
Antimicrobial Potential Activity of Extract Selaginella plana (Desv.
Ex Poir.) Hieron against the Growth of Staphylococcus aureus ATCC
25922 and Methicillin-Resistance Staphylococcus aureus (MRSA)
Juen Carla Warella
1
a
, Agung Dwi Wahyu Widodo
2
b
, Rebekah Juniati Setiabudi
3
c
,
Retno Indrawati Roestamadji
4
d
, Maftuchah Rochmanti
5
e
and Pudji Lestari
6
f
1
Basic Medical Science Study Program, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
2
Department of Medical Microbiology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
3
Department of Oral Biology, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, Indonesia
4
Department of Pharmacology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia
5
Department of Public Health, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia.
Keywords: Antimicrobial, Selaginella plana, Staphylococcus aureus, MRSA.
Abstract: For thousands years, medicinal plants have been used as a source of powerful therapeutic agents, and until
now, many medicines are used from natural products derived from plants or their derivatives. Plants that
contain secondary metabolites can be used as antimicrobials, one of them is Selaginella plana. In this study,
there were 8 treatments consisting of 6 treatments of extract concentration, 1 positive control (vancomycin),
and 1 negative control (distilled water) with 3 replications. The antimicrobial test used was the Tube Dilution
method using Mueller Hinton Broth to determine the MIC and Mueller Hinton Agar to determine the MBC.
Selaginella plana extract showed inhibition against Staphylococcus aureus with MIC values of 12.5% and in
MRSA with MIC value of 50%. In MBC test, the killing power of Selaginella plana extract against
Staphylococcus aureus obtained MBC value of 12.5%. Meanwhile, MRSA bacteria showed negative results,
which were indicated by the growth of colonies. Selaginella plana extract (Desv.ex Poir.) Hieron was able to
show antimicrobial activity on Staphylococcus aureus with the MIC value of 12.5%, and the MBC value of
12.5% while in MRSA, Selaginella plana extract (Desv.ex Poir.) Hieron had an MIC value of 50 %, and the
MBC value was negative.
1 INTRODUCTION
For thousands years, plants have been used as
efficacious therapeutic agents, and until recently,
many medicines are used from natural products from
plants and its derivatives (Kinghorn et al., 2011;
Newman and Cragg, 2012). Almost all ancient
findings regarding medicines are sourced from
natural ingredients (Quiason, 2011). WHO report in
2014 recorded that in 129 countries and 80%
population, natural ingredients were used to meet
a
https://orcid.org/0000-0003-2341-521X
b
https://orcid.org/0000-0002-8538-719X
c
https://orcid.org/0000-0003-2171-8743
d
https://orcid.org/0000-0002-4597-6782
e
https://orcid.org/0000-0002-9222-9376
f
https://orcid.org/0000-0003-4725-4676
treatment needs. Similarly, traditional medicines in
China contributed approximately 18% of all
treatments (WHO, 2014).
It was also discovered that more than a third of
medicines (39.1%) authorized by the Food and Drug
Administration (FDA) were sourced from natural
ingredients (Boy et al., 2018). One of the
continuously developed natural molecules is
secondary metabolite substances, in which
approximately 12,000 have been isolated, and the
estimated number is less than 10% (Cowan, 1999).
Warella, J., Wahyu Widodo, A., Setiabudi, R., Roestamadji, R., Rochmanti, M. and Lestari, P.
Antimicrobial Potential Activity of Extract Selaginella plana (Desv. Ex Poir.) Hieron against the Growth of Staphylococcus aureus ATCC 25922 and Methicillin-Resistance Staphylococcus
aureus (MRSA).
DOI: 10.5220/0010490802450253
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 245-253
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
245

In Indonesia, the utilization of plant-based
medicines is a part of national cultivation and has
been existing for centuries. However, its
effectiveness and safety have not been supported by a
comprehensive study (WHO, 2010). One of the
biological resources in Indonesia is Selaginella Pal.
Beauv (Selaginellaceae Reichb). Selaginella has been
used as an alternative medicine in several traditional
treatments, such as to cure injuries, skin diseases,
cancers (Chen et al., 2005), anti-inflammation (Raj et
al., 2006; Won et al., 2006), rheumatic, and as anti-
microbes.
Plants with antimicrobial potential commonly
have secondary metabolites. Selaginella has species-
dependent molecular bioactivities, such as phenolic
(flavonoid), alkaloid, and terpenoid contents.
However, bioflavonoids (a dimeric form of
flavonoids) are the key bioactive substances of
Selaginella, consisting of 13 substances, particularly
amentoflavone and ginkgetin (Setyawan, 2011).
Antimicrobial substances can be used as a strategy to
tackle health problems related to bacteria, fungi, and
parasites.
According to (WHO, 2015), one of the current
global problems is antimicrobial resistance
threatening public health. Hence, the search for
effective antimicrobial agents can help prevent and
heal the patients. Antimicrobial agent from natural
substances is one of the alternative treatments that is
continuously developed. The antimicrobial agent is
classified into six categories, namely biosynthesis,
biological source, biological function, molecular
properties, structure, composition, and molecular
purpose (Castro-rosas et al., 2017)
From previous studies, it is discovered that only
several species had been observed in detail, such as
Selaginella uncinata (Zou et al., 2013b; Zou et al.,
2014; Taylor et al., 2013; Zou et al., 2016b),
Selaginella doederleinii (Li et al., 2016), Selaginella
involvens (Long et al., 2015), Selaginella tamariscina
(Xu, et a.l, 2011a; Xu et al., 2011b; Xu et al., 2015ab),
Selaginella moellendorffii (Zou et al., 2016a; Zeng et
al., 2017; Zou et al., 2013a), and Selaginella
willdenowii (Chai and Wong, 2012). Meanwhile, the
most distributed Selaginella in Indonesia, i.e.,
Selaginella plana is yet to be observed further.
