
Reversible Imiquimod Effects on Skin Tissue of Psoriasis Mice
Model: An Experimental Study
Thianti Sylviningrum
1
a
, Afifah
2
b
, Dody Novrial
3
c
, Brian Wasita
4
d
, Bambang Purwanto
5
e
,
Harijono K. Sentono
6
f
1
Doctoral Degree of Medical Science Programme, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
2
Department of Pharmacology, Faculty of Medicine, Universitas Jenderal Soedirman, Purwokerto, Indonesia
3
Department of Anatomical Pathology, Faculty of Medicine, Universitas Jenderal Soedirman, Purwokerto, Indonesia
4
Department of Anatomical Pathology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
5
Department of Internal Medicine, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
6
Department of Dermatology and Venereology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia
Keywords: Imiquimod, skin, mice model, psoriasis.
Abstract: Psoriasis is an incurable inflammatory disease with erythematous, scaly, and thick skin. Imiquimod (IMQ)-
induced psoriasis mice have been widely used since it may resemble psoriasis human. Imiquimod effects on
development, peak, and resolution of mice skin are crucial. Thus, this study aims at evaluating IMQ effects
on psoriasis mice model skin tissue changes. Twenty-seven female Balb/c mice 8-11 weeks, 20-25 grams
body weight (BW) were randomize equally into control (A) and experimental groups (B,C). Over 25 grams
BW mice after acclimatization were excluded. The mice had shaved their back then topically applied
emollient (A) and IMQ (B,C) for 7 consecutive days. On day 8 (A,B) and 15 (C), mice were terminated and
back skin harvested for histopathological examination. Psoriasis Area Severity Index (PASI) and Baker’s
scores were used to measure macroscopic and microscopic skin changes. All groups score differences were
assessed using Kruskal Wallis then Mann Whitney tests. Skin changes gradually appeared from day 2 to 7 of
IMQ applications and faded after 2 days IMQ discontinuation. On day 7, all mice showed means of PASI
scores 0(A);9,00±2,69(B);8,11±1,62(C) with significant differences between experimental to control groups
(p = 0.00). Means of PASI scores from mice group C on day 15 showed similar to group A (p = 0.10). Means
of Baker’s scores from all groups were 1,16±0,25(A); 3,33±1,25(B);1,39±0,22(C) and group B mice showed
different significant scores from others (p = 0.00). As conclusion, the IMQ showed a reversible effect on skin
tissue changes in psoriasis mice model.The abstract should summarize the contents of the paper and should
contain at least 70 and at most 200 words. It should be set in 9-point font size, justified and should have a
hanging indent of 2-centimenter. There should be a space before of 12-point and after of 30-point.
1 INTRODUCTION
Psoriasis is an autoimmune disease characterized by
the erythematous plaque covered by the thick, silvery,
and scaly skin lesions with the chronic inflammatory
background (Boehncke & Schön, 2015). The disease
has complex pathophysiological pathways involving
a
https://orcid.org/0000-0003-0349-0087
b
https://orcid.org/0000-0002-5703-7061
c
https://orcid.org/0000-0002-3807-852X
d
https://orcid.org/0000-0002-5501-3541
e
https://orcid.org/0000-0003-3389-9043
f
https://orcid.org/0000-0001-5338-6005
the interactions of multi-genetic and environmental
factors. Psoriatic patients who have skin and/or
systemic manifestations may decrease their quality of
life, and need long-term treatment possibly associated
with higher side effects and lower compliance
(Belinchón et al., 2016).World Health Organization
(WHO) reported that there were approximately 2% of
psoriasis patients throughout the world and tended
260
Sylviningrum, T., Afifah, ., Novrial, D., Wasita, B., Purwanto, B. and Sentono, H.
Reversible Imiquimod Effects on Skin Tissue of Psoriasis Mice Model: An Experimental Study.
DOI: 10.5220/0010491002600266
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 260-266
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
increasing in the last 20 years (WHO, 2016). This
condition indicates that more studies are required to
investigate the pathways involved in the development
of psoriasis and treatment method associated with a
good response and minimum side effects.
Studies on pathophysiological and drug
development in human psoriasis patients may face the
ethical problems. Thus, psoriasis animal model is
preferable. Imiquimod (IMQ)-induced psoriasis
mouse model is the most widely used research
instrument because it is inexpensive, easy to perform,
and immediately induce the acute inflammatory
process resembles human psoriasis. This procedure
may also trigger the psoriasis skin manifestations,
such as erythematous, skin hyperplasia, and scaling.
