
Blood Transfusion, Serum Total Iron Binding Capacity and Iron in
Hemodialysis Patients Margono Soekarjo Hospital
Vitasari Indriani
1
a
, Aditiawarman
2
b
, Yunanto Dwi Nugroho
2
c
and Fania Salsabila
3
d
1
Departement of Clinical Pathology, Faculty of Medicine, Jenderal Soedirman University, Purwokerto, Indonesia
2
Department of Internal Medicine, Faculty of Medicine, Jenderal Soedirman University, Purwokerto, Indonesia
3Student of Faculty of Medicine, Jenderal Soedirman University, Purwokerto, Indonesia
Keywords: Chronic kidney disease, hemodialysis, serum iron, TIBC, transfusion
Abstract: In the final stages, chronic kidney disease (CKD) patients require renal replacement therapy like hemodialysis
(HD). Packed Red Cell (PRC) transfusion is an anaemia management therapy that can increase serum Total
Iron Binding Capacity (TIBC) and iron levels. This study analyzed blood transfusions, TIBC serum and iron
serum levels in hemodialysis patients with repeated transfusions at Margono Soekarjo Hospital to assess the
value of TIBC in the iron excess and deficiency diagnosis. An observational analytic design with cross-
sectional findings was used. We investigated 85 hemodialysis patients; (73.3% men and 26.7% women aged
20 to 70 years) visited hemodialysis clinics from January 2018 to 2020. Correlation studies showed a linear
relationship between the number of blood transfusions and the level of iron serum in hemodialysis patients
(value 𝑃: 0.0001), a positive correlation between the number of blood transfusions and TIBC serum
concentration (value 𝑃: 0.04); the significant relationship between TIBC serum and iron serum (value: 0.003).
The prevalence of excess iron was 10.6%, and 2.3% in iron deficiency. Non-iron therapy patients with a
maximum of one transfusion with iron therapy, patients with transfusions who are not on iron therapy, and
patients on oral iron therapy were compared. The Kruskal-Wallis test showed that iron levels varied
significantly between groups (𝑃 value: 0.0001). TIBC serum is not a reliable marker of excess iron. For
patients with regular transfusions, periodical checking of TIBC and iron serum is recommended.
1 INTRODUCTION
Chronic kidney disease (CKD) is a worldwide public
health problem with a high prevalence and an
increasing incidence every year. CKD is the 27th
cause of death in the world in 1990 and increased to
18th in 2010. According to (Hall 2016), globally, the
prevalence of CKD is 13.4% for stage 1-5 patients
and 10.6% for stage 3- patients 5 (Hall et al., 2016;
Urrechage et al., 2013). In Malaysia, 18 million
people, an estimated 1800 new kidney failure cases
per year. The Ministry of Health (2017) reports that
CKD's prevalence reaches 12.5% in Indonesia's adult
population. In Central Java, CKD's prevalence
reached 0.3% of Central Java's population (Depkes,
2017)
a
https://orcid.org/0000-0001-8563-1781
b
https://orcid.org/0000-0003-1786-211X
c
https://orcid.org/0000-0002-7104-7901
d
https://orcid.org/0000-0002-2825-2571
CKD patients with hemodialysis generally have
low Hb levels, and anaemia is not uncommon. The
indication for transfusion in CKD patients is anaemia
with a Hb level <7 g / dL. CKD anaemia occurs due
to erythropoietin (EPO) deficiency, decreased
intestinal iron absorption, iron deficiency, and iron
loss during HD. During hemodialysis, there is
frequent hemodynamic instability and decreased
oxygen perfusion to body tissues due to a sudden
decrease in blood volume during blood filtration.
CKD also decreases catecholamine hormones'
sensitivity because the damaged kidneys cannot clear
the blood vessels' hormone. The results in a decrease
in heart rate. Combining these two things will reduce
the stroke volume, resulting in hypoperfusion of
organs (McGuire et al., 2018). Hemoglobin is the
284
Indriani, V., Aditiawarman, ., Nugroho, Y. and Salsabila, F.
Blood Transfusion, Serum Total Iron Binding Capacity and Iron in Hemodialysis Patients Margono Soekarjo Hospital.