Based on the background, the authors were
interested in conducting a study regarding the
“Antimicrobial Potential Activity of Extract
Selaginella plana (Desv. Ex Poir.) Hieron against the
Growth of Staphylococcus aureus ATCC 25922 and
Methicillin-Resistance Staphylococcus aureus
(MRSA)”.
2 MATERIAL AND METHODS
In this section, the authors explain the steps in testing
the potential antimicrobial activity of Selaginella
plana (Desv. ex poir.) Hieron extract against the
growth of Staphylococcus aureus ATCC 25922 and
methicillin-resistance Staphylococcus aureus
(MRSA). This study was conducted in the
Pharmacology Laboratory, Faculty of Medicine,
Universitas Airlangga and Medical Microbiology
Laboratory, Faculty of Medicine, Universitas
Airlangga since 15 February 2020 to 14 March 2020.
2.1 Materials
The primary material used was Selaginella plana.
The solvent used in the extraction process was
ethanol 96%. Antimicrobial materials consisted of
Mueller Hinton Agar, Mueller Hinton broth,
vancomycin, test bacteria Staphylococcus aureus
ATCC 25922, methicillin-resistance Staphylococcus
aureus (MRSA), suspension of 0.5 McFarland, and
distilled water.
Equipment used were autoclave GEA FSF-24LDJ
(Hahei, China), oven, refrigerator LG GN-
B215SQMT (Taizhou, China), vortex GEMMY VM-
300 (Taiwan), incubator Memmert UN 33 53L
(Germany), vacuum rotary evaporator Heidolph VV
2000 (Nuremberg, Germany), digital scale FX-300i
(Max 320 g), micropipettes, Bunsen, smear loops, and
glass equipment such as test tubes, petri dish,
Erlenmeyer flask, beaker glass, and volumetric
pipettes.
2.2 Plant Extraction Preparation
In this study, Selaginella plana obtained from Kairatu
Village, West Seram, Maluku Province, on 10
February 2020.
As many as 1 kg of Selaginella plana leaves was
washed using running water, dried under shades,
chopped to pieces, and dried in the oven. Dried leaves
were blended into powders of 600 g. The extraction
process used a maceration method with ethanol 96%
as a solvent. The 600 g powder was soaked in ethanol
of 5400 ml while stirred for 24 hours. The top layer
was taken using Whatman paper no. 41, and the
soaking process was repeated for three times. The
filtrate was dried in the rotary evaporator of 60°C
until the ethanol solution was separated from the
active substance.
The extract was weighed and calculated using the
following formula: Extract % = dried mass / extract
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
246

volume x 1000 ml. The maceration process resulted
in Selaginella plana extracts of 70 g.
2.3 Antimicrobial Activity of the Plant
Extracts
This study used Tube Dilution Test method. This
method was utilized to determine the MIC (Minimum
Inhibitory Concentration) and MBC (Minimum
Bactericidal Concentration). The dilution method
testing was carried out according to the
recommendation of the Clinical and Laboratory
Standards Institute for the determination of MIC and
MBC.
2.3.1 Bacterial Strain
The antimicrobial activity testing of Selaginella
plana extracts used two bacteria strains, i.e.,
Staphylococcus aureus ATCC 25922 and methicillin-
resistance Staphylococcus aureus (MRSA). The
Staphylococcus aureus ATCC 25922 bacteria strain
was obtained from the Health Laboratory Center
Surabaya, and the methicillin-resistance
Staphylococcus aureus (MRSA) bacteria strain was
obtained from the Microbiology Laboratory, Faculty
of Medicine, Universitas Airlangga, Surabaya.
2.3.2 Preparation of Bacterial Suspension
The bacteria rejuvenation process used Mueller
Hinton Agar. The incubation was carried out for 24
hours at the optimum temperature of 37°C. The
bacteria suspension production used Mueller Hinton
broth. One smear of microbes was put into 5 ml media
in the test tube, vortexed, and adjusted to the standard
of 0.5 McFarland (1.5 x 10
8
CFU/ml).
2.3.3 Antimicrobial Activity Assay
There were 6 Selaginella plana (Desv.ex Poir.)
Hieron extracts’ concentrations including 100%,
50%, 25%, 12.5%, 6.25%, and 3.125%. One positive
control used Vancomycin 30 mg, and 1 negative
control used distilled water. The dilution process was
conducted in stages, initiated by the treatment 1 (P1)
group by putting 1 ml of 100% Selaginella plana
extract into 1 ml of Mueller Hinton broth and
vortexed them to be mixed. The treatment 2 (P2)
group was made by putting 1 ml of 50% P1 solution
into 1 ml of Mueller Hinton broth and vortexed them
to be mixed. The same step was applied to P3 group
of 25% sample concentration, the P4 group of 1.25%,
the P5 group of 6.25%, and the P6 group of 3.125%.
Each group was added with 1 ml of bacteria
suspension (1.5 x 10
8
CFU/ml) and repeated three
times. Incubations were carried out for 24 hours and
72 hours with a temperature of 37°C in incubators,
which were then observed and compared with the
positive and negative controls.
2.3.4 Determination of Minimum Inhibitory
Concentration (MIC)
Minimum Inhibitory Concentration (MIC) is the
minimum extract concentration to inhibit microbial
growth after being incubated for 24 hours. The
determination of Minimum Inhibitory Concentration
(MIC) was conducted by taking all incubated
treatment groups, vortexing each tube of different
concentrations, and observing the smallest
concentration to inhibit bacterial growth (visually
marked by three observers) and determined as the
MIC (Brantner and Grein, 1994; Chérigo et al., 2009).