The pathognomonic histopathological changes
of human psoriasis were also found, including munro
abscess, acanthosis, keratinocyte hyperplasia,
parakeratosis (Hawkes et al., 2017). The application
of 62.5mg imiquimod daily may induce the psoriasis
skin lesions on the back-shaved Balb/c mice through
their activation of Interleukin (IL)-23/IL-17 axis
(Bocheńska et al., 2017). The previous studies
showed that IMQ might affect a rapid, temporary, and
reversible inflammation on the human skin (Van der
Kolk et al., 2018), yet the information on the duration
of inflammation development process during the
application of IMQ on mice was various and limited.
The duration of inflammation process in IMQ-
induced psoriasis mouse model is essential for the
momentum to do the treatment procedures for new
drug development.
2 MATERIALS AND METHODS
This study is an experiment study with posttest-only
control design that held in February to April 2020 in
Pharmacology Department, Medical Faculty of
Universitas Jenderal Soedirman, Central Java,
Indonesia. Twenty-seven female Balb/c mice aged 8-
11 weeks and weighed 20-25 grams were divided into
three groups: control group (A), first treatment group
(B), and second treatment group (C) consisting of 9
mice per group. The female Balb/c mice were
obtained from the Department of Pharmacology,
Faculty of Medicine, Universitas Gadjah Mada,
Indonesia.
All mice underwent 7-day acclimatization
period and were under-maintained in 12-12 hour light
and dark cycle with ad libitum food and drink. After
the acclimatization period, all mice were then
anesthetized with 0.1 ml intramuscular injection per
10 gram BW, 80mg/kgBW with ketamine cocktail;
12.5 mg/kg BW with xylazine cocktail; 3mg/kg BW
with acepromazine cocktail (Vertebrate Animal
Research, 2020). After anesthetized, 2x2 cm2 of all
mice’s back was shaved using an animal shaver and
applied with the depilatory cream Veet®. For the next
consecutive 7 days, each mouse in group A was
applied with 62.5 mg Noroid cream® (Soho Global
Health, Indonesia) on the shaved back skin, and
62.5mg of Aldara cream® (3M Pharmacy, United
Kingdom) that contains 5% imiquimod was applied
on the shaved back skin of each mouse in group B and
C. On day 8, the PASI scores of mice in all groups
were evaluated and terminated using the cervical
dislocation for those mice in group A and B followed
by back skin tissue harvesting. This step was repeated
on day 15 for mice in group C.
All skin tissues underwent a histopathological
examination using the hematoxylin and eosin
staining. The components of PASI scores for mice
were interpreted as erythematous (0-4), scaling (0-4),
thickness (0-4) with the total score of 0-12 by 2
certified dermatologists (Luo et al., 2016). To
minimize the subjectivity in evaluating the erythema,
the erythema level was scored based on color, 0 = no
color changes; 1 = light pink; 2 = pink; 3 = red; and 4
= dark red/violet. The scaling was scored based on the
observance, while the skin thickness was evaluated
by the histopathological examination. Two
pathologists evaluated and scored the
histopathological examination results using Baker’s
scoring system as presented in Table 1 (Baker et al.,
1992).