DOI: 10.5220/0010491502840288
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 284-288
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

blood's oxygen carrier. If the levels decrease, there
can be a decrease in the oxygenation of the body's
organs. Anaemia with a Hb level that is too low (<7 g
/ dL) puts CKD patients at high risk for doing HD
because the patient's hemodynamic function becomes
increasingly unstable, so it is too risky to do HD
(Hider et al., 2013; Kdigo, 2012).
One of the treatments for anaemia in CKD is by
transferring Packed Red Cell (PRC) components so
that the patient's Hb level increases. In the body, after
one erythrocyte life cycle, iron is released from heme.
If it is excess, iron will be stored in the endoplasmic
reticulum tissue as ferritin and hemosiderin, but the
body does not have an active iron secretion system.
As the amount of PRC transfused increases, the serum
iron accumulates. The results in less transferrin,
which is not bound by iron. Simultaneously, levels of
non-transferrin bound iron (NTBI) increased. It can
cause excess iron (Gao et al., 2014).
Chronic renal failure is often associated with
Renal anaemia due to iron-restricted erythropoiesis
(Hall et al., 2016; Urrechaga E., 2013). Kidneys
secrete erythropoietin, a protein that is involved in
erythropoiesis. The secretion of erythropoietin
decreases when kidneys are damaged, resulting in
renal anaemia (Depkes, 2017). Because iron is also
required for erythropoiesis, iron deficiency may also
cause anaemia (Depkes 2017; McGuire et a., 2018).
The principal means to treat renal anaemia in most
settings are Blood transfusion, erythropoietin (EPO),
and iron therapy (Hider et al., 2013; Kdigo, 2012).
Renal anaemia correction in chronic renal failure
patients could carry a risk for iron overload (Gao et
al., 2014; Thavarajah et al., 2019) and increase the
risk of adverse events such as hypertension,
congestive heart failure, myocardial infarction, and
vascular access thrombosis (Depkes, 2019; Milic et
al., 2016). Correction of renal anaemia to be done by
blood transfusions and iron therapy. Although
transfusions are considerably safer nowadays
(Bozhuizen et al., 2019), transfusion reactions must
be prevented as a risk in blood transfusion therapy
(Robinson et al., 2017; Tanhehco et al., 2012; Gao et
al., 2014). These risks include transmission of
infectious agents (robinson et al., 2017; Alla et al.,
2016; Rerambiah et al, 2014), the development of
alloimmunization (Tagny et al., 2013; Baby et al.,
2010) and iron overload (Esther et al., 2018). The
present study aimed to evaluate iron status in patients
with renal failure undergoing hemodialysis and assess
the value of Total Iron Binding Capacity (TIBC) in
diagnosing iron overload and iron deficiency in an
RSUD Prof Dr. Margono Soekarjo Hospital.
2 MATERIALS AND METHODS
We investigated 85 hemodialysis patients (73.3%
male and 26.7% female aged 22 to 73 years) who
attended Prof. Dr. Margono Soekarjo Hemodialysis
Center Hospital from January 2018 to January 2020,
using cross-sectional studies. Patients were divided
into four (4) groups; (1) patients with none or single
transfusion without iron therapy; (2) patients with
none or single under iron therapy; (3) polytransfused
patients without iron therapy; (4) polytransfused
patients with iron therapy. Blood samples were taken
before the hemodialysis session. 3 mL of venous
blood was collected in straight tubes. The collected
blood samples were then centrifuged (3000 rpm for
15 minutes. Iron and TIBC in the serum used Cobas
c501 to interpret. Table 1 shows TIBC and iron
concentration ranges in the serum and interpretation
of the results. Characteristics data and research
variables showed in the form of descriptive analysis.
The independent variables are including blood
transfusion and iron serum. The independent variable
of this study is TIBC.
We used the Spearman test to assess the
correlation between the number of transfusions, iron,
and TIBC levels. The strength of TIBC and iron
serum concentrations compatibility was assessed
based on the Kappa coefficient (𝜅) that was calculated
using the formula 𝜅 = (𝑃𝑜 − 𝑃𝑒)/1 − 𝑃𝑒, where 𝑃𝑜 is
the relative observed agreement among assessor and
𝑃𝑒 is the theoretical probability of chance agreement.
