
The Lingzhi Mushroom (Ganoderma lucidum) Extract Reduce the
Number of Lymphocyte in Diabetics Rats with Periodontitis: In Vivo
Experimental on Sprague dawley Rats
Pratitis Widi Seno
1a
, Amilia Ramadhani
2b
, Saryono
3c
, Haris Budi Widodo
4d
and Christiana C. Prihastuti
4e
1
School of Dentistry, Medical Faculty of Jenderal Soedirman University, Purwokerto, Indonesia
2
Departement of Biomedicine, School of Dentistry, Medical Faculty of Jenderal Soedirman University, Purwokerto,
Indonesia
3
Departement of Biochemistry, Nursing of Jenderal Soedirman University, Purwokerto, Indonesia
4
Departement of Oral Biology, School of Dentistry, Medical Faculty of Jenderal Soedirman University, Purwokerto,
Indonesia
Keywords: Garnoderma lucidum, lymphocyte, periodontitis, diabetes mellitus.
Abstract: Periodontitis is a chronic inflammation on the periodontal tissue that is characterized by attachment loss and
bone loss. Diabetes mellitus deteriorated the condition of periodontitis. Ganoderma lucidum has the potential
as anti-inflammation and anti-oxidant agents that can hasten the convalescence of periodontitis disease. This
research aims to understand the effect of oral administration of Ganoderma lucidum extract on the number of
lymphocytes in Sprague dawley rats induced with diabetes mellitus and periodontitis. The results showed that
the estimated number of lymphocytes in groups that given the extract decreased as the doses increased. The
group with 20 mg/kgBW dose has the lowest lymphocyte count and approached the healthy control group.
The results of the Kruskal-Wallis test showed that there are significant differences among the five treatment
groups for the estimation of lymphocyte counts (p <0.05). The Mann-Whitney test showed a significant
difference between groups in estimating lymphocyte counts (p <0.05). This research concludes that there is
an effect of G. lucidum extract to the amount of lymphocyte in Sprague dawley rats designed with diabetes
mellitus and periodontitis.
1 INTRODUCTION
Diabetes mellitus is a chronic disease that disrupts the
body's metabolism so the body can’t regulate blood
glucose levels. Diabetes mellitus has several
complications in the oral cavity, such as periodontitis,
dry mouth, gingivitis, calculus, and alveolar bone
resorption. Periodontitis occurs in patients with
diabetes mellitus, with a prevalence of 75%.
According to research conducted by Sari et al. (2017),
88.24% of people with diabetes in Sardjito Regional
Hospital suffer from periodontitis.
a
https://orcid.org/0000- 0002-9957-501X
b
https://orcid.org/0000- 0001-8902-5164
c
https://orcid.org/0000- 0002-3012-5328
d
https://orcid.org/0000- 0003-2154-902X
e
https://orcid.org/0000- 0002-0611-7651
Periodontitis is a chronic inflammatory disease
caused by specific microorganism or groups of
specific microorganisms. This infection was resulting
in progressive destruction of the periodontal ligament
and alveolar bone with increased probing depth
formation, recession, or both (Newman et al, 2015).
Lymphocytes, plasma cells and macrophages mediate
the body's response during chronic inflammation
(Stryer et al., 2012). Lymphocytes are specific
immune defences to remove antigens, release
antibodies, and provide cytokines for other immune
cells (Kumar et al., 2015).
254
Seno, P., Ramadhani, A., Saryono, ., Widodo, H. and Prihastuti, C.
The Lingzhi Mushroom (Ganoderma lucidum) Extract Reduce the Number of Lymphocyte in Diabetics Rats with Periodontitis: In Vivo Experimental on Sprague dawley Rats.
DOI: 10.5220/0010490902540259
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 254-259
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

The hyperglycemia condition in diabetes mellitus
can worsen the periodontal disease. High blood
glucose level induces high secretion of pro-
inflammatory cytokines and decreases the activity of
chemotaxis, phagocytosis, and intracellular activity
of lymphocyte. This alteration of the immune system
stimulates delayed wound healing (Daniel et al.,
2012; Mealey et al., 2006)
Diabetes mellitus and periodontitis have a two-
way relationship. Diabetes mellitus produces
Advanced Glycation End Products (AGEs), which
can increase local expression of pro-inflammatory
cytokines and enhanced the destruction of periodontal
tissue (Preshaw et al. 2012). Otherwise, high levels of
pro-inflammatory cytokines dysregulated blood
glycemic levels and also increased risk of diabetic
nephropathy, retinopathy, and heart disease. Diabetes
mellitus and periodontitis conditions will increase the
expression of IL-6. The high level of IL-6 will also
increase the number of lymphocytes in the gingival
tissue and worsen the periodontal inflammation (Wu
et al., 2015).