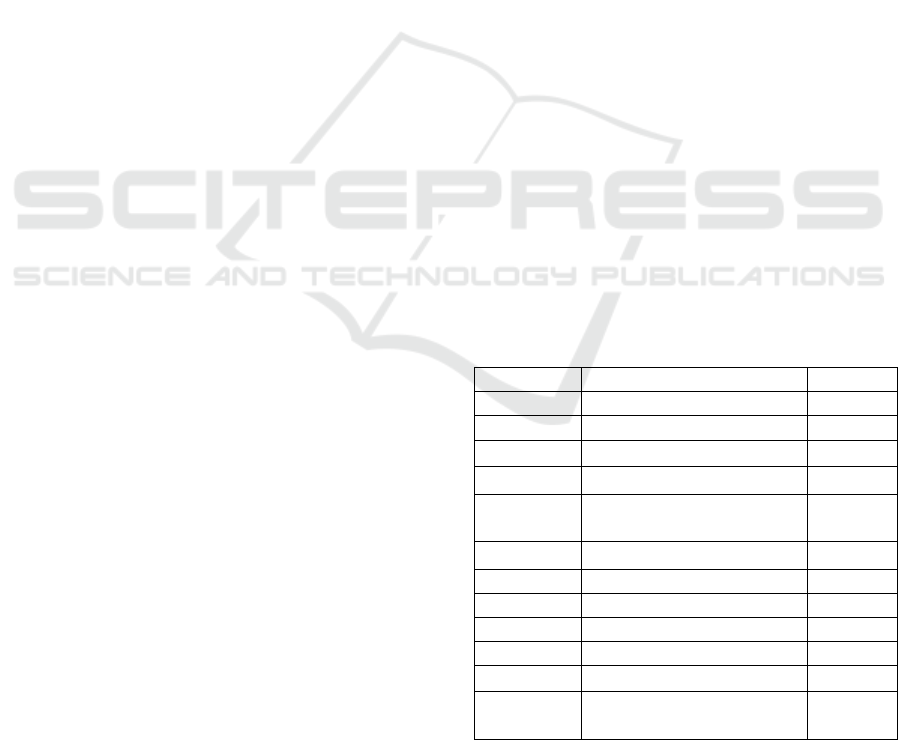
Table 1. Baker’s scoring system
Items Score
Keratin Munro Abscess 2.0
Hyperkeratosis 0.5
Parakeratosis 1.0
Epidermis Thinning above papillae 0.5
Lengthening and
clubbin
g
of rete rid
g
es
1.5
Acanthosis 0.5
Lack of
g
ranular la
y
e
r
1.0
Dermis L
y
mphoc
y
tic infiltrate
Mil
d
0.5
Moderate 1.0
Marked 2.0
Papillary congestion 0.5
Kruskal Wallis test and Mann Whitney test were
used to measure the difference between PASI and
Baker’s scores of the groups. The p value of < 0.05
Reversible Imiquimod Effects on Skin Tissue of Psoriasis Mice Model: An Experimental Study
261
was considered having a significant difference
between variables. This research was approved by the
Health Research Committee, Faculty of Medicine,
Universitas Jenderal Soedirman Number: Ref:
03IIIGPMII/2020.
3 RESULTS
All mice included in this study have completed with
the acclimatization period. The mice were treated in
accordance with the procedure mentioned in the
method section. By the end of day 7, all mice in group
B and C had psoriasis-like skin inflammation lesions,
but those in group A did not. The mice in group C had
the normal skin after 7-day application
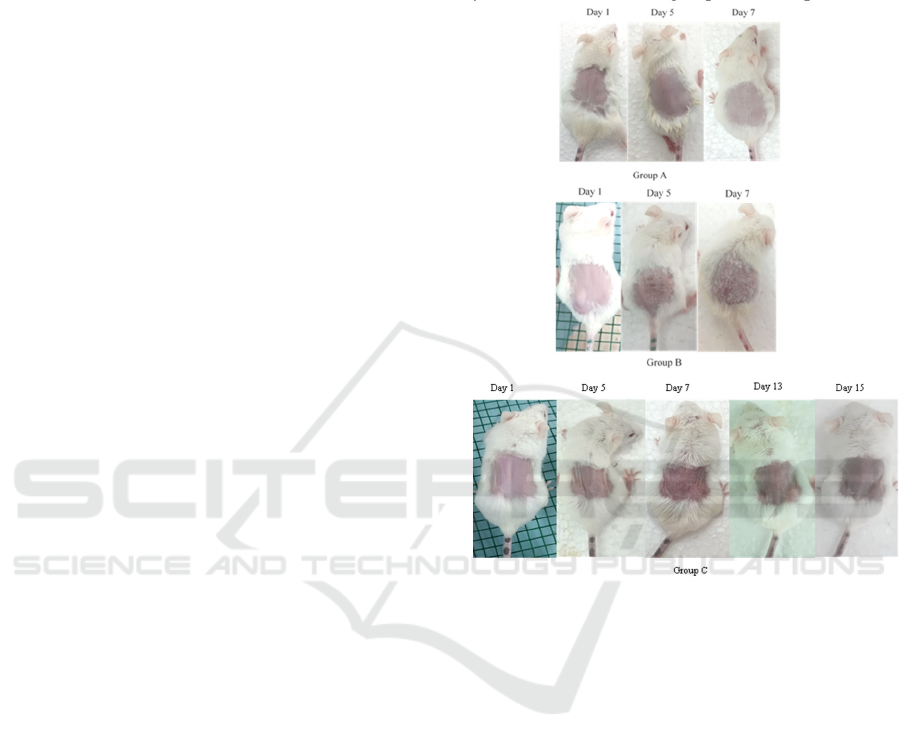
discontinuation of IMQ (Figure 1).
