
Iron for Human Brain Development: A Fulfill Strategy in the First
1,000 Days of Life
Qodri Santosa
1
a
1
Department of Child Health, Faculty of Medicine, Universitas Jenderal Soedirman Purwokerto, Indonesia
Keywords: iron, brain, development, the first 1,000 days of life
Abstract: Every child has the right to live, survive, and optimal growth and development. Iron has a fundamental role
in brain development. Iron deficiency (ID) in the first two years of life harms the child's long-term
development (possibly irreversible), even though ID has been corrected. For optimal child development, the
first two years of life are a crucial period. Iron deficiency during pregnancy can cause adverse pregnancy
outcomes, both maternal and newborn, resulting in long-term disruption to the child's growth and development.
We must pay special attention to the adequacy of iron needs from conception to two years of age. This article
describes the importance of iron for human brain development and what should we do to ensure iron adequacy,
especially in the first 1,000 days of life.
1 INTRODUCTION
Every fetus in pregnancy and the baby (after birth)
has the right to live, survive, and has optimal growth
and development (ILO, 1999). Children are the next
generation of a nation and have a strategic role in
ensuring the nation's existence and continuity (UU RI
23, 2002).
The successful development of children,
especially the brain, determines the future fate of the
country. The first 1,000 days period, from conception
to her child's second birthday, offers a brief critical
brain's window of opportunity (as the golden period)
(Bellieni, 2016) to shape the development of children.
The fulfilment of both macro and micronutrient
nutrients is very fundamental for the successful
development of a child.
Iron, as an essential micronutrient, has a strategic
role in developing the human brain. Iron deficiency/
iron deficiency anaemia (ID/IDA) at the end of the
fetal period and early in the infant period can cause
decreased cellular respiration in the hippocampus and
frontal cortex. It also may lead to abnormal
neurotransmitter concentrations, altered fatty acid
profiles, and impaired myelination, potentially
disrupting infant growth and development (Georgieff,
2007).
a
https://orcid.org/0000-0001-7712-2549
Poorly child fares of iron fulfil in this period,
potentially causing neurological disorders (Georgieff,
2007), interfering with the child's long-term
development (Mattei & Pietrobelli, 2019; Pietrobelli
et al., 2017)
and might irreversible, across the lifespan
(Lozoff, 2006; Halterman, 2009). The success of
nutrition management in the first 1,000 lives provides
a vast opportunity to improve human resources with
transgenerational impacts (Martorell, 2017). It should
be a priority not only for the government but also for
all community groups and individuals, including
academics. This article describes the importance of
iron for human brain development and what we
should do to ensure iron adequacy, especially in the
first 1,000 days of life.
2 THE HUMAN BRAIN
DEVELOPMENT
The human brain develops from the prenatal and
continues to the postnatal period. The brain structures
are designed gradually and begin in the third
gestational week with the differentiation of the neural
progenitor cells (Thompson & Nelson, 2001); (Stiles
& Jernigan, 2010) (See figure 1). Approximately 22
days after conception, the neural plate begins to fold
214
Santosa, Q.
Iron for Human Brain Development: A Fulfill Strategy in the First 1,000 Days of Life.