Scaling, root planning, curettage of gingiva, and
Host Modulating Therapy (HMT) were the golden
standard of periodontitis therapy (Andriani 2012;
Notoharjo & Sihombing 2015). Non-steroid anti-
inflammation medicines such as ibuprofen and
flurbiprofen can be used as host modulating therapies.
In the contrary, the long-term use of anti-COX2 may
lead to stomach ulcers and haemorrhage (Newman et
al., 2015). The Ganoderma lucidum (G. lucidum) has
a potent anti-inflammation effect and has a minimum
long-term side effect (Li et al., 2011).
Ganoderma lucidum (Lingzhi mushroom or
Reishi mushroom) contains triterpenoid, ganoderic
acid, polysaccharide, protein, and unsaturated fat
(Huang et al. 2018). These active substances have a
potential effect of antioxidant, anti-inflammation, and
anti-hyperglycemic (Ma et al. 2015; Ratnaningtyas et
al., 2018; Huang et al., 2018). The previous study
confirmed that ganoderic acid could inhibit the
production of COX-2, and prevent the inflammation
process (Ratnaningtyas et al., 2018).
Huang et al (2018) has proved that the provision
of 10 mg/kg BB doses of G. lucidum extract in 7 days
can decrease the amount of polymorphonuclear cells
(PMN) and in 10 days, it can lead the bone forming.
Polymorphonuclear cells are innate immune system
of non specific body defense system while a specific
immune system takes part in periodontitis (Berglundh
& Donati 2005). One of the cells that takes part in the
specific immune system is lymphocyte (Newman et
al., 2015). The aim of this study was to examine the
effect of oral administration of G. lucidum extract at
a dose of 5 mg/kg BW, 10 mg/kg BW, and 20 mg/kg
BW for 7 days on the total number of lymphocytes in
Sprague dawley rats induced with diabetes mellitus
and periodontitis.
2 MATERIAL AND METHOD
This research was an experimental laboratory with
posttest-only control group design. The study was
conducted for 3 months, started from March to May
2019. This research used 30 rats with inclusion
criteria of male Sprague dawley rats weighing about
200-300 grams, age of 2-3 months, and induced with
periodontitis and diabetes mellitus with blood glucose
levels >126 mg/dL. The rats were divided into 5
groups which include healthy control group (K1),
negative control group (K2), 5 mg/kg BW dose group
(P1), 10 mg/kg BW dose group (P2) and 20 mg/kg
BW dose group (P3).
This study was approved by the Ethics
Commission of Faculty of Medicine, Jenderal
Soedirman University under Ref: 1394 / KEPK / III /
2019. The rats were adapted and placed in a room
with sufficient air flow and light for one week. The
extract was produced by dissolving 300 grams of G.
lucidum mushroom simplicia using 96% ethanol in a
ratio of 1: 5, then it was soaked for 3x24 hours. The
mushroom extract filtrate was evaporated using a
rotary evaporator until a thick extract was obtained.
The extract was then dissolved with the dose of 5 mg
/ kg BW, 10 mg/kg B, and 20 mg / kgBB.
All the K2, P1, P2, P3 group samples were
induced with diabetes mellitus by injecting
streptozotocin (STZ) intraperitoneally at a dose of 40
mg / kg in 0.1 M citrate buffer pH 4.5 (Furman et al.,
2015; Zulkarnain et al., 2013). Measurement of blood
glucose was carried out on the third day after STZ
induction (day 10). The diabetics rats were rats with
glucose blood level >126 mg/dL (Su et al., 2006).
On day 11, K2, P1, P2, and P3 groups were
induced with periodontitis by inoculating P.
gingivalis bacteria into the labial maxillary incisors at
a dose of 1 McFarland according to the standard
bacterial test which is equivalent to a density of 108
i.e. 200 microliters or 0.2 mL everyday within 4 days.