During the application of IMQ on the mice in
group B and C, there was an increasing erythema
score from day 2, that is, the preceding scaling and
skin thickness manifested starting from day 3. On day
7 as the last application of IMQ on the mice in groups
B and C resulted in the highest erythema, scaling, and
thickness scores. In group C mice, there was a gradual
decrease in skin scaling and thickness 1 day after
IMQ exposure discontinuation. Meanwhile the
erythema score began to subsided on day 2 after the
last application of IMQ. All PASI score components
had returned to 0 score on day 13 (Figure 2). The mice
in group A did not show any changes in their skin
erythema, scaling, and thickness during the study.
In this study, the PASI mean scores in all
groups were respectively 0 (group A); 9.00 ± 2.69
(group B); 8.11 ± 1.62 (group C on day 7); and 0 for
group C on day 14. The Kruskal Wallis test result
showed the significant difference of PASI scores on
day 7 among all groups (p = 0.000). Post hoc study
showed the significant difference of PASI scores
between groups A and B, groups A and C on day 7,
group B and C on day 14, also groups C on day 7 and
day 14 (p = 0.000). Meanwhile, no significant
difference in PASI scores was found between those in
group B and C on day 7 (p = 0.465), also those in
group A and C on day 14 (p = 1.000).
The skin tissue was stained using hematoxylin
and eosin further scored using Baker’s system. Mice
in group A and C showed normal epidermis and
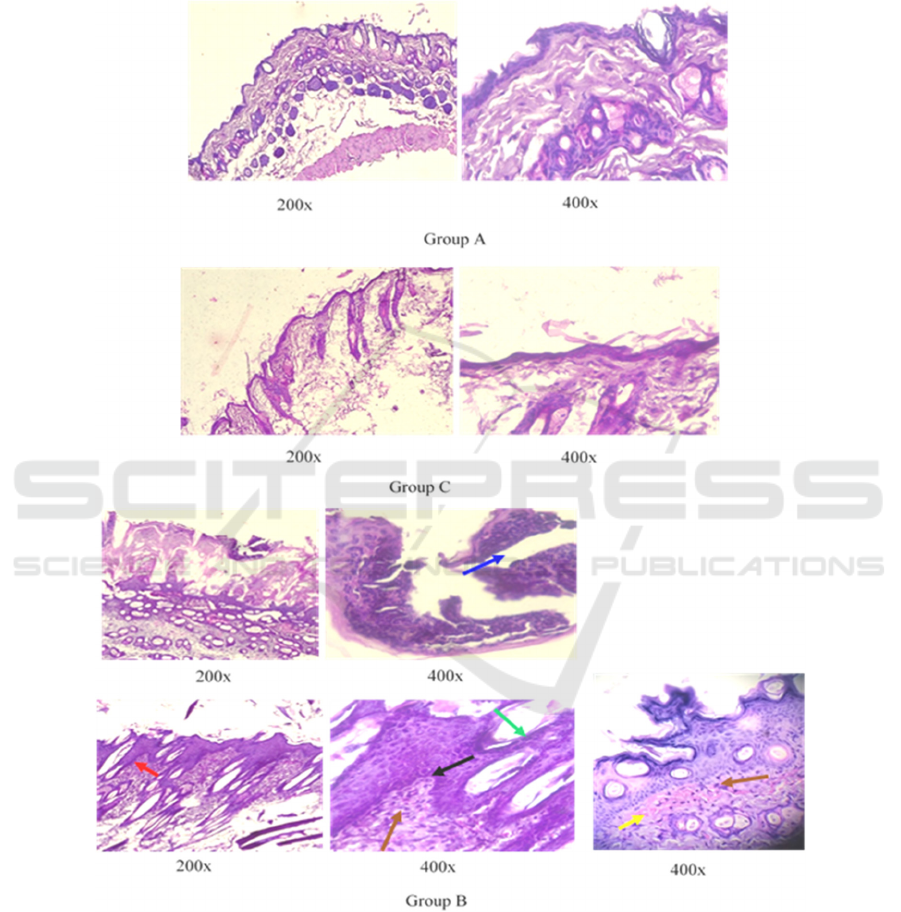
dermis layers. Psoriasis-like histopathological
changes were found in the mice in group B, such as
parakeratosis, Munro microabscess, acanthosis,
thinning above papillae, neutrophils and lymphocytes
infiltrate, and papillary congestions (Figure 3). The
following data were mean of Baker’s scores ±
standard deviation in group A, B, and C; 1.16 ± 0.25;
3.33 ± 1.25; and 1.39 ± 0.22. Baker’s scoring system
used in this study found significant differences among
all groups (p = 0.000). Further tests showed the
statistically significant differences of the mice in
group A and B (p = 0.000); group B and C (p = 0.001),
yet not between mice in group A and C ( p = 0.065).