DOI: 10.5220/0010490302140222
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 214-222
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
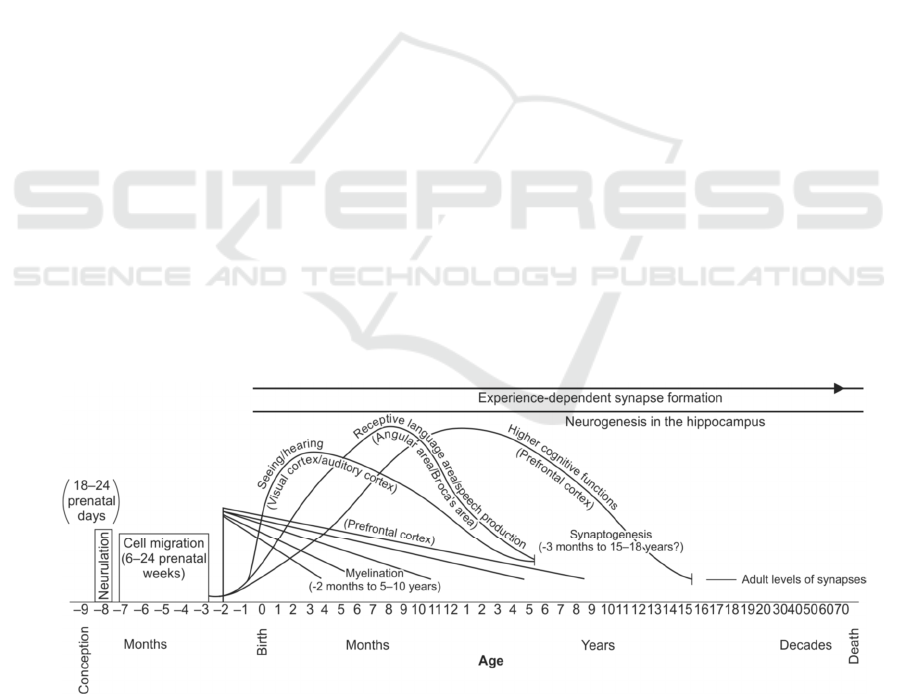
inward, forming the neural tube, which eventually
becomes the brain and spinal cord (Prado & Dewey,
2014). In the prenatal period, neurulation (i.e., the
formation of the neural tube from which eventually
evolves the central nervous system) occurred at 18-24
prenatal days, followed by the generation,
proliferation, migration, and, finally, differentiation
of neurons. Seven weeks after conception, cell
division begins within the neural tube, creating nerve
cells (neurons) and glial cells (cells that support
neurons). After a neuron is made, it migrates to its
place in the brain, where it then grows axons and
dendrites projecting out from its cell body (Prado &
Dewey, 2014).
At the beginning of the last (third) trimester of
fetal life, both myelinations (the fatty insulation of
neurons) runs up to 5-10 years of age. It is continuing
into adult life, and also the synaptogenesis (forming
relationships between cells) continues up to the age
of 15 -18 years). These events are essential to
developing the functional architecture of the brain
(Thompson & Nelson, 2001; Stiles & Jernigan, 2010;
Mattei & Pietrobelli, 2019).
Especially in the third trimester and the first two
years after birth, the brain faces extraordinary growth,
increasing its dimension, differentiating gradually in
a highly specialized organ, and slowly losing
plasticity. It is known that the higher rate of growth
in this period, the greater the risk of damage due to
insufficient nutrients (Fox et al., 2010; Mattei &
Pietrobelli, 2019). The brain has unique
developmental trajectories and a set of nutrient
requirements, so we must pay more attention to it.
The brain regions or processes have
developmental trajectories that begin and accelerate
in fetal life or shortly after birth. Every area of the
brain and every process has two crucial moments, (i)
the critical period and (ii) the sensitive period.
Conceptually, they can be defined as follows: the
former is an early life period where irreversible long-
term consequences follow insults. The latter
represents broader periods when the brain is more
susceptible to environmental factors, such as nutrient
deficiencies (including iron), but the effect is not
inevitably permanent (Mattei & Pietrobelli, 2019).
The logical consequences of failure to construct a
brain region during its critical period can lead to
permanent disorders, such as residual structural
defects (Jorgenson et al., 2003), persistent
neurochemical and electrophysiological
abnormalities, and even altered gene expression
(Tyagi, 2015; Barks et al., 2018; Mattei & Pietrobelli,
2019;). Thus, ensuring adequate nutrient, especially
iron, is necessary to allow a time-coordinated brain
development and create an integrated healthy
working brain structure.
In the human biological system, the brain is the
most complex organ. It contains 100 billion neurons
(information processing cells) and between neuron
cells make connections with other neuron cells
(through synapses) to create the information
processing networks responsible for all of our
thoughts, sensations, feelings, and actions. Since each
neuron has more than 1,000 other neurons, the adult
brain is estimated to have more than 60 trillion
neuronal connections (synapses) (Stiles & Jernigan,
2010).