The groups' discrepancy was analyzed using the
Kruskal-Wallis multiple comparison test Dunn's post-
test to assess therapeutic effectiveness. 𝑃 value below
5% was considered as significant. Regarding ethics,
the health research ethics committee of RSUD Prof
Dr. Margono Soekarjo Number 420/01030/I/2020
approved the study. Besides, all patients consented to
participate in the study.
3 RESULTS
It can be seen in Table 1 that the distribution of iron
and TIBC levels and their interpretation in the study
sample population. Univariate analysis can be seen in
Table 2. It is found that most gender is male. The most
blood group is the patient with blood group B, and the
most age group is the patient aged 51-60 years. Figure
1 shows that the number of PRC units received by
CKD patients undergoing HD by repeated
transfusions is 2 - 14, with a maximum average of 4
Blood Transfusion, Serum Total Iron Binding Capacity and Iron in Hemodialysis Patients Margono Soekarjo Hospital
285
kolf. Correlation between transfusion, TIBC, and Iron
levels are shown here.
Table 1. TIBC and Iron range in the serum and
interpretations.
Iron
deficienc
y
Normal
Iron
overloa
d
Serum
TIBC
>400µg/dL
245-
400
µg
/dL
<245µg/dL
Serum
Iron
<35µg/dL
35-
140
µg
/dL
˃140µg/dL
Data showed a positive and significant correlation
between the number of transfusions and TIBC levels
(Spearman 𝑟: 0.65; 𝑃 value: 0.0001). Although it was
weak, the correlation between the number of
transfusions and iron serum levels was positive and
significant (Spearman 𝑟: 0.32; 𝑃 value: 0.03).
Table 2. Research Characteristics
Characteristics
N=85 %
Gender
Male 53 73.3
Female 32 26.7
Blood type
A 25 33.3
B 28 33.3
AB 10 11.1
O 22 22.2
Age (year)
22
–
30 6 7.1
31
–
40 5 5.8
41
–
50 29 34.1
51
–
60 37 43.5
61
–
70 6 7.1
> 70 2 2.4
Further correlation analysis showed a significant
relationship between TIBC and iron serum
concentration (Spearman 𝑟: 0.32; 𝑃 value: 0.003).
However, the observed Spearman 𝑟 coefficient
suggested that the correlation between TIBC serum
and iron serum concentrations is weak, although
significant. Next is the correlation between TIBC and
Iron serum in excess iron cases. Table 3 shows the
distribution of patients according to TIBC and their
iron levels in this study. The prevalence of excess iron
based on TIBC serum was 46.06%. The prevalence of
excess iron was 15.7% when it was assessed based on
the iron serum levels. The prevalence of excess iron
based on TIBC and the iron concentration in serum
was 10.6%. The prevalence of iron deficiency based
on TIBC serum was 17.4%, while iron deficiency
based on iron serum concentration was 8.9%. The
prevalence of iron deficiency in both TIBC and iron
concentration is 3.3%.
Table 3. Patients Distribution according to their TIBC and
iron levels in serum
Total Iron Binding Capacit
y
Iron
deficienc
y
normal Iron
Overloa
d
Total
Iron
Deficienc
y
3 5 7 15
Normal 15 19 26 60
Iron
Overloa
d
1 5 8 14
Total 19 29 41 89
Cohen's Kappa (𝜅) formula used in this study with
0.14 𝜅 coefficient. As a result, the correlation between
TIBC and iron levels in the serum was considered
weak or inadequate. Regarding transfusion, TIBC,
and Iron serum, a comparison was made on patients
in groups 1 and 2 to patients in groups 3 and 4. The
"Kruskal-Wallis test" showed that TIBC levels varied
significantly between the groups (𝑃 value: 0.0001).
Besides, "Dunn's Multiple Comparison Test" also
showed that (1) on patients without iron therapy,
TIBC level was significantly higher in polytransfused
patients than patients with none or single transfusion
(𝑃 value: 0.0001), (2) on patients under iron therapy,
the TIBC level was significantly higher in
polytransfused patients than on patients who had none
or single transfusion (𝑃 value < 0.05), (3)
polytransfused patients on iron therapy had
significantly higher levels of TIBC compared to
patients who had none or one transfusion and without
iron therapy (𝑃 value: 0.001), and (4) polytransfused
patients who were not on iron therapy had
significantly higher levels of TIBC compared to
patients who had none or single transfusion and who
were under iron therapy (𝑃 value < 0.05).