2.3.5 Determination of Minimum
Bactericidal Concentration (MBC)
Minimum Bactericidal Concentration (MBC) is the
minimum concentration of test materials to kill
bacteria, measured using the colony counter. The
Minimum Bactericidal Concentration (MBC) was
conducted by taking samples and smearing them to
the Mueller Hinton agar and incubated at 37°C for 24
hours. It was then determined for the smallest
concentration where microbial colonies stopped
growing on the media.
The colony growth on the Mueller Hinton agar
was declared with: (-) if more than 10 colonies were
obtained on the Petri dish, (+) if less than 10 colonies
were obtained on the Petri dish, and if colonies were
grouping, it was counted as one colony.
3 RESULTS
3.1 Extraction
Selaginella plana with a wet weight of 1 kg was dried
to obtain a dry weight of 600 grams. Selaginella
plana was then extracted with a maceration method
using ethanol 96% solvent. Extracts from the
maceration process were 70 grams.
3.2 Minimum Inhibitory Concentration
(MIC) of the Plant Extract
The microbial activity test was conducted using broth
dilution method. Concentrations used were 100%,
Antimicrobial Potential Activity of Extract Selaginella plana (Desv. Ex Poir.) Hieron against the Growth of Staphylococcus aureus ATCC
25922 and Methicillin-Resistance Staphylococcus aureus (MRSA)
247
50%, 25%, 12.5%, 6.25%, and 3.125%. The negative
control was distilled water, while the positive control
was vancomycin.
The test results of Minimum Inhibitory
Concentration (MIC) of Selaginella plana (desv.ex
poir.)
Hieron extracts are presented in Table 1. The
Table 1: Minimum Inhibitory Concentration of Selaginella plana.
Bacterial Strain Replication
Test Concentration
100% 50% 25% 12.5% 6.25% 3.125%
Staphylococcus aureus
ATCC 25922
1 + + + + + -
2 + + + + - -
3 + + + + - -
methicillin-resistant
Staphylococcus aureus (MRSA)
1 + + + - - -
2 + + - - - -
3 + + - - - -
results of antimicrobial activity testing showed
turbidity differences on different concentration
levels. Therefore, the Minimum Inhibitory
Concentration (MIC) on a particular concentration
was determined.
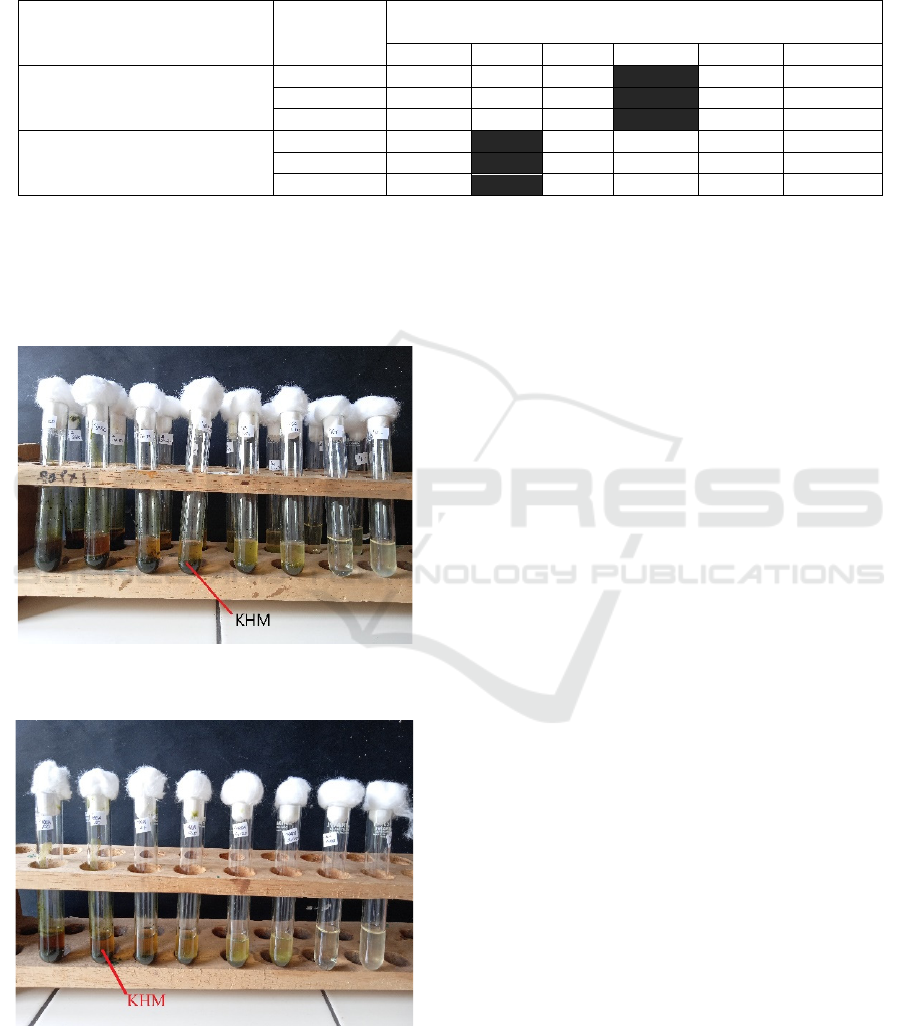
Figure 1: The Minimum Inhibitory Concentration (MIC) of
Staphylococcus aureus ATCC 25922.
Figure 2: The Minimum Inhibitory Concentration (MIC) of
methicillin-resistance Staphylococcus aureus (MRSA).
