
Drug-induced Hypersensitivity Syndrome in a Breast Cancer Patient:
A Case Report
Raditya Bagas Wicaksono
1a
, Wahyu Djatmiko
2b
, Ismiralda Oke Putranti
3c
1
Department of Bioethics and Humanities, Faculty of Medicine Universitas Jenderal Soedirman, Purwokerto, Indonesia
2
Department of Internal Medicine, Faculty of Medicine Universitas Jenderal Soedirman
3
Department of Dermatovenerology, Faculty of Medicine Universitas Jenderal Soedirman
Keywords: Drug Allergy, Drug Eruption, DIHS, Breast Cancer.
Abstract: Drug-induced hypersensitivity syndrome (DIHS) is a life-threatening condition. The diagnosis of DIHS is
quite challenging due to highly variable clinical manifestations. This paper was aimed to describe the
diagnosis criteria, pathogenesis, and relation of DIHS with cancer. We describe a case of DIHS, probably
induced by cefadroxil, in a 50-year-old woman post modified radical mastectomy for her non-specific-type
unilateral breast cancer. After four weeks of cefadroxil therapy, the patient started to develop symptoms of
drug eruption with elevated liver function tests, direct bilirubin, alkaline phosphatase (ALP), and gamma-
glutamyl transferase (GGT). The laboratory tests also showed decreased hemoglobin and albumin. The
patient's clinical manifestations were highly suggestive of DIHS. Discontinuation of drug consumption and
administration of symptomatic therapy did not improve the condition. After four days of postoperative
monitoring in the intensive care unit, the patient did not survive the external and internal bleeding due to
severe thrombocytopenia. Several hypothetical mechanisms involved in this syndrome include defective
detoxifying enzymes, genetic defects related to human leukocyte antigen, viral infections, and concurrent
disease processes, such as a neoplasm.
1 INTRODUCTION
Drug-induced hypersensitivity syndrome (DIHS) is
one of the adverse drug reactions with systemic
manifestation. Approximately 15,1% of adverse drug
reaction happens during hospitalization, and 6,7% of
them are a severe adverse drug reaction (Demoly et
al., 2014). The diagnosis of DIHS is quite challenging
due to highly variable clinical manifestations. The
DIHS is also recently referred to as DRESS (drug
reaction with eosinophilia and systemic symptoms) or
DIDMOHS (drug-induced delayed multi-organ
hypersensitivity syndrome) (Kumari et al., 2011).
A recent report from a tertiary hospital in
Indonesia showed that drug eruption with
maculopapular rash was the most common diagnosis
(29,82%) in drug hypersensitivity reaction patients,
with antibiotics as the most frequent culprit drug
(29,8%). Septic shock was the condition that
a
https://orcid.org/0000-0002-1671-4919
b
https://orcid.org/0000-0002-9024-3086
c
https://orcid.org/0000-0002-4321-9286
increases the mortality of the patients (Soegiarto and
Putra, 2020).
The diagnosis of DIHS/DRESS is sometimes
difficult due to its similar characteristics with viral
exanthems. Physicians are often more aware of other
severe adverse reactions to drugs such as Stevens-
Johnson Syndrome and Toxic Epidermal Necrolysis
(SJS–TEN) Acute Generalized Exanthematous
Pustulosis (AGEP) than the DIHS. We want to raise
awareness of DIHS, which could happen to any
patient, including cancer patients. A better
understanding of this condition might improve
survival and life expectancy. In this case report, we
want to describe the diagnosis criteria and
pathogenesis of the DIHS.
Wicaksono, R., Djatmiko, W. and Putranti, I.
Drug-induced Hypersensitivity Syndrome in a Breast Cancer Patient: A Case Report.
DOI: 10.5220/0010490702410244
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 241-244
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
241
2 CASE PRESENTATION
We describe a case of DIHS, probably induced by
cefadroxil, in a 50-year-old woman post modified
radical mastectomy for her non-specific-type
unilateral breast cancer. The patient previously
underwent the first operation on August 16th, 2017,
an excisional biopsy for the lump in her right breast.