Figure 1. Development course of the human brain (Thompson & Nelson, 2001)
Iron for Human Brain Development: A Fulfill Strategy in the First 1,000 Days of Life
215

Neuron populations are linked by fibres extending
from individual neurons' cell bodies, namely
dendrites and axons (see Figure 2). Dendrites are
short visible fibres like the branches of a tree, which
function to receive electrochemical input signals from
other neurons. In contrast, axons are long connecting
fibres that extend over great distances and make
connections with other neurons (often at the
dendrites) to send electrochemical signals to neurons
located at distant locations (Stiles & Jernigan, 2010).
Figure 2. Schematically illustration of a neuron (Stiles & Jernigan, 2010)
Individual axon collections from many different
neurons (in one brain region) form fibre channels that
extend to and make connections with groups of
neurons in the other areas of the brain, developing
information-processing networks. The axons are
encased in a fatty substance called myelin, like the
insolation in telephone wires, making the
transmission of electrochemical signals between
regions efficient (Stiles & Jernigan, 2010). The
efficiency of information transmission in the
pathways is greatly enhanced by myelin, which
unsheathes the axons. This myelination process needs
iron so that ID during the rapid growth period (the
first 1,000 days of life) will harm the child's future.
3 HOW DOES IRON
DEFICIENCY INTERFERE
WITH BRAIN
DEVELOPMENT?
Iron deficiency is the most prevalent nutritional
deficiencies in the world (Bastian, 2020)
.
Iron is an
essential micronutrient for normal cellular function in
roles as varied as oxygen transportation, energy
metabolism/energy production, cellular respiration,
cell signalling, gene expression/ DNA synthesis and
the regulation of cell growth and differentiation, and
more (Musallam & Taher, 2019; Ferreira et al., 2019).
Heme is an iron complex with protoporphyrin IX,
which is essential for all aerobic cells' function. Cells
need heme as a prosthetic part for key hemoprotein,
including haemoglobin, cytochromes, and
myoglobin. Another ones are catalase, peroxidases,
and nonheme-containing enzymes involved multiple
metabolic activities. The dominance function of iron
as the cofactor for intracellular processes is due to the
chemistry and redox properties of iron, which enable
it to bind oxygen, transfer electrons and catalyze
various reactions (Aisen, 2001; Musallam & Taher,
2019; Ferreira et al., 2019). In case the brain, as a
metabolically active organ, is susceptible to iron
homeostasis changes, there is still much uncertainty
(Ferreira et al., 2019).
Cells in the brain do not directly access nutrients,
including iron, in the systemic circulation because the
blood-brain barrier and the blood–cerebrospinal fluid
barrier separate the CNS from the systemic
circulation (Ferreira et, 2019). The iron can cross the
blood-brain border by binding to transferrin. The
transferrin-iron complex will attach to the transferrin
receptor on the capillary endothelium. It results in
further internalization by forming endocytic vesicles.
The iron is then pumped out via the expression
divalent metal transporter 1 (DMT1) in the "ferrous"
form. In the cytosol, ceruloplasmin oxidase Ferro to
ferric form. It releases to the extracellular space by
ferroportin (Rouault, 2013).
Disruption of iron homeostasis in the brain
significantly impairs oxidative metabolism of neural
cells, with dramatic consequences for synaptic
plasticity, myelination, and synthesis of
neurotransmitters (Beard & Connor, 2003; Nnah &
Wessling-Resnick, 2018). Iron is a double-edged
sword, "deficiency" and "overload" of iron lead to
detrimental consequences. It means that both
deficiency and iron overload are associated with
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
216

disruption of neurophysiological mechanisms
previously associated with impaired cognition,
altered social behaviour, and other brain functions
(Ferreira et al., 2019). Iron deficiency further
threatens the body's physiological processes during
the fast-growing life period.