On day 15, clinical examination showed that the rats’
gingiva were reddish and suppurating. One rat of each
groups were euthanized to examine the alveolar bone
resorption radiographically (Figure 1). The oral
administration of G. lucidum extracts and distilled
water were given every 24 hours for 7 consecutive
days using gastric tube. On day 25, the rats’ blood
samples were taken from the tail to measure post-
The Lingzhi Mushroom (Ganoderma lucidum) Extract Reduce the Number of Lymphocyte in Diabetics Rats with Periodontitis: In Vivo
Experimental on Sprague dawley Rats
255
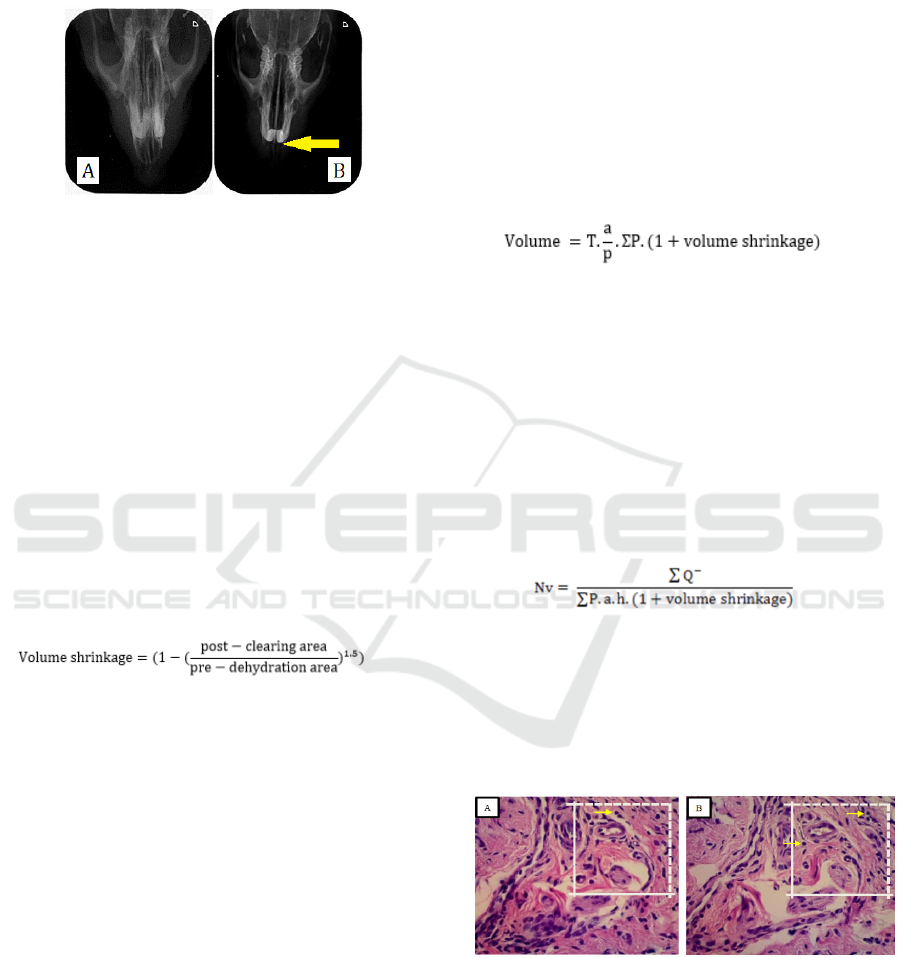
treatment blood sugar levels. The rats were
euthanized using ether and the anterior maxillary
gingival tissues were carefully extracted and stored in
10% formalin solution.
Figure 1: Radiograph image of rats alveolar bone (A)
healthy rats ; (B) periodontitis rats; Yellow arrow showed
bone resorption.