6 months before the excision, she had felt the tumor
but hesitated to see the doctor. The size of the tumor
was approximately 5x7 cm. Cefadroxil and
mefenamic acid were given two weeks after the
excisional biopsy. Histopathology results showed a
non-specific type of adenocarcinoma with invasion to
local lymph vessels. The patient then underwent the
second operation on September 2nd, 2017, unilateral
modified mastectomy and axillary
lymphadenectomy, thus given another two weeks of
cefadroxil and mefenamic acid. The patient started to
have a fever, jaundice, and maculopapular rash all
around her skin. She also had facial edema, scaling,
anorexia, and nausea. The suspected culprit,
cefadroxil, was directly stopped. The patient did not
have any history of a previous allergic reaction. She
was hospitalized on September 17th, 2017, for the
next five days. Increased levels of liver function tests,
ALP, GGT, and direct bilirubin were observed. The
attending physician administered dexamethasone,
diphenhydramine, cetirizine, Curcuma, and
ursodeoxycholic acid. The patient was hospitalized
for the second time on October 11th, 2017, due to
severe anemia and hypoalbuminemia. We found the
liver function test level was too high, and the rash was
reappeared all around her body, despite the
discontinuation of the culprit drug consumption.
An abdominal CT scan showed a sign of
cholestasis and paraaortic mass. She went to the third
operation on October 27th, 2017, a cholecysto-
jejunostomy shunt with a planned biopsy for the mass
above. During the procedure, we did not found any
intraabdominal mass. The liver surface was clean and
smooth. Hence, there was possibly no sign of liver
metastasis. The patient was monitored thoroughly in
the Intensive Care Unit after the operation was done.
Day by day, she showed marked deterioration of vital
signs. There was also significant bleeding inside her
respiratory tract in which the blood clot disturbed her
airway. Laboratory tests showed a considerable
increase of leukocytes with prolonged hemostasis
profile, hypoglycemia, and hypoalbuminemia. We
had given human albumin, packed red cells, and
thrombocyte concentrate. Endotracheal intubation
was done to support the patient's airway.
Norepinephrine and dopamine were also
administered for her fluid refractory shock.
Unfortunately, the patient passed away on October
31st, 2017, due to cardiorespiratory failure. Skin
manifestation and macroscopic examination of the
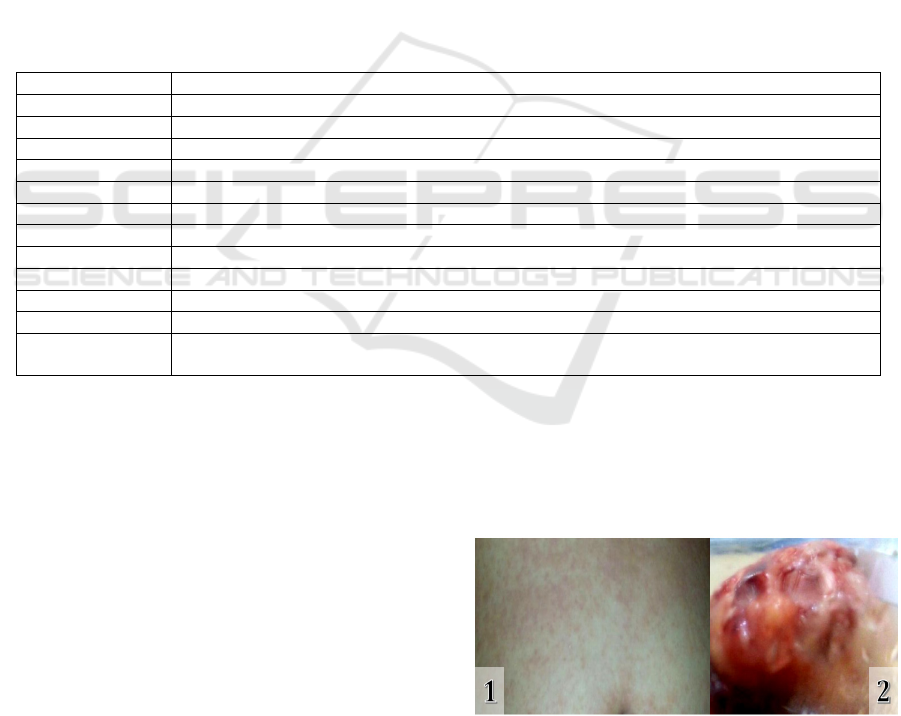
breast tumor can be seen in figure 1. The patient's
clinical course is described in table 1, and her
laboratory results are shown in Table 2.
Figure 1: (1) Maculopapular skin rash; (2) The excised
breast tumor.
Table 1: Patient’s clinical course.