Figure 1. Psoriasis-skin lesion like developed by the mice
in group B and C after the application 5% imiquimod cream
(Aldara®), while mice in group A had no changes in their
skin appearances. This psoriasis-like skin manifestation
was gradually decreased after the application
discontinuation of 5% imiquimod cream in group C.
4 DISCUSSIONS
Psoriasis is an inflammatory disease that may be
induced by multifactor, including drugs. Imiquimod
is known as one of the drugs that may induce psoriasis
in humans (Balak et al., 2017), meanwhile, IMQ
application in mice could trigger psoriasis-like skin
inflammation (Bocheńska et al., 2017). The use of
IMQ to induce psoriasis mice may provide the
benefits of investigating the pathogenesis and
developing new drugs for psoriasis.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
262
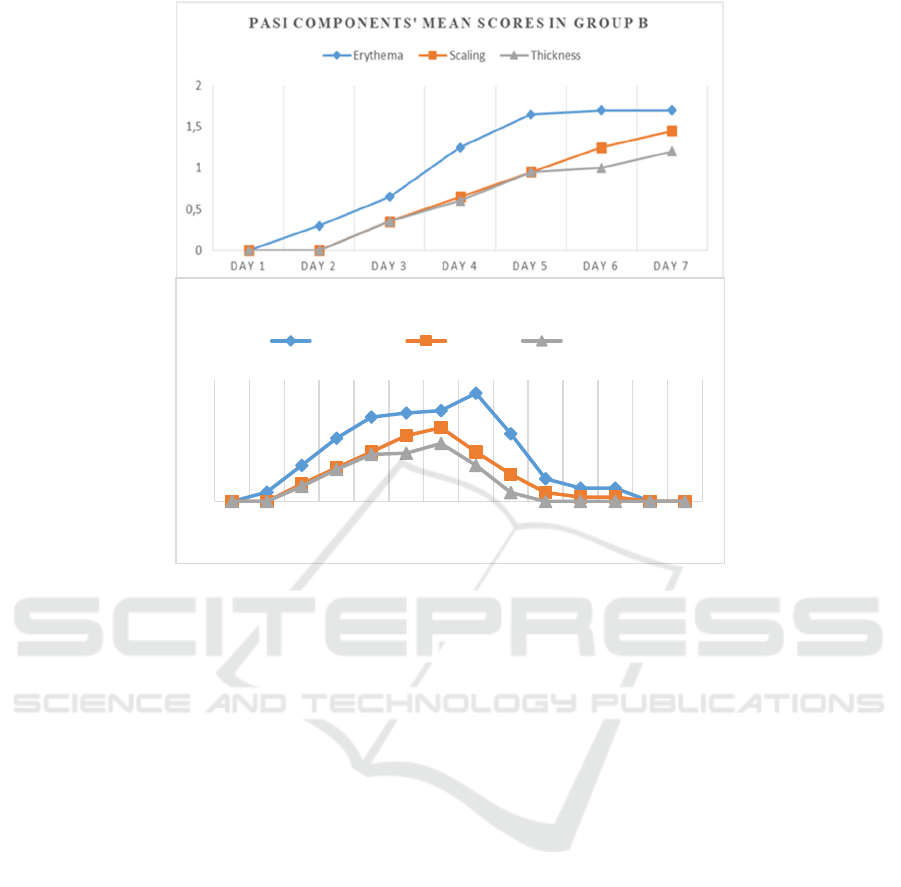
Figure 2. Progressively increasing mean of PASI scores components in groups B and C in the 7-day application of IMQ were
as the culmination point. In the mice of group C, these PASI score components also showed gradually after discontinuation
of IMQ applications.