The dehydration, clearing, and embedding phases
were performed in a tissue processor (Tissue Tex®,
USA). The embedding process was carried out by the
embedding center tool (Leica®, Germany). Pre-
dehydration and post-clearing images of gingival
tissue were documented for the calculation of the
volume shrinkage. A grid composed of regularly
spaced array of points made up using Image J®
program was placed over the gingival tissue pre-
dehydration and post-clearing images. The distance
between points were 1.5 mm. The number of points
falling on the images of gingival tissues were counted
and used to calculate the volume shrinkage using the
following formula (Nyengaard, 1999):
(1)
Paraffin blocks were sectioned at nominal
thickness of 5µm using a Systematic Uniform
Random Sampling (SURS) technique (Altunkaynak
et al., 2012; Tschanz et al., 2014). A number between
1 and 20 was randomly chosen and this number
pointed to the number of the section to be sampled
together with its adjacent section. The following 18
sections were discarded and the subsequent pairs of
section were sampled. This procedure was continued
until the whole gingival tissue was exhaustively
sectioned. The average number of pairs of section was
6 pairs. All sample sections were mounted on to glass
slides and stained with Hematoxylin-eosin.
The gingival tissue volume was estimated using
the Cavalieri principle. Images of the gingival tissue
of one section from each pair of section of each rat
were viewed under Olympus CX21FS1 (Olympus
Singapore PTE, LTD) light microscope at 40X
magnification and captured using Optilab CX-21
camera (PT Minocos, Indonesia). These images were
combined in order to make a complete picture of
gingival tissue using Adobe photoshop® CS6
software (Adobe System Incorporated, United
States). The complete image was then viewed using
ImageJ
®
software (NIH Image; National Institutes of
Health, Bethesda, MD) and superimposed with
regularly spaced array of test points at a distance of
3.4 mm between points. The areas represented by
each point (a/p) were 11.56 mm
2
. All points (P) which
hit the gingival tissue were counted. The volume of
gingival tissue was estimated using the following
formula (Pulungan et al., 2018) :
(2)
where “T” is the distance between sections (mm),
“a/p” is the area per point (mm
2
), and ∑P is the
total number of points.
The numerical densities of gingival tissue’s
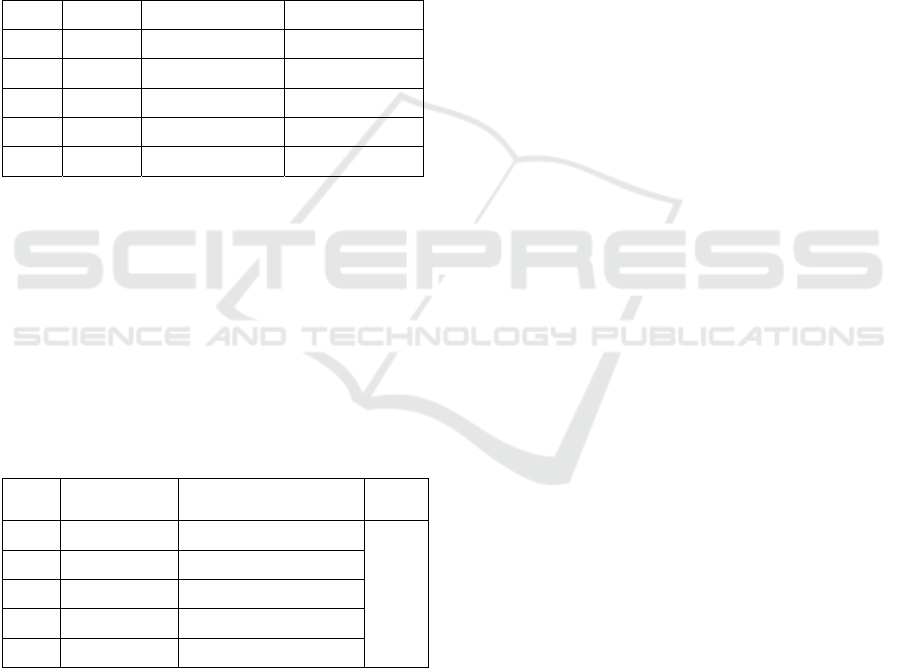
lymphocytes were determined using a physical
dissector probe. The gingival tissue were viewed and
captured at 400x magnification. The counting frame
of 60 x 60 mm
2
was used for counting the profile of
gingival tissue’s lymphocytes (Figure 2). The
numerical density (Nv) of the lymphocytes was
calculated using the following formula (Pulungan et
al., 2018).