Timeline
(
2017
)
Descri
p
tion
August 16
th
Excisional biopsy for right breast tumo
r
Consumption of cefadroxil (two weeks)
September 1
st
Histopathology: nonspecific type of adenocarcinoma with invasion to local lymph vessel
Se
p
tember 2
nd
Unilateral modified radical mastectom
y
Consum
p
tion of cefadroxil
(
another two weeks
)
Se
p
tember 17
th
Fever,
j
aundice,
g
eneralized maculo
p
a
p
ular rash, facial edema, scalin
g
, anorexia and nausea
Discontinuation of cefadroxil consumption and hospitalization (5 days)
October 11
th
Severe anemia and hypoalbuminemia - rehospitalization
Abdominal CT scan : cholestasis and paraaortic mass
October 27
th
Cholec
y
stic-
j
e
j
unostom
y
shunt with
p
lanned bio
p
s
y
Posto
p
erative ICU monitorin
g
October 31
st
Patient passed away due to severe hypoalbuminemia, external-internal bleeding, and
cardiores
p
irator
y
failure
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
242

Table 2: Patient's laboratory results.
Parameter
11/10/17
(hospital
admission)
14/10/17
(post-
transfusion)
28/10/17
(post-
operation)
Hemoglobin (g/dL) 7,1 (L) 11,3
Leukocyte (U/L) 10.870 11.570 (H)
Haematocrit (%) 21 (L) 33
Erhythrocyte
(cells/µL)
2,8x106
(L)
4,18x106
(L)
Thrombocyte
(cells/µL)
296.000 430.000
Eosinophil (%) 0,0% 0,0%
Lymphocyte (%) 14,5% (L) 18,2%
MCV (fL) 72,7 (L) 80,4
MCH (pg/cell) 25,2 (L) 27,4
MCHC (%) 34,6 34
Serum Iron
(µg/dL)
208 (H)
TIBC (ng/dL) 91 (L)
CEA (ng/mL) 2,4
AFP (ng/mL) 1,4
SGOT (U/L) 202 (H) 184 (H) 102 (H)
SGPT (U/L) 279 (H) 286 (H) 121 (H)
ALP (U/L) 371 (H) 249 (H) 102
GGT (U/L) 434 (H) 282 (H) 105 (H)
Albumin (g/dL) 2,47 (L) 3,05 (L) 1,78 (L)
Total Bilirubin
(mg/dL)
8,27 (H) 27,27 (H)
Direct Bilirubin
(mg/dL)
6,41 (H) 18,91 (H)
Indirect Bilirubin
(mg/dL)
1,86 (H) 8,36 (H)
3 DISCUSSION
Incidence of drug-induced hypersensitivity syndrome
ranges from 1:1.000 to 1:10.000 drug exposures. This
syndrome can turn into a fatal condition in 10% of
patients. It can be related to difficulties in diagnosing
the patient. Diagnosis of DIHS is indeed quite
challenging. Delay of diagnosis can happen because
of variable clinical manifestations and late-onset
symptoms (Cacoub et al., 2011). A maculopapular
rash can develop three weeks after starting the culprit
drug. Discontinuation of the drug consumption did
not directly eliminate the symptoms. They may
prolong more than 15 days (Shiohara et al., 2009).
European Registry of Severe Cutaneous Adverse
Reactions to Drugs and Collection of Biological
Samples (RegiSCAR) Group Criteria can be used to
diagnose DIHS by using a scoring system accurately.
Table 3: Diagnostic criteria from the RegiSCAR group
(Kardaun et al., 207)
Clinical Features -1 +1 +2
Fever No or
unknown
≥38,5°C
Lymphadenopathy ≥2 sites,
≥1 c
m
Atypical
lymphocytes
Present
Eosinophilia 10%-
19,9%
≥20%
Skin rash
- Body surface
area involve
d
>50%
- Edema,
infiltration,
purpura,
scaling
No Minimum
two
- Biopsy
suggesting
DIHS
No
Internal organ
involvement
1 organ ≥2
organs
Resolution in more
than 15 days
No or
unknown
Yes
More than 3
biological
investigations
and negative to
exclude alternative
dia
g
nosis
Yes
From the scoring system, patients are then
classified into definite (>5), probable (4-5), possible
(2-3), or no cases (50% (+1), edema and scaling (+1),
liver and gallbladder involvement (+2), also the
resolution in more than 15 days (+1). Treatment for
DIHS includes discontinuation of the culprit drug
consumption followed by administration of steroid.