In this study, psoriasis-like skin inflammation
induced by IMQ in mice was successful, evidenced
by the mean of PASI scores of mice in treatment
groups significantly higher than control one. The
results of this study showed similar findings from Luo
et al. (2016) that stated IMQ was a strong inducer of
psoriasiform changes in the mice skin tissue.8 The
IMQ exposures will bond to the TLR7 on the surface
of keratinocytes (Li et al., 2013), macrophages, and
plasmacytoid dendritic cells5 followed by NF-κβ,
mitogen-activated kinase protein, and activations of
the inflammatory signaling pathways (Ma et al.,
2020).
The IMQ exposure may cause the maturation of
dendritic cells and the mature ones will release IL-23.
The IL-23 may induce the differentiation and
activation of Th17 cells (Ueyama et al., 2014). The
activated Th17 cells will continue releasing
inflammatory cytokines and the chemo-attractants
that possibly causing the neutrophil migrations from
the dermal vascular to the epidermal layers, and
induce the keratinocyte cells to produce antimicrobial
peptides (AMPs) including cathelicidin. In psoriasis,
cathelicidin may induce inflammation by activating
immune cells via multiple pathways (Takahashi et al.,
2020). All of these processes may produce gradual
and prolonged inflammation in psoriasis (Kelhälä et
al., 2014). The skin tissue inflammation in mice
induced by the IMQ has gradually appeared. In this
study, the inflammation signs were initiated by
erythematous from day 2, followed by skin scaling
and thickness that visible from day 3. These skin
inflammation changes also showed disappeared
sequentially after discontinuation of IMQ exposures.
Unlike erythematous score that showed reduced later,
skin scaling and thickness disappeared earlier started
from day 1 after IMQ exposure cessation. These
gradual changes could be explained by
immunological circuits involving immune cells as
described above. Therefore, it is important to
determine the peak occurring inflammation to
become the point where tre Previous studies showed
that psoriasis histopathological features may have
similarities with chronic dermatitis (Ghasemi Basir et
al., 2018) and other psoriasiform dermatitis
(Chanadanwale et al., 2015). Previous study
mentioned that different mice strains that induced
with IMQ may give distinct responses that correspond
to other human skin disorders such as wounds or
infections (Swindell et al., 2017). Therefore, a
0
1
2
3
DAY
1
DAY
2
DAY
3
DAY
4
DAY
5
DAY
6
DAY
7
DAY
8
DAY
9
DAY
10
DAY
11
DAY
12
DAY
13
DAY
14
PASI COMPONENTS' MEAN SCORES IN GROUP C
Erythema Scaling Thickness
Reversible Imiquimod Effects on Skin Tissue of Psoriasis Mice Model: An Experimental Study
263
histopathological examination should be done to
determine psoriasiform changes apart from clinical
features evaluation in psoriasis-like inflammation
mice. Based on human psoriasis and psoriasiform
dermatitis skin biopsies, a prior study mentioned that
acanthosis, parakeratosis, hyperkeratosis, Munro
microabscess, dilated blood vessels, and
inflammatory infiltrates in the upper dermis including
neutrophils and lymphocytes consistent with
psoriasis (Chanadanwale et al., 2015).
Figure 3. Histopathological changes was not found in the mice in group A and C. Mice in group B showed the Munro abscess
(blue arrow), acanthosis (red arrow), parakeratosis (green arrow), thinning above papillae (black arrow), mild-moderate
lymphocyte infiltration (brown arrow), and papillary congestion (yellow arrow) which was pathognomonic to the psoriasis.