The testing of Selaginella plana extract on
Staphylococcus aureus ATCC 25922 bacteria was
conducted on different concentrations, i.e., 100%,
50%, 25%, 12.5%, 6.25%, and 3.125%. The results in
Table 1 present that the first (100%), second (50%),
third (25%), and fourth (12.5%) tubes showed no
turbidity. Therefore, the fourth (12.5%) tube was
determined as the Minimum Inhibitory
Concentration. On the positive control tube with
vancomycin, no turbidity presented. Meanwhile, the
negative control tube with distilled water showed
turbidity (Figure 1).
The testing for MIC was also applied to the
methicillin-resistance Staphylococcus aureus
(MRSA) bacteria with the same concentration of
100%, 50%, 25%, 12.5%, 6.25%, and 3.125%. The
results in Table 1 show that the 100% and 50%
concentrations had abilities to inhibit MRSA’s
growth, marked by no turbidity in tubes. Therefore,
the 50% concentration was considered as the MIC.
However, the inhibitory potential of Selaginella
plana extract was considered weak because the lower
concentrations of 25%, 12.5%, 6.25%, and 3.125%
showed turbidity and thick lumps. The positive
control tube with vancomycin showed no turbidity,
and the negative control tube with distilled water
showed turbidity (Figure 2).
However, due to the incomplete screening of
Selaginella plana extraction results, it may leave
dregs that pose bias in determining the MIC.
Therefore, the microbes’ growth inhibition was also
tested using selective growth media for each microbe.
It aimed to confirm the presence or absence of
microbes’ growth in a particular concentration
showing the Minimum Inhibitory Concentration
(MIC). The result obtained was determined as the
Minimum Bactericidal Concentration (MBC).
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
248
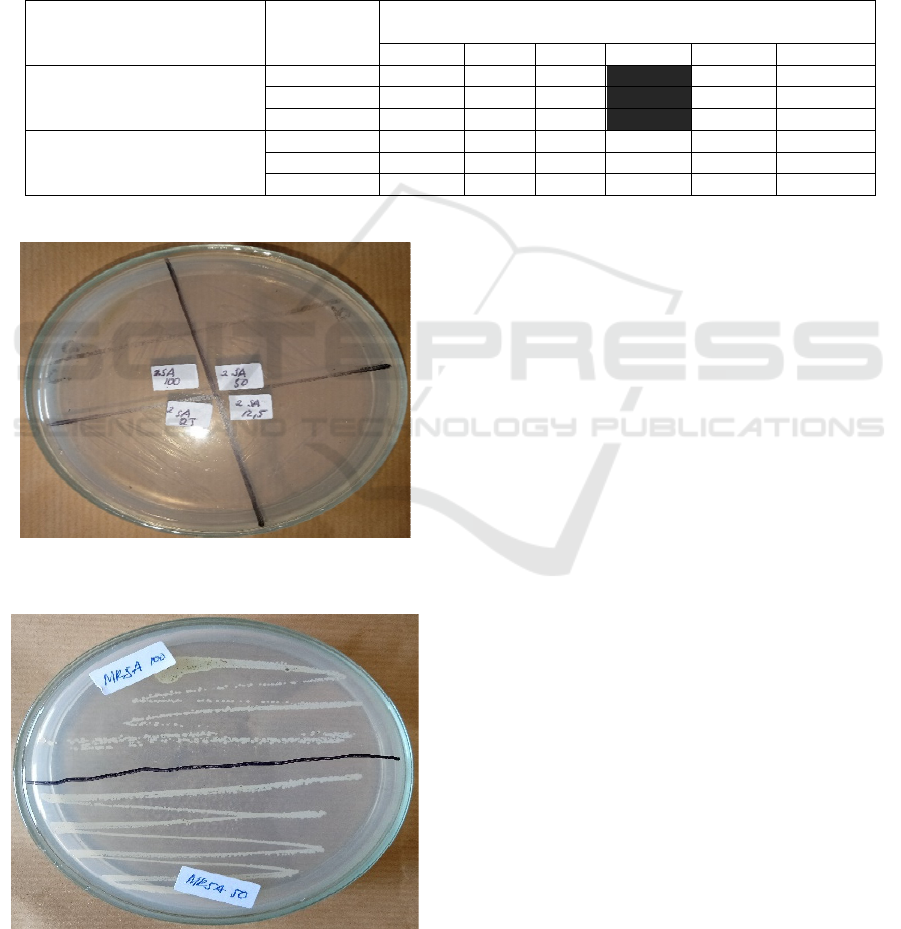
3.3 The Minimum Bactericidal
Concentration (MBC) of the Plant
Extract
The test results of Minimum Bactericidal
Concentration (MBC) of Selaginella plana (desv.ex
poir.) Hieron extracts are presented in Table 2.
The Minimum Bactericidal Concentration (MBC)
test to Staphylococcus aureus ATCC 25922 bacteria
on the concentrations of 100%, 50%, 25%, and 12.5%
showed positive results, marked with zero growth of
Staphylococcus aureus ATCC 25922 colony on all
test concentrations (Figure 3).
The Minimum Bactericidal Concentration (MBC)
test to MRSA bacteria on the concentrations of 100%
and 50% showed negative results, marked with
MRSA colony growth on all concentrations. It shows
that Selaginella plana extracts are incapable to kill
MRSA (Figure 4).
Table 2: Minimum Bactericidal Concentration of Selaginella plana.