Table 4. Discrepancy between groups
Grou
p
sf
p
1 20 < 0.001
2 21
3 22
4 22
Comparing the same groups of patients for their
serum iron concentrations, the "Kruskal-Wallis test"
showed no significant differences between the groups
(table 4).
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
286
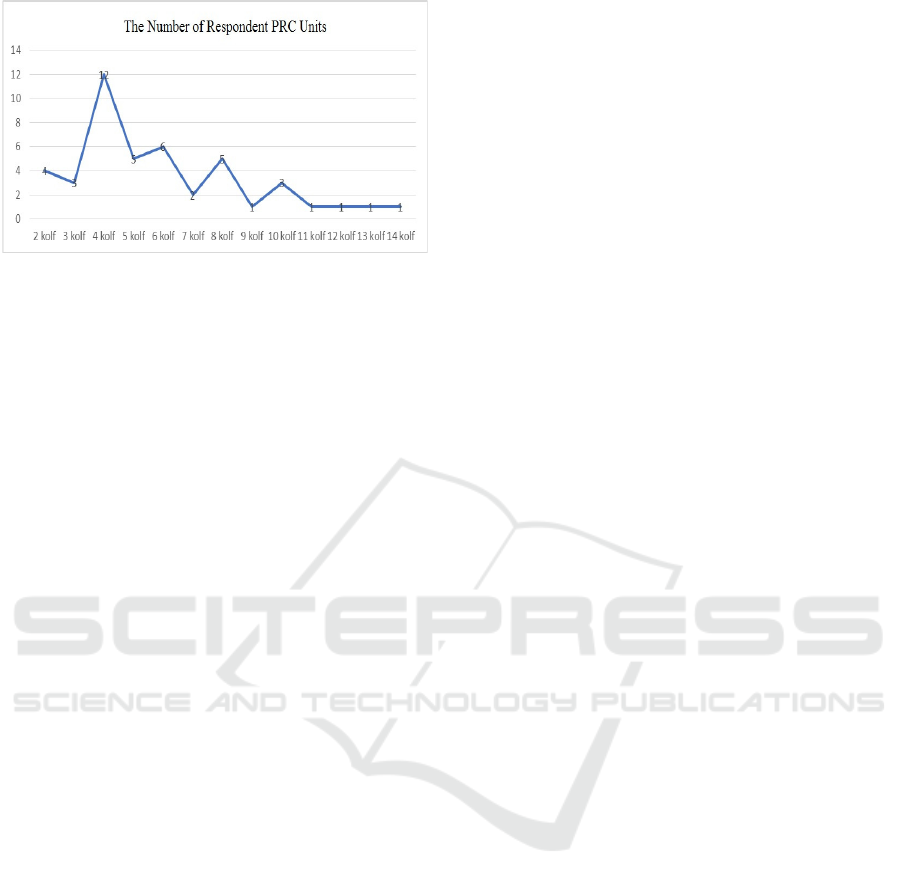
Figure 1 Distribution of data on the number of respondent
PRC units
4 DISCUSSION
The present study aimed to evaluate iron status in
patients with renal failure undergoing hemodialysis
and assess the value of TIBC in the diagnosis of
excess iron and iron deficiency. In developing
countries such as Indonesia, TIBC serum level in
patients is often used as a marker for excess or
deficiency iron diagnosis in hemodialysis patients.
This is because iron-binding capacity is the
transferrin's capacity to bind with iron. When iron
stores are depleted, the transferrin levels increase in
blood. As only one-third of transferrin is saturated
with iron, so the transferrin present in serum has an
extra binding capacity (67%). It is called unsaturated
iron-binding capacity (UIBC). TIBC is the total of
iron serum and UIBC. Iron status in hemodialysis
patients monitored using serum TIBC may rarely be
performed by hemodialysis service personnel
because confounding factors such as acute, chronic
inflammation, and malnutrition can cause different
TIBC serum value interpretation (Gujja et al., 2010).
This study showed an intense positive linear
relationship between the number of blood
transfusions with TIBC serum levels. It also indicated
a weak association between the number of blood
transfusions and iron concentrations in the serum.