(3)
where “ΣQ-”is the sum of nucleoli profiles; “a” is the
area of counting frames (μm
2
); “h” is the height of the
disectors which is equal to the section thickness (5
μm); and “ΣP” is the total number of counting frames.
The total number of gingival tissue’s lymphocytes
were estimated by multiplying the volume with the
numerical density.
Figure 2 : Example of paires of adjacent section; (A) Look
up section; (B) Reference section. Dashed line of counting
frame represent inclusion line, while full drawn line
represent exclusion line. Yellow arrow showed counted
lymphocyte.
The differences of total lymphocytes’ number of
in all groups were analyzed using Kruskal-Wallis test
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
256
with confidence level of 95% (p<0,05), since the data
were not normally distributed. The Mann Whitney
test were carry out with a confidence level of 95% (p
<0.05) to observe the differences of total
lymphocytes’ number between groups.
3 RESULT
The table 1 showed the rats’ blood glucose level pre-
treatment and post-treatment. The Levene test
showed that the hyperglycemic state of each rat was
different at the beginning of the study (p<0.05).
Tabel 1: Blood glucose level (mg/dL) ± SD
No. Group Pre treatment Post treatment
1. P1 214,2±43,2 240,2±52,9
2. P2 220,8±73,0 188,0±50,9
3. P3 439,4±97,6 352±76,9
4. K1 79,8±24,0 -
5. K2 363,2±151,76 312,75±107,2
P1: Groups of treatment with a dose of 5 mg/KgBB, P2:
Groups of treatment with a dose of 10 mg/KgBB, P3:
Groups of treatment with a dose of 20 mg/KgBB), K1:
Healthy control, K2: Negative control
The total number of lymphocytes in each groups were
shown in table 2. The total number of lymphocytes in
the treatment group decreased as the doses of G.
lucidum extract increased.
Oral administration of 20
mg/KgBW G.lucidum extract decreased the total
number lymphocytes related to healthy control.
Table 2: Total number of lymphocytes each groups
No. Groups Mean of estimated
l
y
m
p
hoc
y
te ± SD
P
1. P1 992,64 ± 262,43 0,04
2. P2 848,70± 358,06
3. P3 322,85± 226,73
4. K1 325,74 ± 57,8
5. K2 1.006,49 ± 488,08
P1: Groups of treatment with a dose of 5 mg/kgBW, P2:
Groups of treatment with a dose of 10 mg/kgBW, P3:
Groups of treatment with a dose of 20 mg/kgBW), K1:
Healthy control, K2: Negative control; p: Result of
Kruskal-Wallis test
The Kruskal-Wallis test results showed that there
were significant differences of the total number of
lymphocytes between all groups (p <0.05). Mann-
Whitney Test results show that there is no significant
differences of total number of lymphocytes between
the 5 mg / kgBW dose group (P1), 10 mg / kgBW
dose group (P2), and the negative control group (K2).
Otherwise, the total number of lymphocytes of the 20
mg / kgBB dose group (P3) was significant difference
to the P1, P2, and K2 groups. This result showed that
the total number of lymphocytes in group P3 similar
to the healthy control group, since there were no
significant difference with the healthy control group.
4 DISCUSSION
Inflammatory responses of periodontitis in
conjunction with DM were different than
periodontitis alone. DM condition accompanied by
periodontitis reduces the function of neutrophils,
monocytes, and macrophages in chemotaxis and
phagocytosis (Mealey, 2006). According to Otton et
al. (2002), the DM conditions affect lymphocyte
metabolism. Glucose and glutamine metabolism of
lymphocytes were altered so it requires more glucose
but cannot oxidize their metabolites efficiently and
caused decreased function of lymphocyte in immune
system.
Hyperglycemic conditions caused some
disruption in healing process such as a longer
inflammatory phase. Mirza et al. (2014) stated that in
the condition of diabetes mellitus, the inflammatory
phase will reach the peak on the 5th day and start to
decrease on the 10th day. Meanwhile, in the non-
diabetes mellitus condition, the proliferation phase
has begun on the 6th day. Changes in the period of
inflammation will affect the healing process. In
hyperglycemic conditions, reparative cells in the
periodontium and fibroblast cannot work optimally so
that the newly formed-collagen fibers were easily
destroyed by MMP (Mealey, 2006). The DM
condition modify the microvascular integrity, that is
the formation of AGEs (Mirza et al., 2014).