Figure 3: The clinical course of DIHS. Symptoms like
maculopapular rash and fever appear three weeks after the
culprit drug was initiated (Shiohara et al., 2009
).
Drug-induced Hypersensitivity Syndrome in a Breast Cancer Patient: A Case Report
243

Two main hypotheses involved in DIHS
pathogenesis are the (pro) hapten hypothesis and the
pharmacoimmunological (p-i) hypothesis. The drug
can act as a hapten or prohapten, covalently bind with
larger molecules in vivo - such as protein – forming a
brand new antigen. This newly formed antigen will be
presented by antigen-presenting cells (APC). Hence,
it activates the drug-specific T cells, leading to
lymphocyte proliferation. Meanwhile, the p-i
hypothesis is proposing a non-covalent interaction
between drug and APC. The interaction will activate
HLA alleles (probably HLA-B) and T-cell receptors,
subsequently trigger an immune response
(Choudhary et al., 2013; Schrijvers et al., 2015). Viral
infection - such as human herpesvirus 6 (HHV-6),
cytomegalovirus (CMV), Epstein Barr virus (EBV),
and paramyxovirus – can also induce inflammation
and activate the proliferation of drug-specific T cells.
Viral infection may lower the threshold for T cell
activation. There may be a cross-reaction between
activated T cells and the culprit drug. Several
individuals can be more susceptible to DIHS due to
defects in the detoxification mechanism, resulting in
reactive metabolite formation and subsequent
immune reaction (Cacoub et al., 2011; Shiohara et al.,
2009).
4 CONCLUSIONS
Drug-induced hypersensitivity syndrome is a
complicated and fatal condition. The patient
presented in this case report is classified into a
probable case of DIHS by using the RegiSCAR
scoring system. This patient underwent a different
path and eventually passed away, despite
discontinuing the culprit drug consumption and
administration of steroids and other symptomatic
drugs. The culprit drugs may act as a (pro) hapten or
non covalently interact with APC, resulting in severe
immune reaction. The pathogenesis of DIHS may be
related to breast cancer via the activity of T helper-2
cells and eosinophils.
ACKNOWLEDGEMENTS
We want to express our gratitude to the family of the
patient and the hospital staff, who had been very
supportive of this work.
REFERENCES
Cacoub P, Musette P, Descamps V, Meyer O, Speirs C,
Finzi L, Roujeau. The DRESS Syndrome: A Literature
Review. The American Journal of Medicine. 2011; 124:
588-597.
Choudhary S, McLeod M, Torchia D, Romanelli P. DRESS
Syndrome. The Journal of Clinical Aesthetic
Dermatology. 2013; 6(6): 31-37.
Demoly, P., Adkinson, N.F., Brockow, K., Castells, M.,
Chiriac, A.M., Greenberger, P.A., Khan, D.A., Lang,
D.M., Park, H.S., Pichler, W. and Sanchez‐Borges, M.,
2014. International Consensus on drug
allergy. Allergy, 69(4), pp.420-437.
Kumari, R., Timshina, D.K., and Thappa, D.M., 2011. Drug
hypersensitivity syndrome. Indian Journal of
Dermatology, Venereology, and Leprology, 77(1), p.7.
Peyriere, H., Dereure, O., Breton, H., Demoly, P., Cociglio,
M., Blayac, J.P., Hillaire‐Buys, D., and Network of the
French Pharmacovigilance Centers, 2006. Variability in
the clinical pattern of cutaneous side‐effects of drugs
with systemic symptoms: does a DRESS syndrome
really exist?. British Journal of Dermatology, 155(2),
pp.422-428.
Schrijvers R, Gilissen L, Chiriac AM, Demoly P.
Pathogenesis and diagnosis of delayed-type drug
hypersensitivity reactions from bedside to bench and
back. Clinical and Translational Allergy. 2015; 5(31):
1-10.
Shiohara T, Kano Y, Takahashi R. Current concepts on the
diagnosis and pathogenesis of drug-induced
hypersensitivity syndrome. Japanese Association of
Medical Sciences. 2009; 52(5): 347-352.
Soegiarto, G., and Putra, R.P.S., 2020. Profile of drug
hypersensitivity patients in a tertiary hospital in
Indonesia. World Allergy Organization Journal, 13(8).
81-82
Stern, R.S., 2012. Exanthematous drug eruptions. New
England Journal of Medicine, 366(26), pp.2492-2501.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
244