During pregnancy and the early phases of human
life (first 1,000 days of life), the needs for macro and
micronutrients (including iron), energy, and other
resources increase. The effects of ID are profound on
cells with the highest metabolic rates, possibly
because ID disrupts mitochondrial and cellular
energy (Beard & Connor, 2003). Mitochondrial
function can be severely impaired because iron is a
cofactor for both heme and nonheme-containing
enzymes in the mitochondrial electron transport chain
(Georgieff et al., 2019). The consequences of ID are
more significant during development when the
oxygen consumption rates of cells are highest, driven
by the energy demands of growth and differentiation
(Kuzawa, 1998; Georgieff et al., 2019), such as
during the late stages of pregnancy and early life.
As an illustration, the total-body oxygen
consumption of a neonate is three to four times
greater than that of an adult. The infant's brain is
estimated to consume 50–60% of the total metabolic
expenditure. The neonatal human brain alone utilizes
60% oxygen consumption, compared with 20% in the
adult brain (Kuzawa, 1998; Georgieff et al., 2019). As
a result, because of the high use of iron in rapid brain
development, the early life period is the most
susceptible to ID.
Potential mechanisms contributing to this
disorder include deficits in brain energy metabolism,
nerve transmission, and myelination. A
comprehensive review shows the brain's reduced
energetic capacity as a mechanical driver of impaired
neurobehavioral development due to fetal-neonatal
ID. Permanent metabolic reprogramming, which
occurs during the ID period, results in chronic
disruption of neuronal energy and mitochondrial
capacity in adulthood, limiting neuroplasticity and
neuro-behaviour in adults (Bastian et al., 2020).
Unfortunately, ID in the late fetal and early life period
can cause abnormal cognitive performance and
emotional regulation, which can persist into
adulthood despite iron repletion.
4 STRATEGY FULFILL IRON
NEED 1,000 DAYS OF LIFE
The strategy to fulfil the iron need in the first 1,000
days of life is a meaningful way to prevent the adverse
effects of ID that harms the child's long-term
development, even though ID has been corrected. So,
clear that prevention is the best solution.
General ID Prevention Strategies Approaches
In principle, ID prevention must involve various
sectors, both government and non-government
organizations. Individual preventive approaches will
not have an impact on the broader community.
General strategies approach of ID prevention is
including food-based procedures, infection disease
control program, and iron supplementation (WHO,
2001).
1. Food-based approaches
a. Dietary improvement
Add substances that enhance iron absorption and
remove inhibitors of iron absorption substances in the
diet menu (WHO, 2001). Iron absorption enhancers
(such as ascorbic acid, Muscle tissue)
(Seriki et al.,
2017; Cappellini et al., 2020) or inhibitors (such as
calcium, phytates [cereals], polyphenols [black tea],
tannins [tea and coffee], proteins [milk proteins, egg
proteins]) should also be aware of when supplying
iron-rich food menus (WHO, 2001; Seriki et al.,
2017; Cappellini et al., 2020).
b. Provide a diet menu that is heme iron-rich food
sources
Low dietary intake of bioavailable iron is an essential
factor in the development of ID. Increased access to
and consumption of iron-rich foods should always be
a priority (WHO, 2018a; Cappellini et al., 2020).
Complementary foods should not forget to provide
animal food sources rich in heme iron which is easily
absorbed.
c. Food fortification
The food fortification (or enrichment of food) is
adding micronutrients (including iron) to food. It is
usually considered the deliberate addition of one or
more micronutrients to particular foods to increase
these micronutrient (s) intake to prevent or correct a
demonstrated deficiency (WHO & FAO, 2006). For
Iron for Human Brain Development: A Fulfill Strategy in the First 1,000 Days of Life
217

example, enrichment of food (rice, maize flour,
cornmeal) with iron. Food manufacturers can carry it
out (or by governments as a public health policy) to
reduce the number of people with a low iron diet and
risk of ID within a population (WHO & FAO, 2006).
2. Infection disease control program
In particular, this effort to hookworm,
schistosomiasis, and malaria control, can enhance
IDA control program effectiveness in a population
with moderate to severe levels of infection (WHO,
2001).
3. Iron supplementation
Supplementation is the most common strategy
currently and often used to treat existing IDA (WHO,
2001). Iron supplementation program has
successfully reduced the prevalence of ID/ However,
we must realize that iron is a "double-edged sword,"
deficiency and iron overload lead to detrimental
consequences (Georgieff, 2007).