Inflammatory cells have AGEs receptor on the cell
surface. When AGEs bound to inflammatory cells
such as macrophages, monocytes, and lymphocytes,
the production of proinflammatory cytokines will
increase and resulted in the hyper-responsiveness of
the immune system (Mealey, 2006).
The oral administration of 20 mg/kgBW G.
lucidum extract showed a significant decrease of total
number of lymphocytes compared to negative control
group. Dudhgaonkar et al. (2009) argued that
ganoderic acid and polysaccharides from G. lucidum
extract decreased the proinflammatory cytokines
such as TNF-α, IL-6, and PGE2 from LPS induced
macrophages. As in vivo study showed that G.
The Lingzhi Mushroom (Ganoderma lucidum) Extract Reduce the Number of Lymphocyte in Diabetics Rats with Periodontitis: In Vivo
Experimental on Sprague dawley Rats
257

lucidum extract act as anti-inflammatory and
antiproliferative agents via inhibition of the NF-κB
and AP-1 signaling cycles in macrophages. Izzaty et
al., (2014) study showed that hyperglycemic
conditions cause a longer inflammatory phase,
regarding to high number of lymphocytes observed
on the 7th day of healing process. Our recent study
showed that oral administration of G. lucidum extract
for 7 consecutive days reduce the number of
lymphocytes, so that the healing process accelerated.
Triterpenoids as antioxidants play a role to bind
reactive ROS compounds such as superoxide anions
(O2-), so that the inflammatory response will
decrease (Chen et al., 2016). The decrease in ROS
will result in acceleration of proliferation phase by
decreasing the production of IL-7, so the migration of
leukocyte cells such as neutrophils and macrophages
will decrease (Pushparani, 2015; Sari et al., 2017).
Polysaccharides optimize the macrophages,
lymphocytes, and plasma cells to modulate the
inflammatory process (Ratnaningtyas et al., 2018).
Polysaccharide extract of G. lucidum has a
hypoglycemic effect by reducing glucose levels in
plasma in which it enhanced the activity and
metabolism of lymphocytes. The treatment groups of
the 5 mg/kgBW and 10 mg/kgBW G. lucidum extract
statistically showed no significant difference with the
negative control group. This shows that both doses
have not reached the maximum effect in reducing the
number of lymphocytes.
The oral administration of 20 mg/kg BW G.
lucidum extract was the most optimum dose to reduce
the number of lymphocytes in rats with periodontitis
and diabetes mellitus condition. This result different
from Huang et al. (2018) study that showed decreased
number of neutrophils of periodontitis rats on day 7
after administration of 10 mg/kgBW G. lucidum
extract. The persistent inflammatory response of
neutrophils triggered lymphocytes to form a chronic
inflammatory response. In this study, the
hyperglycemic condition is responsible for the
increase in the severity of periodontitis and the
delayed of healing response.
The oral administration of 5 mg/kgBW, 10
mg/kgBW and 20mg/kgBW G. lucidum extract did
not affect the hyperglycemic conditions of the rats.
The post treatment glucose levels were decreased, but
still remained in hyperglycemic conditions. Previous
study confirmed antidiabetic effect of G. lucidum
extract in higher dose. The administration of 50
mg/kg BW and 100 mg/kgBW G. lucidum extract
prove to provide antidiabetic effects as measured by
blood glucose levels and insulin levels of rats
(Sirisidthi et al., 2016; Zhang & Lin, 2004).
This recent study showed that the oral
administration of 20 mg/kgBW G. lucidum extract
reduced the total number of gingival tissues’
lymphocytes pronounced to the healthy control
group. As the decreased number of lymphocytes
indicate the reduced in periodontal inflammation
process (Taubman & Kawai, 2001). The higher dose
of G. lucidum extract will comprised the higher
concentration of triterpenoids and polysaccharides
and escalated the anti-inflammatory effect.
5 CONCLUSION
In conclusion, the G. lucidum extract intensify the
inflammatory phase of healing process of
periodontitis with diabetes mellitus. The total number
of lymphocytes were decline resembling the healthy
groups after oral administration of 20 mg/kg BW G.
lucidum extract. Further study is required to examine
the potential of G. lucidum extract to the next phases
of healing process of periodontitis with diabetes
mellitus.