Bacterial Strain Replication
Test Concentration
100% 50% 25% 12.5% 6.25% 3.125%
Staphylococcus aureus
ATCC 25922
1 + + + + + -
2 + + + + - -
3 + + + + - -
methicillin-resistant
Staphylococcus aureus
(MRSA)
1 +
- - - - -
2 - - - - - -
3 - - - - - -
Figure 3: Minimum Bactericidal Concentration (MBC) of
Staphylococcus aureus ATCC 25922.
Figure 4: Minimum Bactericidal Concentration (MBC) of
and methicillin-Resistance Staphylococcus aureus (MRSA)
4 DISCUSSION
4.1 Minimum Inhibitory Concentration
(MIC) of Selaginella plana
Staphylococcus aureus and methicillin-resistance
Staphylococcus aureus (MRSA) bacteria are
pathogenic microorganisms commonly infecting
humans, and many researchers suggested that these
microbes are resistant to medicines (Kumar, 2016;
Passàli et al., 2007; Ksiezopolska, 2018; Onanuga,
2011). It was discovered that secondary metabolite
substances in plants play a vital role as antimicrobial,
especially phenolic (Gechev et al., 2014).
On the MRSA bacteria, the MIC test showed
positive results, marked by the absence of turbidity in
a high concentration. The previous study discovered
that a flavonoid substance of 7-O-Butyl Naringenin
had activity against MRSA strains on a lower MIC
than natural flavonoids (Lee et al., 2013a). Glabrol
elements in flavonoids disturb membrane potentials
and permeability, hence, potential to be used as an
anti-microbe against MRSA (Wu et al., 2019).
The study by (Cao et al., 2010b) on active
substances in Selaginella pulvinata have good and
significant inhibitory activity against Staphylococcus
aureus with a MIC value of 9.6 μg/ml. According to
(Zou et al., 2016a), flavonoid compounds can inhibit
S.aureus growth with a MIC value of 12.5 μg/ml.
Flavonoids ability as anti-microbes depends on
the aromatic ring structure (Xie et al., 2014).
Antimicrobial Potential Activity of Extract Selaginella plana (Desv. Ex Poir.) Hieron against the Growth of Staphylococcus aureus ATCC
25922 and Methicillin-Resistance Staphylococcus aureus (MRSA)
249

Flavonoid activities disturb membrane integrity due
to an interaction with phospholipids that change the
membrane protein’s structure and function, adhere to
the membrane’s hydrophobic and hydrophilic sides,
and cause dysfunction of plasm membrane’s works
(Górniak et al., 2019). It also causes cell agglutination
(Babii et al., 2016), energy metabolism disruption,
nucleic acid synthesis, coenzyme metabolism, and
cell leaking (Cushnie and Lamb, 2011).
Bacteria used in this study were gram-positive
bacteria, i.e., Staphylococcus aureus and methicillin-
resistance Staphylococcus aureus (MRSA), which are
also influenced by flavonoids. The reason is because
positive gram bacteria cell’s walls contain a high
amount of peptidoglycans. On the outer cell part,
phosphate groups contain ≥ 80% negative charges
(Cha et al., 2006). It causes interaction between
negative and positive charges on the carbon atom of
1.3-dithiolium flavonoid ring (Bahrin et al., 2014). As
a result, an intracellular leak. Another study also
found that a particular dosage of saponins was
effective in damaging Staphylococcus aureus cell
walls (Khan et al., 2018). However, terpenoids do not
pose activities on Staphylococcus aureus, while on
methicillin-resistance Staphylococcus aureus
(MRSA), terpenoids posed activities as anti-MRSA,
although not effective as standard medicines
(Nzogong et al., 2018)
.
4.2 Minimum Bactericidal
Concentration (MBC) of
Selaginella plana
The MBC value test in Table 2 shows that Selaginella
plana extracts on the concentrations of 100%, 50%,
25%, 12.5%, and 6.25% had positive results against
Staphylococcus aureus.
Different results were presented by methicillin-
resistance Staphylococcus aureus (MRSA) bacteria.
Selaginella plana extracts had no ability as
bactericidal, marked by bacteria colony growth on
Petri dishes. Phytochemical substances such as tannin
and polyphenol are major contributors to inhibit
methicillin-resistance Staphylococcus aureus
(MRSA) bacteria. Therefore, these substances'
absence affects the non-synergized multi-target
effects against methicillin-resistance Staphylococcus
aureus (MRSA) bacteria (Chew et al., 2018). The
mixture of constituents may act on several
antibacterial targets concurrently, i.e. depolarizing
the cell membrane, inhibiting the efflux pump,
disintegrating the genetic materials (Coutinho et al.,
2009; Efferth and Koch, 2010).
A study conducted by (Chew et al., 2018) found
that tannins in plants could contribute to MRSA
inhibitory activity. The potency of the phytochemical
compound can be increased if it is combined with
other medicines since it has different targets in
MRSA. Phytochemical compounds can change the
permeability of the outer cell membrane, inhibit the
efflux pump, change the active site, and β-lactamase
inhibitors (Kubo et al., 2003).
Multi-target effects of phytochemical substances
are known to act as anti-MRSA by depolarizing cell
membranes, inhibiting efflux pumps, and damaging
genetic materials (Coutinho et al., 2009; Efferth and
Koch, 2010). Methicillin-resistant Staphylococcus
aureus (MRSA) resistance towards extracts is caused
by mucosa layer thickness surrounding cell walls.
The cell wall layer produced by resistant isolates is
thicker than the sensitive walls of strains (Amira,
2016). It was caused by the decreased penicillin-
binding proteins (PBP) activity affecting the cross-
link in peptidoglycan and an increase in gene
expression related to cell wall synthesis caused an
increase in the production of teichoic acid in the cell
wall (García, et al., 2017).