In this present study, these histopathological
features are also found in the skin tissue of group B
mice. The mice which were not applied with IMQ
(group A) or 7-day application discontinuation of
IMQ (group C) showed that the histopathological
images mimicking psoriasis were not found. The
mean of Baker's score 3.33 ± 1.25 in the group B mice
significantly differed from other groups. These
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
264

outcomes proved that imiquimod induction in mice
for 7 days succeeded in providing the same
psoriasiform histopathological features as those
found in human psoriasis. The results of this study are
consistent with those conducted by Luo et al. (2016),
that the repeated applications of IMQ could cause
changes in the histopathological features resembling
psoriasis.8 These histopathological changes could be
initiated by skin irritation after application of IMQ
following the mice back shave that leads to the
increase of infiltrating lymphocytes, monocytes, and
dermal dendritic cells including plasmacytoid
dendritic cells (Luo et al., 2016; Ueyama et al., 2014;
Chiricozzi et al., 2018).
Furthermore, imiquimod induced maturation of
plasmacytoid dendritic cells into activated myeloid
dendritic cells which acted as the main source of IL-
23 (Girolomoni et al., 2017). The increase of IL-23
will direct Th17 to release IL-17 and IL-22 that
trigger epidermal hyperplasia, acanthosis, and
parakeratosis.21 Hereafter, IL-17, and IL-22 also
induce the keratinocytes to produce IL-8 and AMPs
as chemoattractants for neutrophils migration from
dermal vascular to epidermis layers which may
appear as the Munro/Kogoj microabscess (Luo et al.,
2016; Kelhälä et al., 2014; Chiricozzi et al., 2018;
Moos et al., 2019). These steps of the inflammation
process in psoriasis may elucidate the
histopathological changes induced by IMQ in the skin
of mice.
This study also indicated that the mice skin tissues
exposed to IMQ may cause the temporary induction
of psoriasiform histopathological changes. The
limitation of this study is we cannot identify the
stages of psoriasiform histopathological changes
during IMQ exposure or discontinuation, unlike the
clinical manifestation of gradual inflammation
changes that can be observed and scored.
Nevertheless, to the best of our knowledge, this is the
first study that shows the inflammatory effects of
IMQ in Balb/C female mice are temporary and the
period for these inflammatory signs subsided. The
duration of IMQ-induced inflammation in psoriasis
mice model is useful for further research to identify
pathogenesis and drug development for psoriasis.
5 CONCLUSIONS
The IMQ may induce reversible acute inflammation
with clinical and histopathological changes
resembling psoriasis in humans as treated on the
female Balb/c mice.
ACKNOWLEDGMENTS
We thank the following individuals, namely Lantip
Rujito who help us to write this article, Shinta Prima
Ardinas and Nirwan for their proficiencies,
experiences, and assistance throughout laboratory
aspects of our study.
REFERENCES
Baker, B.S., Brent, L., Valdimarsson, H., Powles, A.V., al-
Imara, L., Walker, M., et al. 1992. Is epidermal cell
proliferation in psoriatic skin grafts on nude mice
driven by T-cell derived cytokines? Br J Dermatol.,
126(2), 105-110. doi: 10.1111/j.1365-
2133.1992.tb07805.x.
Balak, D.M.W., Hajdarbegovic, E., 2017. Drug-induced
psoriasis: clinical perspectives. Psoriasis (Auckl)., 7,
87–94. doi: 10.2147/PTT.S126727.
Belinchón, I., Rivera, R., Blanch, C., Comellas, M., Lizán,
L., 2016. Adherence, satisfaction and preferences for
treatment in patients with psoriasis in the European
Union: a systematic review of the literature. Patient
Prefer Adherence, 10, 2357-2367. doi:
10.2147/PPA.S117006.
Bocheńska, K., Smolińska, E., Moskot, M., Jakóbkiewicz-
Banecka, J., Gabig-Cimińska, M., 2017. Models in the
Research Process of Psoriasis. Int J Mol Sci., 18(12),
2514. doi: 10.3390/ijms18122514.
Boehncke, W.H., Schön, M.P., 2015. Psoriasis. Lancet,
386(9997), 983-994. doi: 10.1016/S0140-
6736(14)61909-7.
Chanadanwale, S.S., Panicker, N.K., Kulkarni, S.P., Shah,
K.R., Kumar, H., Sharma, Y.K., et al., 2015.
Morphometry analysis of psoriasis and psoriasiform
dermatitis: A retrospective study of 50 cases. Med J DY
Patil Univ., 8, 43-47. doi: 10.4103/0975-2870.148843.
Chiricozzi, A., Romanelli, P., Volpe, E., Borsellino, G.,
Romanelli, M., 2018. Scanning the
Immunopathogenesis of Psoriasis. Int J Mol Sci., 19(1),
179. doi: 10.3390/ijms19010179.
