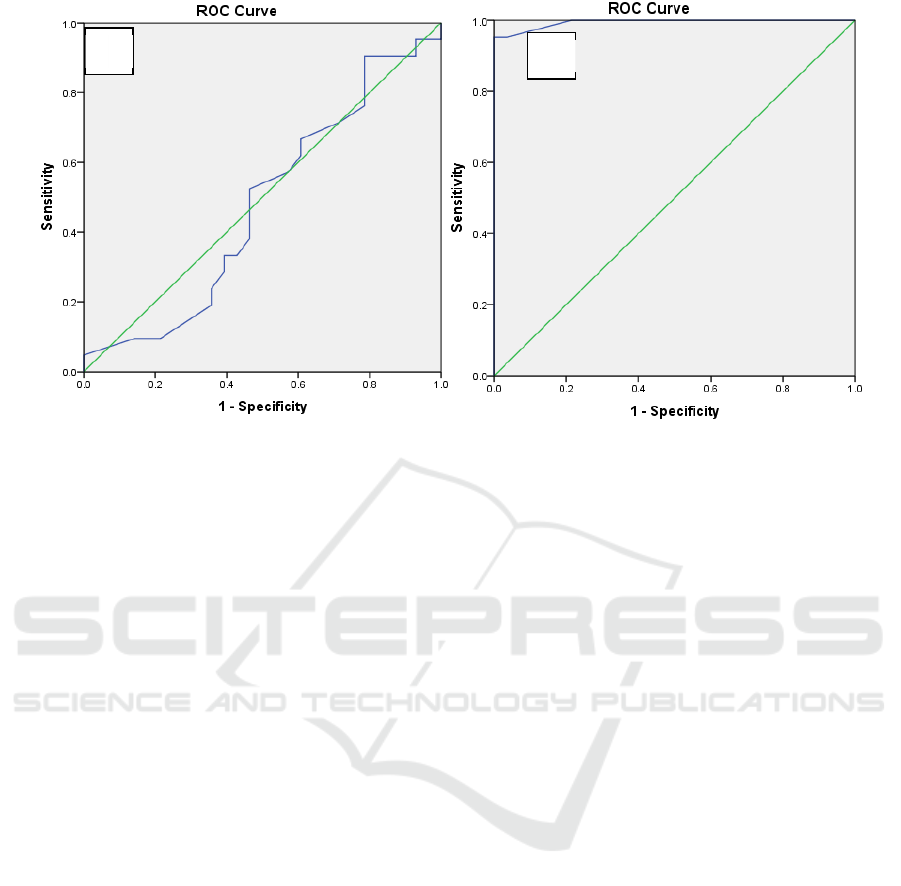
Figure 1. Analysis of the ROC curveCurve IFN-γ (A)and CurveIL-17 (B) using cut-off-point IFN-γ 6850 pg / mL and cut-
off-point IL-17 85 pg / mL.
The sensitivity value of IFN-γ levels was 80.95%
with a specificity of 78.57%. Postive Predictive Value
(PPV) of 98.63%. Negative Predictive Value (NPV)
of 17.84%. Meanwhile, the sensitivity value of IL-17
levels was 85.71% with a specificity of 85.71%.
Postive Predictive Value (PPV) of 85.71%. Negative
Predictive Value (NPV) of 85.71%.
Table 5 informs that IFN-γ levels were higher in
active TB, namely 6700 (2000-9100) pg / mL while
latent TB was 6000 (2500-9000) pg / mL but
statistically there was no significant difference
between the two groups after stimulation of Fusion
Antigen EC610 with a value of ρ> 0.05, namely
0.769.IL-17 levels were higher in active TB, namely
160 (80-210) pg / mL and latent TB 60 (40-90) pg /
mL.
The area under the ROC curve of IFNγ levels to
predict active TB and latent TB is 0.475 (95% CI =
0.311 to 0.639) while the IL-17 level to predict active
TB and latent TB is 0.994 (95% CI = 0.980 s / d
1,000) (Figure 1). The ROC curve also shows that the
IFNγ level has a very low diagnostic value while the
IL-17 level is categorized as <85pg / mL and> 85 pg
/ mL has a good diagnostic value because the curve
moves away from the 50% line and approaches the
100% line. This suggests that IL-17 levels can be used
as a predictor or diagnosis of active TB and latent TB.