A quantitative study against the inhibitory of
phytochemical compounds needs to be conducted in
determining Minimum Inhibitory Concentration
(MIC) and Minimum Bactericidal Concentration
(MBC) by observing the Optical Density (OD) value
in each tested concentration and further investigation
is conducted against the specific phytochemical
compound in preventing, inhibiting, and degrading
the biofilm growth in each microbe.
5 CONCLUSION
Based on the study results, conclusions can be drawn
as follow:
1. Selaginella plana (Desv.ex Poir.) Hieron extracts
have the potential as anti-microbes on the
Minimum Inhibitory Concentration (MIC) test
with the concentrations of 100%, 50%, 25%, and
12.5% could inhibit Staphylococcus aureus’
growth, and the concentrations of 100% and 50%
can inhibit MRSA’s growth.
2. Selaginella plana (Desv.ex Poir.) Hieron extracts
have the potential as bactericidal on the Minimum
Bactericidal Concentration (MBC) test with the
concentrations of 100%, 50%, 25%, and 12.5%
can kill Staphylococcus aureus’ growth.
However, the results are negative against MRSA
with colony growth on the concentrations of
100% and 50%.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
250

REFERENCES
Amira, H. A. A. A. (2016). Effect of plants extracts on the
growth of Candida albicans and Staphylococcus aureus.
African Journal of Pharmacy and Pharmacology,
10(16), 337–345.
https://doi.org/10.5897/ajpp2016.4522
Babii, C., Bahrin, L. G., Neagu, A. N., Gostin, I., Mihasan,
M., Birsa, L. M., & Stefan, M. (2016). Antibacterial
activity and proposed action mechanism of a new class
of synthetic tricyclic flavonoids. Journal of Applied
Microbiology, 120(3), 630–637.
https://doi.org/10.1111/jam.13048
Bahrin, L. G., Apostu, M. O., Birsa, L. M., & Stefan, M.
(2014). The antibacterial properties of sulfur containing
flavonoids. Bioorganic and Medicinal Chemistry
Letters, 24(10), 2315–2318.
https://doi.org/10.1016/j.bmcl.2014.03.071
Balouiri, M., Sadiki, M., & Ibnsouda, S. K. (2016).
Methods for in vitro evaluating antimicrobial activity :
A review. Journal of Pharmaceutical Analysis, 6(2),
71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Boy, H. I. A., Rutilla, A. J. H., Santos, K. A., Ty, A. M. T.,
Yu, A. I., Mahboob, T., … Nissapatorn, V. (2018).
Recommended Medicinal Plants as Source of Natural
Products: A Review. Digital Chinese Medicine, 1(2),
131–142. https://doi.org/10.1016/s2589-
3777(19)30018-7
Cao, Y., Chen, J., Tan, N., Oberer, L., Wagner, T., Wu, Y.,
… Wang, Q. (2010b). Antimicrobial selaginellin
derivatives from Selaginella pulvinata. Bioorganic &
Medicinal Chemistry Letters, 20(8), 2456–2460.
https://doi.org/10.1016/j.bmcl.2010.03.016
Castro-rosas, J., Ferreira-grosso, C. R., Gómez-aldapa, C.
A., Rangel-vargas, E., Rodríguez-marín, M. L.,
Guzmán-ortiz, F. A., & Falfan-cortes, R. N. (2017).
Recent advances in microencapsulation of natural
sources of antimicrobial compounds used in food - A
review. Food Research International, 102(September),
575–587.
https://doi.org/10.1016/j.foodres.2017.09.054
Cha, T. W., Guo, A., & Zhu, X. Y. (2006). Formation of
supported phospholipid bilayers on molecular surfaces:
Role of surface charge density and electrostatic
interaction. Biophysical Journal, 90(4), 1270–1274.
https://doi.org/10.1529/biophysj.105.061432
Chai, T., & Wong, F. (2012). Antioxidant properties of
aqueous extracts of Selaginella willdenowii. Journal of
Medicinal Plants Research, 6(7), 1289–1296.
https://doi.org/10.5897/JMPR11.1376
Chen, J., Duh, C., & Chen, J. (2005). New Cytotoxic
Biflavonoids from Selaginella delicatula. Planta
Medica, 71(7), 659–665. https://doi.org/10.1055/s-
2005-871273
Chew, Y. L., Mahadi, A. M., Wong, K. M., & Goh, J. K.
(2018). Anti-methicillin-resistance Staphylococcus
aureus (MRSA) compounds from Bauhinia kockiana
Korth. And their mechanism of antibacterial activity.
BMC Complementary and Alternative Medicine, 18(1),
1–9. https://doi.org/10.1186/s12906-018-2137-5
Coutinho, H. D. M., Costa, J. G. M., Lima, E. O., Falcão-
Silva, V. S., & Siqueira, J. P. (2009). Herbal therapy
associated with antibiotic therapy: Potentiation of the
antibiotic activity against methicillin - Resistant
Staphylococcus aureus by Turnera ulmifolia L. BMC
Complementary and Alternative Medicine, 9, 1–4.
https://doi.org/10.1186/1472-6882-9-13
Cowan, M. M. (1999). Plant Products as Antimicrobial
Agents. Clinical Microbiology Reviews, 12(4), 564–
582. https://doi.org/10.1128/CMR.12.4.564
Cushnie, T. P. T., & Lamb, A. J. (2011). Recent advances
in understanding the antibacterial properties of
flavonoids. International Journal of Antimicrobial
Agents, 38(2), 99–107.
https://doi.org/10.1016/j.ijantimicag.2011.02.014
Efferth, T., & Koch, E. (2010). Complex Interactions
between Phytochemicals. The Multi-Target
Therapeutic Concept of Phytotherapy. Current Drug
Targets, 12(1), 122–132.
https://doi.org/10.2174/138945011793591626
García, A. B., Viñuela-Prieto, J. M., López-González, L., &
Candel, F. J. (2017). Correlation between resistance
mechanisms in Staphylococcus aureus and cell wall and
septum thickening. Infection and Drug Resistance, 10,
353–356. https://doi.org/10.2147/IDR.S146748
Gechev, T. S., Hille, J., Woerdenbag, H. J., Benina, M.,
Mehterov, N., Toneva, V., Mueller-roeber, B. (2014).