REFERENCES
Altunkaynak, B. Z., Önger, M. E., Altunkaynak, M. E.,
Ayranci, E., & Canan, S., 2012. A Brief Introduction
To Stereology And Sampling Strategies: Basic
Concepts Of Stereology. Neuroquantology, 10(1), Pp.
31–43.
Andriani, I., 2012. Efektivitas Antara Scaling Root Planing
(Srp) Dengan Dan Tanpa Pemberian Ciprofloxacin Per
Oral Pada Penderita Periodontitis. Insiva Dental
Journal, 1(2), Pp. 81–89.
Berglundh, T., & Donati, M., 2005. Aspects Of Adaptive
Host Response In Periodontitis. Journal Of Clinical
Periodontology, 32(Suppl. 6), Pp. 87–107.
Chen, T.-Q., Yang, C., Wu, J.-G., & Wu, J.-Z., 2016. Total
Triterpenoids From The Ultrasonic-Circulating Extract
Powder Of Ganoderma Lucidum And Its Antioxidant
Activity In Vitro. Ournal Of Chemical And
Pharmaceutical Research, 8(5), Pp. 730–735.
Daniel, R., Gokulanathan, S., Shanmugasundaram, N.,
Lakshmigandhan, M., & Kavin, T., 2012. Diabetes And
Periodontal Disease. Journal Of Pharmacy And
Bioallied Sciences, 4(6), Pp. 280–283.
Dudhgaonkar, S., Thyagarajan, A., & Sliva, D., 2009.
Suppression Of The Inflammatory Response By
Triterpenes Isolated From The Mushroom Ganoderma
Lucidum. International Immunopharmacology, 9(11),
Pp. 1272–1280.
Furman, B. L., 2015. Streptozotocin-Induced Diabetic
Models In Mice And Rats. Current Protocols In
Pharmacology, 70(1), Pp. 5471-54720.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
258

Huang, P.-H., Hsieh, M.-C., Weng, P.-W., Cheng, W.-C.,
Chu, C.-L., Chen, D.-C., Sung, C.-E., & Huang, R.-Y.,
2018. Ganoderma Lucidum Reduces Inflammation-
Induced Bone Loss : A Pilot Study In Rats. 1(1), Pp.
35–40.
Izzaty, A., Dewi, N., & Pratiwi, D. I. N., 2014.
Ekstrakharuan (Channa Striata) Secara Efektif
Menurunkan Jumlah Limfosit Fase Inflamasi Dalam
Penyembuhan Luka. Journal Of Dentomaxillofacial
Science, 13(3), 176.
Kumar, V., Abbas, A. K., & Aster, J. C., 2015. Robbins And
Cotran Pathologic Basic Of Disease (Ninth Edit).
Elsevier Ltd.
Li, F., Zhang, Y., & Zhong, Z., 2011. Antihyperglycemic
Effect Of Ganoderma Lucidum Polysaccharides On
Streptozotocininduced Diabetic Mice. International
Journal Of Molecular Sciences, 12(9), Pp. 6135–6145.
Ma, H. T., Hsieh, J. F., & Chen, S. T., 2015. Anti-Diabetic
Effects Of Ganoderma Lucidum. Phytochemistry, 114,
Pp. 109–113.
Mealey, B. L., 2006. Periodontal Diseases And Diabetes: A
Two-Way Street. The Journal Of American Dental
Association, 137(12), Pp. 26s-31s.
Mirza, R. E., Fang, M. M., Weinheimer-Haus, E. M., Ennis,
W. J., & Koh, T. J., 2014. Sustained Inflammasome
Activity In Macrophages Impairs Wound Healing In
Type 2 Diabetic Humans And Mice. Diabetes, 63(3),
Pp. 1103–1114.
Newman, M. G., Takei, H. H., & Klokkevold, P. R., 2015.
Carranza’s Clinical Periodontology (F. A. Carranza
(Ed.); 12th Ed.). Elsevier.
Notoharjo, I. T., & Sihombing, M., 2015. Faktor Resiko
Pada Penyakit Jaringan Periodontal Gigi Di Indonesia
(Riskesdas 2013). Buletin Penelitian Sistem Kesehatan,
18(1), Pp. 87–94.