4 DISCUSSION
The proportion of subjects in this study based on
gender was found to be more women than men in
active TB and latent TB. As for several factors,
namely social and economic factors (financial
conditions) that cause women of childbearing age to
suffer from tuberculosis (TB) are more common,
these are found in Afghanistan, Pakistan and
Iran(Dotulong J.F.J et al, 2015)
The active TB and latent TB groups in this study
were found in the age range between 25-36 years.
This result is in accordance with the provisions of the
Ministry of Health of the Republic of Indonesia,
which is mostly found in the 25-34 year age group.
productive age is very dangerous to the level of
transmission because patients easily interact with
other people, high mobility allows it to be transmitted
to other people and the environment. In this study,
there were in the range of 25-36 years of age who
were classified as having a regular job every day
outside the home, especially the latent TB group
because they were nurses / hospital staff. This makes
it easy for patients to interact with other people,
thereby increasing the risk of contracting TB
(Dotulong J.F.J et al, 2015)
Based on the level of education in this study, the
most active TB was obtained with a high school
background while latent TB with a recent diploma
education. Higher education does not always behave
A
B
Secretion of IFN- and IL-17 after Stimulation of ESAT-6-CFP10 (EC610) Fusion Antigen from PBMC in Groups Active TB and Latent TB
293

well. Therefore education is not an indicator of
healthy life behavior (Marieta K.S, 2014).
Most of the patients with Active TB and Latent
TB have received BCG immunization when they
were children. However, BCG immunization does not
fully protect children from tuberculosis attacks. The
factors that cause someone to be infected with TB
include household contact with tuberculosis patients,
low nutritional status so that the body's immune
system is not optimal, high humidity that makes
tuberculosis bacteria thrive and an unclean
environment(Rachim R, 2014).
Body Mass Index (BMI) is a way of directly
assessing nutritional status using height and weight.
In this study most were found in BMI which was
classified as normal. The increase in BMI in TB
patients is a good marker of decreasing the likelihood
of relapse (relapse) from TB infection and a sign that
the TB infection process is reduced (Priyantomoet al
, 2014).
In this study all latent TB with TST induration ≥
10 mm (positive). The tuberculin test is done to find
out whether a person has immunity to TB bacilli or
not so it is very good for detecting TB infection. If the
tuberculin test result is positive or abnormal, it means
that the person is infected with TB bacilli and there
are antibodies to the TB bacilli that can become
active. Positive tuberculin test results should be
confirmed by chest X-ray and sputum examination. If
the chest X-ray is normal, then latent TB therapy can
be done, but if the chest X-ray is abnormal and shows
TB, it can be included in active Mycobacterium
tuberculosis (Kenyorini et al,. 2012).
Chest X-ray laboratory data shows abnormal
results of active pulmonary TB which is common
with minimal lesions and normal latent TB. This is
related to one's immunity and the virulence of
Mycobacterium tuberculosis. The lower the
immunity and virulence of a person against
Mycobacterium tuberculosis, the more damage there
is to the radiological image (chest X-ray) (Afif E et al
, 2013).
In this research, BTA examination was mostly
found in BTA +1. There are several factors that affect
the results of the sputum BTA examination including
too few germs due to sputum extraction (not
according to operational procedures (SOP), methods
and methods of examination that are not in
accordance with the SOP, and the effect of anti-
tuberculosis drug treatment. Coughing is effective in
removing sputum can help find BTA germs on
sputum examination in the pulmonary TB group (Ors
et al.,2007)
Two commercial kits that can be used to test for
M. Tuberculosis are QuantiFERON and T-SPOT.
The basic principle of this examination is that the
cells produced by TCD4 lymphocytes if these cells
are incubated with M. Tuberculosis antigen when the
examination uses QuantiFERON, whereas when
using T-SPOT, the number of spots formed in the
membrane is calculated which indicates the presence
of IFN-producing TCD4 lymphocytes. states that this
examination cannot distinguish between active and
latent TB infections so that in order to be able to
diagnose TB in addition to the results of the IGRA
examination, it still takes into account the clinical
situation, laboratory results and other examinations
(CDC, 2010).
Where in this study the latent TB group was
declared if the TST or IGRA results were positive,
both (TST and IGRA) were positive. QuantiFERON
in this study was used for screening latent TB. In
measuring the levels of IFN and IL-17 in this study
using T-SPOT. This QFT and T-SPOT examination
must be accompanied by other supporting
examinations such as Latent TB, TST examination
and active TB must be BTA examination and chest
X-ray to detect TB. QFT and T-SPOT are indirect
markers of M. tuberculosis exposure and indicate a
cellular immune response to M. tuberculosis.