Natural products from resurrection plants : Potential for
medical applications. Biotechnology Advances, 32(6),
1091–1101.
https://doi.org/10.1016/j.biotechadv.2014.03.005
Górniak, I., Bartoszewski, R., & Króliczewski, J. (2019).
Comprehensive review of antimicrobial activities of
plant flavonoids. Phytochemistry Reviews, 18(1), 241–
272. https://doi.org/10.1007/s11101-018-9591-z
Khan, M. I., Ahhmed, A., Shin, J. H., Baek, J. S., Kim, M.
Y., & Kim, J. D. (2018). Green Tea Seed Isolated
Saponins Exerts Antibacterial Effects against Various
Strains of Gram Positive and Gram Negative Bacteria,
a Comprehensive Study In Vitro and In Vivo. Evidence-
Based Complementary and Alternative Medicine, 2018,
12. https://doi.org/10.1155/2018/3486106
Kinghorn, A. D., Pan, L., Fletcher, J. N., & Chai, H. (2011).
The relevance of higher plants in lead compound
discovery programs. Journal of Natural Products,
74(6), 1539–1555. https://doi.org/10.1021/np200391c
Ksiezopolska, E., & Gabaldón, T. (2018). Evolutionary
emergence of drug resistance in candida opportunistic
pathogens. Genes, 9(9).
https://doi.org/10.3390/genes9090461
Kubo, I., Fujita, K. I., & Nihei, K. I. (2003). Molecular
design of multifunctional antibacterial agents against
methicillin resistant Staphylococcus aureus (MRSA).
Bioorganic and Medicinal Chemistry, 11(19), 4255–
4262. https://doi.org/10.1016/S0968-0896(03)00433-4
Kumar, M. (2016). Multidrug-resistant Staphylococcus
aureus, India, 2013–2015. Emerging Infectious
Diseases, 22(9), 1666–1667.
https://doi.org/10.3201/eid2209.160044
Antimicrobial Potential Activity of Extract Selaginella plana (Desv. Ex Poir.) Hieron against the Growth of Staphylococcus aureus ATCC
25922 and Methicillin-Resistance Staphylococcus aureus (MRSA)
251

Lee, K. A., Moon, S. H., Lee, J. Y., Kim, K. T., Park, Y. S.,
& Paik, H. D. (2013a). Antibacterial activity of a novel
flavonoid, 7-O-butyl naringenin, against methicillin-
resistant Staphylococcus aureus (MRSA). Food
Science and Biotechnology, 22(6), 1725–1728.
https://doi.org/10.1007/s10068-013-0272-9
Li, J., Yu, X., Cao, D., Li, D., Zeng, W., Zhang, G., & Tan,
G. (2016). NU SC. Fitoterapia.
https://doi.org/10.1016/j.fitote.2016.11.014
Long, H., Zou, H., Li, F., Li, J., Luo, P., Zou, Z., & Hu, C.
(2015). Fitoterapia Involven fl avones A – F , six new
fl avonoids with 3 ′ -aryl substituent from Selaginella
involven. Fitoterapia, 105, 254–259.
https://doi.org/10.1016/j.fitote.2015.07.013
Newman, D. J., & Cragg, G. M. (2012). Natural products as
sources of new drugs over the 30 years from 1981 to
2010. Journal of Natural Products, 75(3), 311–335.
https://doi.org/10.1021/np200906s
Nzogong, R. T., Ndjateu, F. S. T., Ekom, S. E., Fosso, J. A.
M., Awouafack, M. D., Tene, M., … Tamokou, J. de D.
(2018). Antimicrobial and antioxidant activities of
triterpenoid and phenolic derivatives from two
Cameroonian Melastomataceae plants: Dissotis
senegambiensis and Amphiblemma monticola. BMC
Complementary and Alternative Medicine, 18(1), 1–11.
https://doi.org/10.1186/s12906-018-2229-2
Onanuga, A., & Temedie, T. C. (2011). Multidrug-resistant
intestinal Staphylococcus aureus among self-medicated
healthy adults in Amassoma, South-South, Nigeria.
Journal of Health, Population and Nutrition, 29(5),
446–453. https://doi.org/10.3329/jhpn.v29i5.8898
Passàli, D., Lauriello, M., Passàli, G. C., Passàli, F. M., &
Bellussi, L. (2007). Group A streptococcus and its
antibiotic resistance. Acta Otorhinolaryngologica
Italica : Organo Ufficiale Della Società Italiana Di
Otorinolaringologia e Chirurgia Cervico-Facciale,
27(1), 27–32. https://doi.org/10.1155/2019/5739247
Quiason, S. (2011). Concise History of Drug Discovery
Drug Discoveries and Invention. A Global
Perspective. 3-23, 154-225.