Ghasemi Basir, H.R., Alirezaei, P., Hamian, Z.,
Khanlarzadeh, E., 2018. Are quantitative
histopathologic criteria capable of differentiating
psoriasis from chronic dermatitis?, Clin Cosmet
Investig Dermatol., 11, 239-244. doi:
10.2147/CCID.S160697.
Girolomoni, G., Strohal, R., Puig, L., Bachelez, H., Barker,
J., Boehncke, W.H., et al., 2017. The role of IL-23 and
the IL-23/T
H
17 immune axis in the pathogenesis and
treatment of psoriasis. J Eur Acad Dermatol Venereol.,
31(10), 1616-1626. doi: 10.1111/jdv.14433.
Hawkes, J.E., Gudjonsson, J.E., Ward, N.L., 2017. The
Snowballing Literature on Imiquimod-Induced Skin
Inflammation in Mice: A Critical Appraisal. J Invest
Dermatol., 137(3), 546-549. doi:
10.1016/j.jid.2016.10.024.
Reversible Imiquimod Effects on Skin Tissue of Psoriasis Mice Model: An Experimental Study
265

Kelhälä, H.L., Palatsi, R., Fyhrquist, N., Lehtimäki, S.,
Väyrynen, J.P., Kallioinen, M., et al. 2014. IL-17/Th17
pathway is activated in acne lesions. PLoS One., 9(8),
e105238. doi: 10.1371/journal.pone.0105238.
Li, Z.J., Sohn, K.C., Choi, D.K., Shi, G., Hong, D., Lee,
H.E., et al. 2013. Roles of TLR7 in activation of NF-κB
signaling of keratinocytes by imiquimod. PLoS One.,
8(10), e77159. doi: 10.1371/journal.pone.0077159.
Luo, D.Q., Wu, H.H., Zhao, Y.K., Liu, J.H., Wang, F.,
2016. Original Research: Different imiquimod creams
resulting in differential effects for imiquimod-induced
psoriatic mouse models. Exp Biol Med (Maywood).
241(16), 1733-1738. doi: 10.1177/1535370216647183.
Ma, F., Zhang, J., Zhang, J., Zhang, C., 2010. The TLR7
agonists imiquimod and gardiquimod improve DC-
based immunotherapy for melanoma in mice. Cell Mol
Immunol., 7(5), 381-388. doi: 10.1038/cmi.2010.30.
Moos, S., Mohebiany, A.N., Waisman, A., Kurschus, F.C.,
2019. Imiquimod-Induced Psoriasis in Mice Depends
on the IL-17 Signaling of Keratinocytes. J Invest
Dermatol., 139(5),1110-1117. doi:
10.1016/j.jid.2019.01.006.
Swindell, W.R., Michaels, K.A., Sutter, A.J., Diaconu, D.,
Fritz, Y., Xing, X., et al. 2017. Imiquimod has strain-
dependent effects in mice and does not uniquely model
human psoriasis. Genome Med., 9(1), 24. doi:
10.1186/s13073-017-0415-3.
Takahashi, T., Yamasaki, K., 2020. Psoriasis and
Antimicrobial Peptides. Int J Mol Sci., 21(18), 6791.
doi: 10.3390/ijms21186791.
Ueyama, A., Yamamoto, M., Tsujii, K., Furue, Y., Imura,
C., Shichijo, M., et al. 2014. Mechanism of
pathogenesis of imiquimod-induced skin inflammation
in the mouse: a role for interferon-alpha in dendritic cell
activation by imiquimod. J Dermatol., 41(2), 135-143.
doi: 10.1111/1346-8138.12367.
Van der Kolk, T., Assil, S., Rijneveld, R., Klaassen, E.S.,
Feiss, G., Florencia, E., et al. 2018. Comprehensive,
Multimodal Characterization of an Imiquimod-Induced
Human Skin Inflammation Model for Drug
Development. Clin Transl Sci., 11(6), 607-615. doi:
10.1111/cts.12563.
Vertebrate Animal Research. 2020. Anesthesia (Guideline),
Available from :
https://animal.research.uiowa.edu/iacuc-guidelines-
anesthesia.
World Health Organization. 2016. Global Report on
Psoriasis. World Health Organization.
https://apps.who.int/iris/handle/10665/204417.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
266