The compatibility level between TIBC and iron levels
in the serum was low. The data suggest that multiple
transfusions increase TIBC serum substantially and at
lower iron serum levels. We found that in
hemodialysis patients, TIBC serum is strongly
overestimated iron level. TIBC serum also
overestimates iron deficiency cases, but on a lower
level. In this study, the prevalence of excess iron
based on TIBC serum was 42.3, whereas the
prevalence of excess iron based on iron serum levels
was 21.2%. The prevalence of iron deficiency based
on TIBC serum and iron serum was, respectively,
17.4% and 10.6%. Based on the data, 65% of patients
had moderately high TIBC serum levels, and 67% of
patients with very high TIBC levels had their iron
serum level within the normal range. High TIBC
serum is not a reliable marker of excess iron (Petkova
et al., 2019; Pfeiffer et al., 2017). Because the use of
TIBC serum as a marker for excess iron or deficiency
could lead to (1) holding iron therapy in patients that
need it and (2) giving iron treatment to patients who
do not require it, accurate assessment of the body iron
load is essential to prevent iron toxicity and to
manage iron chelation therapy. Although we did not
assess liver iron concentration (LIC) by magnetic
resonance imaging (MRI), based on the published
report (Depkes, 2017; Milic et al., 2018; Hoffbrand et
al., 2012).
The Anemia monitoring due to retention in
dialyzers was not possible in this study. This study
also did not monitor the nutritional intake and diet
consumed by patients. The type of food consumed by
the patient can affect iron serum levels. Red meat is
the most effective food (40%) absorbed by the
intestine because it is a heme iron source.
Consumption of vitamin C also increases the
absorption of iron in the intestines.
Meanwhile, intake containing calcium and
tannins inhibits iron absorption, thereby reducing
serum iron levels. Therefore, this can be a
confounding factor in this study. The suggestion is to
do similar research by controlling for confounding
factors such as nutritional intake, Erythropoiesis
Stimulating Agents (ESAs) therapy. Future research
will use the cohort method to see the disease's course
and the effect of transfusion on iron levels in a more
usual manner. We would suggest patients under
regular transfusion therapy to do TIBC and iron
serum measurement periodically in these countries.
5 CONCLUSIONS
TIBC serum is not a reliable marker of excess iron.
For patients with regular transfusions, periodical
checking of TIBC and iron serum is recommended.
ACKNOWLEDGEMENTS
The author would like to acknowledge RSUD Prof
Dr. Margono Soekarjo hemodialysis centre for giving
access to the medical record and hemodialysis
patients.
Blood Transfusion, Serum Total Iron Binding Capacity and Iron in Hemodialysis Patients Margono Soekarjo Hospital
287

REFERENCES
Hall, J.E., 2016. Blood Cells, Immunity, and Blood
Coagulation: Red Blood Cells, Anemia, and
Polycythemia. In: JE Hall (Ed.). Guyton and Hall
Textbook of Medical Physiology 13th Edition. USA:
Elsevier. Page: 445-52..
Urrechaga, E., Borque, L and Escanero, J. F., 2013.
"Erythrocyte and reticulocyte indices in the assessment
of erythropoiesis activity and iron availability".
International Journal of Laboratory Hematology,
[online] 35(2), pp.144–149.
Depkes, 2017. Info DATIN Pusat Data dan Informasi
Kementerian Kesehatan RI Situasi Penyakit Ginjal
Kronis. ISSN 2442-7659
McGuire, S., Horton, E. J., Renshaw, D., et al., 2018
Hemodynamic Instability during Dialysis: The
Potential Role of Intradialytic Exercise. Biomed
Research International, [online], 8276912,
2018pmid:29682559.
Hider, R.C. & Kong, X., 2013. Iron: Effect of Overload and
Deficiency. In Astrid Sigel, Helmut Sigel and Roland
K. O. Sigel (ed.). Interrelations between Essential Metal
Ions and Human Diseases. Metal Ions in Life Sciences.
13. Springer. pp. 229–294, 2013.
International Society of Nephrology. Kidney disease
improving global outcome: Clinical practice guideline
for anemia in chronic kidney disease. Vol 2. Page: 283-
335, 2012.
Gao, C., Li, L., Chen, B., et al., 2014. Clinical outcomes of
transfusion-associated iron overload in patients with
refractory chronic anemia. Patient preference and
adherence. [online] 22(8), Pp. 513–517.