4.1 Secretion of IFN-γ after EC610
Stimulation in Active TB and
Latent TB
IFN-γ is a pro-inflammatory cytokine produced by
activated T cells due to an immune response to a
specific antigen stimulus. These cytokines play an
important role in the activation of macrophages to
eliminate Mycobacterium tuberculosis by recruiting
phagocytic cells to eliminate Mycobacterium
tuberculosis. IFN-γ strengthens the phagocyte
potency of macrophages by stimulating
phagolisosome fusion which can destroy M.
tuberculosis bacteria(Widjaja JT et al, 2010;
Wahyuniati N, 2017)
In this study, IFN-γ levels after stimulation with
EC-610 antigen were higher in patients with active
TB than latent TB but statistically there was no
significant difference between the two groups.. These
results are in line with Setiawan and Nugraha that
active TB levels of IFN-γ were higher than latent TB
but there was no statistically significant difference.
IFN-γ levels are higher because there is a protective
immune response against infection with TB germs.
And the results of IFN-γ levels showed no significant
difference between active TB and latent TB. This is
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
294

due to the nutritional status of active TB sufferers
who tend to be malnourished so that the immune
response does not function optimally. Patients who
have been diagnosed with TB but did not immediately
seek anti-TB treatment. This condition results in a
decrease in immune response, so that the IFN-γ level
is not too high (Setiawan H and Nugraha J, 2016
). The
development of recent research is that IFN-can induce
the autophagy mechanism in cells infected with
mycobacteria. The induction of autophagy will
deliver mycobacteria into the lysosome and there will
be a phagolysosome fusion which functions as an
anti-microbial so that the bacteria will be killed
(Wahyuniati N, 2017 )
The results of this study differ from previous
studies possibly because the sample in this study were
new TB patients who had received OAT <1 month
where the inflammatory response was still increasing
in the early phase of TB infection. When the initial
TB infection, the immune system will respond by
carrying out an inflammatory reaction that occurs
within 2-10 weeks after exposure to bacteria by
forming a body defense system called granuloma,
thusrecruiting immune cells to eliminate bacteria by
increasing the production of proinflammatory
cytokines. TB patients who consume OAT <1 month,
the possibility of developing M. tuberculosis bacteria
will decrease because the patient's immune system
increases so that macrophage activation occurs which
is marked by increased production of cytokines. The
increase in proinflammatory cytokines, namely the
levels of IFN-γ and IL-17 can also occur due to the
influence of memory T cells that have been
previously described (in vivo) and then stimulated
with a more specific EC610 antigen so that the
expression of IFN-IL and IL-17 levels increases. The
effect of the strength of antigen presentation is
thought to determine the antigenity that affects
stimulation of T cell proliferation and cytokine
production(Wibowo R.Y et al.,2017).
Low IFN-γ levels in latent TB are due to the
reduced number of effector memory T-cells secreting
IFN-in individuals with latent TB infection, due to the
absence of M. tuberculosis replication and antigen
load. This suggests that IFN-γ secreting T-cells
predominatse during active TB disease (Biselli R et
al.,2010)
4.2 Secretion of IL-17 after EC610
Stimulation in Active TB and
Latent TB
IL-17 is a pro-inflammatory cytokine that plays a role
in the pathogenesis of TB. The role of IL-17, among
others, is to induce an optimal Th1 response and form
granulomas, which are protective immunity against
MTB infection. IL-17 also plays a role in attracting
and activating neutrophils (Torado E and Cooper
M.A, 2010).
IL-17 levels in this study in patients with active
TB and latent TB showed a significant difference in
the two groups where the IL-17 levels in active TB
were higher than latent TB. This study is in line with
Luo et al. Stated that IL-17 levels were increased in
active TB compared to latent TB. In the early phase
of active TB, Th17 will produce IL-17 which will
recruit neutrophils to the infected site. In addition, as
TB disease progresses, there is an increase in the
production of IL-17 in peripheral blood for
aprotective immune response against M.Tuberculosis
(Luo et al., 2017)
Based on the results of previous studies, an
increase in IL-17 levels in TB patients stimulated
with an antigen increased IL-17 production, in
response to M. tuberculosis. IL-17 plays a significant
role in the protection induced by the ESAT-6 antigen.