Raj, Y., Won, J., Young, J., Woo, H., Gwang, H., Woo, E.,
& Wook, K. (2006). Potent inhibition of the inductions
of inducible nitric oxide synthase and cyclooxygenase-
2 by taiwania X avone. 15, 217–225.
https://doi.org/10.1016/j.niox.2006.01.001
Setyawan, A. D., & Darusman, L. K. (2008). REVIEW :
Senyawa Biflavonoid pada Selaginella Pal. Beauv . dan
Pemanfaatannya Review : Biflavonoid compounds of
Selaginella Pal . Beauv. and its benefit. Biodiversitas,
9, 64–81. https://doi.org/10.13057/biodiv/d090115
Taylor, P., Zou, H., Xu, K., Li, F., Zou, Z., Long, H.,Tan,
G. (2013). Journal of Asian Natural Products
Uncinataflavone , a new flavonoid with a methyl
benzoate substituent from Selaginella uncinata.
(March), 37–41.
https://doi.org/10.1080/10286020.2013.771345
Won, J., Raj, Y., Kim, M., Woo, E., Kyoon, H., & Wook,
K. (2006). Inhibition of inducible nitric oxide synthase
by sumaflavone isolated from Selaginella tamariscina.
105, 107–113.
https://doi.org/10.1016/j.jep.2005.10.001
World Health Organization (WHO). (2010). Traditional
Medicine in Republic of Indonesian Traditional
Medicine. 23–36. Retrieved from
http://www.searo.who.int/entity/medicines/topics/tradi
tional_medicines_in_republic_of_indonesia.pdf
World Health Organization (WHO). (2014). WHO
Traditional Medicine Strategy Plan 2014-2020. WHO
Traditional Medicine Strategy, (March 2014), 120–
125. Retrieved from
https://www.who.int/medicines/publications/traditiona
l/trm_strategy14_23/en/
World Health Organization (WHO). (2015). Global Action
Plan on Antimicrobial Resistance. Microbe Magazine,
10(9), 354–355.
https://doi.org/10.1128/microbe.10.354.1
Wu, S., Yang, Z., Liu, F., Peng, W., Qu, S., & Li, Q. (2019).
Antibacterial Effect and Mode of Action of Flavonoids
From Licorice Against Methicillin-Resistant
Staphylococcus aureus. Frontiers in Microbiology,
10(November), 1–14.
https://doi.org/10.3389/fmicb.2019.02489
Xie, Y., Yang, W., Tang, F., Chen, X., & Ren, L. (2014).
Antibacterial Activities of Flavonoids: Structure-
Activity Relationship and Mechanism. Current
Medicinal Chemistry, 22(1), 132–149.
https://doi.org/10.2174/0929867321666140916113443
Xu, K., Zou, H., Tan, Q., Li, F., Liu, J., & Xiang, H.
(2011a). Selaginellins I and J , two new alkynyl phenols
, from Selaginella tamariscina ( Beauv .) Spring. 13(2),
93–96. https://doi.org/10.1080/10286020.2010.536535
Xu, K., Zou, H., Li, F., & Xiang, H. (2011b). Journal of
Asian Natural Products Two new selaginellin
derivatives from Selaginella tamariscina ( Beauv .)
Spring. (December 2014), 37–41.
https://doi.org/10.1080/10286020.2011.558840
Xu, K., Li, J., Zhu, G., He, X., & Li, F. (2015a). Journal of
Asian Natural Products New Selaginellin derivatives
from Selaginella tamariscina. (April), 37–41.
https://doi.org/10.1080/10286020.2015.1016001
Xu, K., Li, J., Zhu, G., He, X., & Li, F. (2015b). New
Selaginellin derivatives from Selaginella tamariscina.
Journal of Asian Natural Products Research, (April),
37–41.
https://doi.org/10.1080/10286020.2015.1016001
Zeng, W., Yao, C., Xu, P., Zhang, G., Liu, Z., Xu, K., …
Tan, G. (2017). A new neolignan from Selaginella
moellendorffii Hieron. Natural Product Research,
6419(March),0.
https://doi.org/10.1080/14786419.2017.1297935
Zou, Z., Xu, K., Li, F., Zou, H., Liu, M., Zhang, Q., … Tan,
G. (2013a). Original article A new pyrrole alkaloid
from Selaginella moellendorfii Hieron. Chinese
Chemical Letters, 24(2), 114–116.
https://doi.org/10.1016/j.cclet.2013.01.028
Zou, H., Xu, K., Zou, Z., Long, H., Li, F., Li, J., … Tan, G.
(2013b). Journal of Asian Natural Products A new
flavonoid with 6-phenyl substituent from Selaginella
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
252

uncinata. (October), 37–41.
https://doi.org/10.1080/10286020.2012.745515
Zou, H., Xu, K., Li, F., Zou, Z., Liu, R., Liu, R., … Tan, G.
(2014). Fitoterapia Uncifl avones A – F , six novel fl
avonoids from Selaginella uncinata ( Desv .) Spring.
Fitoterapia, 99, 328–333.
https://doi.org/10.1016/j.fitote.2014.10.012
Zou, Z., Xu, P., Wu, C., Zhu, W., Zhu, G., He, X., … Tan,
G. (2016a). Fitoterapia., Carboxymethyl fl avonoids
and a chromone with antimicrobial activity from
Selaginella moellendorffii Hieron. 111, 124–129.
https://doi.org/10.1016/j.fitote.2016.04.022
Zou, H., Xu, P., iu, R., Zou, Z., Li, J., Zhong, A., & Hu, J.
(2016b). Selayclicbiflavone A , an unusual macrocyclic
biflavone from Selaginella uncinata (Desv .) Spring.
Tetrahedron Letters, 37, 37–39.
https://doi.org/10.1016/j.tetlet.2016.01.038
Antimicrobial Potential Activity of Extract Selaginella plana (Desv. Ex Poir.) Hieron against the Growth of Staphylococcus aureus ATCC
25922 and Methicillin-Resistance Staphylococcus aureus (MRSA)
253