Thavarajah, S., Choi, M.J., 2019. "The Use of
Erythropoiesis-Stimulating Agents in Patients With
CKD and Cancer: A Clinical Approach". Am J Kidnes
Dis. [online] 74(5), pp.667-674.
Milic, S., Mikolasevic, I., Orlic, L., et al., 2016. "The Role
of Iron and Iron Overload in Chronic Liver Disease",
Medical science monitor. Med Sci Monit. [online] 22,
pp. 2144–2151.
Boshuizen, M., Van Hezel, M.E., Van Manen, L., Straat,
M., Somsen, Y.B., Spoelstra‐de Man, A.M. Juffermans,
N. P., 2019. The effect of red blood cell transfusion on
iron metabolism in critically ill patients. Transfusion,
[online] 59(4), 1196-1201.
Robinson, S., Harris, A., Atkinson, S, et al., 2017. The
administration of blood components: a British Society
for Haematology Guideline. Transfusion Medicine.
[online] 28(1), pp. 3–21.
Tanhehco, Y.C and Berns, J. S., 2012. Red blood cell
transfusion risks in patients with end-stage renal
disease. Seminars in Dialysis, [online], 25(5), pp. 539–
544.
Gao, C., Li, L., Chen, B., et al., 2014. Clinical outcomes of
transfusion-associated iron overload in patients with
refractory chronic anemia", Patient preference and
adherence, [online] 8, pp. 513–517.
Alla, M.B.A.A., Adam, K.M. and Mohammed, N.E.A.,
2016. Assessment of Iron Profile among Transfused
Dependent Chronic Renal Failure Sudanese Patients",
Journal of Biosciences and Medicines, [online] 4, pp.
52-56.
Rerambiah, L.K., Rerambiah, ., L.E., Bengone, C and
Djoba Siawaya, J. F., 2014. The risk of transfusion-
transmitted viral infections at the Gabonese National
Blood Transfusion Centre. Blood Transfusion, [online]
12(3), pp. 330–333.
Tagny, C.T., Murphy, E.L and Lefrere, J.-J., 2013. The
Francophone ` Africa blood transfusion research
network: a five-year report (2007–2012). Transfusion
Medicine, [online] 23(6), pp. 442–444.
Baby, M., Fongoro, S., Cisse, M., et al., 2010. Frequency
of red blood cell ´alloimmunization in polytransfused
patients at the university teaching hospital of Point G,
Bamako, Mali. Transfusion Clinique et Biologique,
[online] 17(4), pp. 218–222.
Esther, I.O., Crosdale, O.P., Richard, I.O.,2018. Red blood
cell alloimmunization in multi-transfused patients with
chronic kidney disease in Port Harcourt, South-South
Nigeria. Afr Health Sci, [online] 18(4), pp. 979-987.
Gujja, P., Rosing, D.R., Tripodi, D.J., et al., 2010. Iron
overload cardiomyopathy: better understanding of an
increasing disorder. Journal of the American College of
Cardiology, [online] 56(13), pp.1001–1012
Petkova, N.Y., Raynov, J.I., Petrova, D.Y., Ramsheva,
Z,N., Petrov, B,A., 2019. Diagnostic Significance of
Biomarkers of Iron Deficiency for Anemia in Clinical
Practice. Folia Med (Plovdiv), [online], 61(2), pp. 223-
230.
Pfeiffer, C.M, Looker, A.C., 2017. Laboratory
methodologies for indicators of iron status: strengths,
limitations, and analytical challenges. Am J Clin Nutr,
[online] 106(Suppl 6),pp. 1606S-1614S.
Milic, S., Mikolasevic, I., Orlic, L., et al., 2018. The Role
of Iron and Iron Overload in Chronic Liver Disease",
Medical science monitor. Med Scie Monit, [online] 22,
pp.2144–2151.
Hoffbrand, A.V., Taher, A and Cappellini, M.D., 2012.
How I treat transfusional iron overload. Blood, [online]
120(18), pp. 3657– 3669.
Andy, M., Henry, R.W., Andrea, D., Matt, K., Michael,
L.G., Stefan, N., Jimmy D.B., Rajarshi, B.E., Louise T.,
2018. Measurement of liver iron by magnetic resonance
imaging in the UK Biobank population. PLoS ONE,
[online] 13(12), pp. e0209340.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
288