However IL-17 production in the lungs is generally
immunosuppressive to IFN-γ. Thus, T cells in the
presence of ESAT-6 reduce the proliferation and
production of Th1 cytokines, but increase IL-17
production. ESAT-6 plays a role in changing the
function of T cells to suppress protective immunity
and eliciting a potential immunopathological
response. During tuberculosis, IL-17 is a strong
inflammatory cytokine capable of inducing
chemokine expression that promotes cell recruitment
and granuloma formation during infection. A balance
between Th1 and Th17 responses is needed to control
bacterial growth and limit immunopathology (Wang
X et al, 2013). In this study, there was a shift in the
response towards the excessive production of IL-17
which could lead to the recruitment of large numbers
of neutrophils and tissue damage. Thus, regulation of
Th1 and Th17 responses during tuberculosis is
essential for enhancing anti-mycobacterial immunity
and preventing widespread immunopathology. In this
study, T cells inhibited and suppressed the
proliferation of IFN-γ production. It can be seen from
the mean increase in IFN-levels which were not too
high and statistically insignificant. IL-17 as an initial
response to M. tuberculosis plays a role in granuloma
formation and controlling bacterial growth.
Therefore, the role of IL-17-secreting cells during
active TB patient disease represents the highest
proportion of T lymphocytes to produce IL-17 to the
site of infection ( Jurado et al.,2012).
By using the ROC curve, it can be seen that IFN-
γ levels have very low values to predict or diagnose
Secretion of IFN- and IL-17 after Stimulation of ESAT-6-CFP10 (EC610) Fusion Antigen from PBMC in Groups Active TB and Latent TB
295

active TB and latent TB. Another study by Khan et al
(2013) based on the ROC analysis of IFN-γ levels can
be used for tuberculosis biomarkers
The level of IL-17 in this study has a very good
value for predicting or diagnosing active TB and
latent TB because the curve moves away from the
50% line and approaches 100%. This suggests that
IL-17 levels can be used as a predictor or diagnosis of
active TB and latent TB. This is in accordance with
Seyedhosseini et al., (2019) from the area under curve
(AUC) value, it is known that IL-17 is more specific
in differentiating TB infection (Seyedhosseiniet
al.,2019)
Based on the analysis of the ROC curve and the
results of the study, IL-17 levels can be used to
diagnose active TB and latent TB, but must be
investigated for TB because the cytokine IL-17 is not
only in tuberculosis but can occur in other
inflammatory reactions.
5 CONCLUSION
IFN-γ levels and IL-17 levels after stimulation of
ESAT-6-CFP-10Fusion Antigen (EC610) were
higher in active TB than in latent TB. But statistically,
there was no difference in the meaning of IFN-γ
levels in the two groups and IL-17 levels were
significantly different in the two groups.
This shows that IL-17 levels play a role in the
protective immune response against Mycobacterium
tuberculosis during the progression of TB disease.
The author's suggestion for further research can
see the effect of the duration of use of OAT treatment
with the secretion pattern of IFN-γ and IL-17
stimulated by EC610 and compare active TB and
latent TB with healthy groups.
ACKNOWLEDGEMENTS
Thanks to Dr.dr. Francisca SrioetamiTanoehardjo,
SpPK., M.Si at the National Health Research and
Development Center, Kemenkes RI, Jakarta for her
support and assistance in procuring the EC610 fusion
antigen
REFERENCES
Biselli, R., Mariotti, S., Sargentini,V., Sauzullo, I., Lastilla,
M., Mengoni, F., et al. 2010. Detection of interleukin-2
in addition to interferon-c discriminates active
tuberculosis patients, latently infected individuals, and
controls. Journal Compilation: European Society of
Clinical Microbiology and Infectious Disease.,, 16 (8),
1282-1284. doi: 10.1111/j.1469-0691.2009.03104.x
Centers for Disease Control and Prevention.2010. Updated
Guidelines for Using Interferon Gamma Release
Assays to Detect Mycobacterium tuberculosis
Infection — United States. Department Of Health And
Human Services:CDC., pp, 1-21
Cowan, J., Pandey, S., Fillion, LG., Angel, J.B., Kumar, A.,
Cameron, D., 2011. Comparison of interferon-g-,
interleukin (IL)-17- and IL-22-expressing CD4 T cells,
IL-22-expressing granulocytes and proinflammatory
cytokines during latent and active tuberculosis
infection. Clin Exp Immunol., 167(2), 317–329. doi:
10.1111/j.1365-2249.2011.04520.x
Dotulong, JFJ., Sapulete, MR., and Kandou G., 2015. Isk
factors for age, gender and occupancy density with the
incidence of pulmonary tuberculosis in Wori Village,
Wori District. Journal of Community and Tropical
Medicine.,3(2): 60-62.