Nyengaard, J.R., 1999. Stereologic Methods And Their
Application In Kidney Research. Journal Of The
American Society Of Nephrology : Jasn, 10(5), Pp.
1100–1123.
Otton, R., Mendonça, J. R., & Curi, R., 2002. Diabetes
Causes Marked Changes In Lymphocyte Metabolism.
Journal Of Endocrinology, 174(1), Pp. 55–61.
Preshaw, P., Alba, A., Herrera, D., Jepsen, S.,
Konstantinidis, A., Makrilakis, K., & Taylor, R., 2012.
Periodontitis And Diabetes: A Two-Way Relationship.
International Journal Of Evidence-Based Healthcare,
55, Pp. 21–31.
Pulungan, Z. S. A., Sofro, Z. M., & Partadiredja, G., 2018.
Sodium Fluoride Does Not Affect The Working
Memory And Number Of Pyramidal Cells In Rat
Medial Prefrontal Cortex. Anatomical Science
International, 93(1), Pp. 128–138.
Pushparani, D., 2015. Role Of Cytokines In Periodontal
Wound Healing Process - A Review. Pharmaceutical
Analytical Chemistry: Open Access, 01(01), Pp. 1–5.
Ratnaningtyas, N. I., Hernayanti, H., Andarwanti, S.,
Ekowati, N., Purwanti, E. S., & Sukmawati, D., 2018.
Effects Of Ganoderma Lucidum Extract On Diabetic
Rats. Biosaintifika: Journal Of Biology & Biology
Education, 10(3), Pp. 642–647.
Sari, R., Herawati, D., Nurcahyanti, R., & Wardani, P. K.,
2017. Prevalensi Periodontitis Pada Pasien Diabetes
Mellitus (Studi Observasional Di Poliklinik Penyakit
Dalam Rsup Dr. Sardjito). Majalah Kedokteran Gigi
Indonesia, 3(2), Pp. 98.
Sirisidthi, K., Kosai, P., & Jiraungkoorskul, W., 2016.
Antidiabetic Activity Of The Lingzhi Or Reishi
Medicinal Mushroom Ganoderma Lucidum: A Review.
Sa Pharmaceutical Journal, 83(8), 45–47.
Stryer, D. S., Rubin, E., Saffitz, J. E., & Schiller, A. L.,
2012. Rubin’s Pathology. Wolters Kluwer Health.
Su, H. C., Hung, L. M., & Chen, J. K., 2006. Resveratrol,
A Red Wine Antioxidant, Possesses An Insulin-Like
Effect In Streptozotocin-Induced Diabetic Rats.
American Journal Of Physiology - Endocrinology And
Metabolism, 290(6), Pp. 1339–1346.
Taubman, M. A., & Kawai, T., 2001. Involvement Of T-
Lymphocytes In Periodontal Disease And In Direct
And Indirect Induction Of Bone Resorption. Critical
Reviews In Oral Biology And Medicine, 12(2), Pp.
125–135.
Tschanz, S., Schneider, J. P., & Knudsen, L. (2014).
Design-Based Stereology: Planning, Volumetry And
Sampling Are Crucial Steps For A Successful Study.
Annals Of Anatomy, 196(1), Pp. 3–11.
Https://Doi.Org/10.1016/J.Aanat.2013.04.011
Wu, Y. Y., Xiao, E., & Graves, D. T. (2015). Diabetes
Mellitus Related Bone Metabolism And Periodontal
Disease. International Journal Of Oral Science, 7(2),
Pp. 63–72.
Zhang, H. N., & Lin, Z. Bin., 2004. Hypoglycemic Effect
Of Ganoderma Lucidum Polysaccharides. Acta
Pharmacologica Sinica, 25(2), Pp. 191–195.
Zulkarnain., 2013. Perubahan Kadar Glukosa Darah Puasa
Pada Tikus Sprague Dawley Yang Diinduksi
Streptozotocin Dosis Rendah. Jurnal Kedokteran Syiah
Kuala, 13(2), Pp. 71–76.
The Lingzhi Mushroom (Ganoderma lucidum) Extract Reduce the Number of Lymphocyte in Diabetics Rats with Periodontitis: In Vivo
Experimental on Sprague dawley Rats
259