Eunkyoung you., Kim, M.H., Lee, W.I., Kang Y.2016.
Evaluation of IL-2, IL-10, IL-17 and IP-10 as potent
discriminative markers for active tuberculosis among
pulmonary tuberculosis suspects.J
Tuberculosis.,99,100-108.
doi:10.1016/j.tube.2016.04.009
Javan, M.R., Nezhad, A.A.L., Shahraki, S., Safaf, A., Aali,
H., Kiani, Z., 2016. Cross-talk between the Immune
System and Tuberculosis Pathogenesis; a Review with
Emphasis on the Immune Based Treatment.
International Journal of Basic Science in Medicine.,
1(2), 40-47.doi:10.15171/ijbsm.2016.10
Jurado, J.O., Pasquinelli, V., Alvarez, I.B., Pena,
D., Rovetta, A.I., Tateosian, N.L., et al. 2012. IL-17
and IFN-γ expression in lymphocytes from patients
with active tuberculosis correlates with the severity of
the disease.J Leukoc Biol., 91(6), 991–1002.doi:
10.1189/jlb.1211619.
Ministry of Health of the Republic of Indonesia. 2014.
Indonesia health profile year 2013. ISBN: 978-602-
235-645-5. pp:127-132. Available from:
https://www.kemkes.go.id/folder/view/01/structure-
publikasi-pusdatin-profil-kesehatan.html. Acessed: 2
March 2018.
Ministry of Health of the Republic of Indonesia. 2016.
Indonesia health profile year 2015. pp: ISBN 978-602-
416-065-4. pp: 160-167. Available from:
https://www.kemkes.go.id/folder/view/01/structure-
publikasi-pusdatin-profil-kesehatan.html. Acessed: 2
March 2018.
Ministry of Health of the Republic of Indonesia. 2017.
Indonesia health profile year 2016. ISBN:978-602-416-
253-5. pp: 153-158. Available
from:https://www.kemkes.go.id/folder/view/01/structur
e-publikasi-pusdatin-profil-kesehatan.html. Acessed: 2
March 2018.
Kenyorini., Suradi., Surjanto, E., 2012. Tuberculin test.
Journal of Tuberculosis., 3(2),1-5.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
296

Khan,
F.Y., Hamza, M., Omran, A.H., Saleh,
M., Lingawi, M., Alnaqdy, A., et al., 2013. Diagnostic
value of pleural fluid interferon-gamma and adenosine
deaminase in patients with pleural tuberculosis in
Qatar. Int J Gen Med., 6(13),13-18. doi:
10.2147/IJGM.S39345.
Luo, J., Zhang, M., Yan, B., Zhang, K., Chen, M., Deng,S.,
2017. Imbalance of Th17 and Treg in peripheral blood
mononuclear cells of active tuberculosis patients.
Journal of Infectious Diseases.,21(2),155-161.
doi:10.1016/j.bjid.2016.10.011.
Marieta, K.S., 2014. The Relationship between Education
Level and Knowledge of Pulmonary TB Patients with
Sputum Disposal Behavior in PuskesmasRewarangga,
Ende Timur District, Ende Regency. Journal of Health.
12(1),1-7. doi: 10.31965/infokes.v12i1.39.
Marin, N.D., Paris, S.C., Velez, V.M., Rojas, C.A., Rojas,
M., Gracia, L.F..2010. Regulatory T cell frequency and
modulation of IFN-gamma and IL-17 in active and
latent tuberculosis.J Tuberculosis., 90(4), 252-261.
doi:10.1016/j.tube.2010.05.003.
Afif, E., Taufik., Medison, I., 2013. Analysis of Anti-
ESAT-6 antibody levels in pulmonary tuberculosis
patients. J Respir Indo., 33(1), 17-25.
Ors, F., Deniz, O., Bozlar, U., Gumus, S., Tasar, M.,
Tozkoparan, E., et al. 2007. High-resolution CT
Findings in Patients with Pulmonary Tuberculosis:
Correlation With the Degree of Smear Positivity. J
Thorac Imaging., 22(2), 154 – 159. doi:
10.1097/01.rti.0000213590.29472.ce.
Oxford immunotec. 2017. T-SPOT. TB Frequently Asked
Questions. Oxford Diagnostic Laboratories. pp, 27-30.
Available from: http: //www.oxfordimmunotec.com.
Acessed: 2 March 2018.
Pai, M., Denkinger, C.M., Kik, S.V., Rangaka,
M.X., Zwerling, A., Oxlade, O., et al. 2014. Gamma
interferon release assays for detection of
Mycobacterium tuberculosis infection. Clin Microbiol
Rev27(1),3-20.doi: 10.1128/CMR.00034-13.
Pratomo, I.P., Setyanto, D.B., 2013. Use of Esat-6 and CFP-
10 Antigen Complexes for Diagnosis of Tuberculosis.
J Respir Indo., 33(1), 67-68.
Priyantomo, E.P., Salam A., dan Arundina, A., 2014.
Description of Body Mass Index in Tuberculosis
Patient with Anti Tuberculosis Drugs theraphy in Unit
Lung Disease Medicine (UP4) Pontianak, Pontianak,
Medical Student Journal. pp,1-15.
Rachim, R., 2014. The Relationship between BCG
Immunization and Tuberculosis in Children at
Puskesmas Pandian, Sumenep Regency. Journal of
Health Sciences and Family Medicine., 10(2), 110-114.
doi:10.22219/sm.v10i2.4158.
Saraiva, M., O’Garra A.2010. The regulation of IL-10
production by immune cells. Nat Rev Immunol.,10
(3),170-181. doi: 10.1038/nri2711.
Setiawan, H., and Nugraha, J., 2016. Analysis of IFN-γ and
IL-10 levels in PBMCs for patients with active
tuberculosis, latent and healthy people, after being
stimulated with ESAT-6 antigen. Journal of
Bioscience., 18 (1), 50-66. doi: 10.20473/jbp.v18i1.
Seyedhosseini, F.S., Mohammadi, S., Ebrahimabad,
M.Z., Khodabakhshi, B., Abbasi, A., Yazdani,
Y., 2019. Interleukin-6, interleukin-17 and
transforming growth factor-beta are overexpressed in
newly diagnosed tuberculosis patients; potent
biomarkers of mycobacterial infection. Archives of
Clinical Infectious Diseases., 14(4),1-6. doi:
10.5812/archcid.68417.
Torrado, E., and Cooper, M.A., 2010. IL-17 and Th17 Cells
In Tuberculosis. Cytokine Growth Factor Rev. 21(6):
455–462.doi: 10.1016/j.cytogfr.2010.10.004.
Wahyuniati, N., .2017. The Role of Gamma Interferon in
Mycobacterium Tuberculosis Infection. Journal of
Medicine Syiah Kuala., 17(2),126-132. doi:
10.24815/jks.v17i2.8992.
Wang, X., Barnes, P.F., Huang, F., Alvarez, I.B.,
Neuenschwander, P.F., Sherman, DR., et al. 2012.
Early Secreted Antigenic Target of 6- kD Protein of
Mycobacterium tuberculosisPrimes Dendritic Cells to
Stimulate Th17 and Inhibit Th1 Immune Respones. J
Immunol. 189(6), 3092–3103. doi:
10.4049/jimmunol.1200573.
Wibowo, R.Y., Tambunan, B.A., Nugraha, J.,
Tanoerahardjo, F.S., 2017. Expression of IFN-γ by
CD4 + and CD8 + T Cells After Stimulation of ESAT-
6- CFP-10 Fusion Antigen in Active Pulmonary
Tuberculosis Patients. Surabaya: Airlangga
University., 45(4), 223-226. doi:
10.22435/bpk.v45i4.6874.
Widjaja, J.T., Jasaputra, D.K., and Roostati, R.L., 2010.
Analysis of gamma interferon levels in patients with
pulmonary tuberculosis and healthy people. J Respir
Indo., 30(2), 119-120.
Secretion of IFN- and IL-17 after Stimulation of ESAT-6-CFP10 (EC610) Fusion Antigen from PBMC in Groups Active TB and Latent TB
297