The WHO (2016) recommends iron
supplementation (without screening) to prevent DI /
IDA in a population where the prevalence of anaemia
is 40% or higher (: children 6–23 months (10–12.5
mg elemental iron daily), three consecutive months in
a year), 24–59 months (30 mg elemental iron daily),
three straight months in a year), 5–12 years (30–60
mg essential iron daily), three consecutive months in
a year) (WHO, 2016).
Recommendation from WHO, pregnant women
are given a daily oral iron and folic acid
supplementation with 30 - 60 mg of elemental iron
and 400 g (0.4 mg) of folic acid to prevent maternal
anaemia, puerperal sepsis, low birth weight, and
preterm birth (WHO, 2018c).
When daily iron is not acceptable due to side-
effects, and in populations with anaemia prevalence
among pregnant women of less than 20%,
intermittent oral iron and folic acid supplementation
with 120 mg of elemental iron and 2800 µg (2.8 mg)
of folic acid once weekly (WHO, 2018b).
Controversy Iron Supplementation of Pregnant
Women and Children
Although iron supplementation programs have
successfully reduced ID prevalence, blindly iron
supplementation without detecting iron status is still
controversial. Iron has a narrower adequacy range, so
iron supplementation might even cause health
problems regardless of iron status. Iron
supplementation to iron-sufficient individuals is
likely unnecessary or has a little additional benefit
and may carry health risks for iron-sufficient
individuals and potentially some iron-deficient
populations (Georgieff, 2007; Georgieff et al., 2019).
However, emerging and preliminary evidence
shows a U-shaped risk at both deficiency and iron
status overload for birth and infant adverse health
outcomes (Dewey & Oaks, 2017). This fact raises
questions about the effects of high iron intakes
through supplementation or food fortification during
pregnancy and infancy, particularly in iron-replete
individuals (Brannon & Taylor, 2017). However, the
inability to reliably distinguish total-body iron status
from three iron-replete states of haemoglobin in
nonanemic women (namely: nonanemic ID, optimal
iron status, and iron overload) raises a significant
problem in determining the "benefit-risk analysis"
since the effects of iron supplementation on these
three states likely differ (Georgieff et al., 2019).
During pregnancy, high iron status is associated with
increased risk for maternal and fetal adverse
outcomes, related to preterm birth, low birth weight
and small for gestational babies (Brannon & Taylor,
2017; Breymann, 2015).
There is preliminary
evidence that supplementation or high iron status is
associated with gestational diabetes mellitus (Zhang
& Rawal, 2017). Iron supplementation on iron-
replete children increased the risk of vomiting and
fever (Pasricha et al., 2013), impaired linear growth
(Lönnerdal, 2017), and disturbing microbiome
profiles (Brannon & Taylor, 2017; Paganini &
Zimmermann, 2017). Iron supplementation without
screening is allowed by WHO in populations wit a
high prevalence of ID / IDA. The implications of this
risk of iron supplementation deserve serious
discussion relative to screening and supplementation
in these "vulnerable" populations (which are likely
iron-replete) (Brannon & Taylor, 2017), not only
population in developed countries but also every iron-
replete individuals.
The Phase of Human Life, Associated With a
Vicious Cycle of Iron Deficiency
Neonates women born to iron deficient mothers will
potentially grow up to be children, adolescents, and
women of childbearing age with ID and subsequently
become pregnant women with ID, thus giving birth to
babies with ID. Continuity of care is not only
necessary throughout the lifecycle (adolescence,
pregnancy, childbirth, the postnatal period, and
childhood) but also between places of caregiving
(including households and communities, outpatient
and outreach services, and clinical-care settings)
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
218

(Kerber et al., 2007). It is like a vicious cycle, so to
prevent the ID / IDA must cut it at every phase of
human life and need an effective continuum of care.
See figure 3.
Figure 3. The phase of human life, associated with a vicious cycle of ID
All life periods need to get attention to fulfil
human iron needs for brain growth and development
in the golden period, the first 1,000 days of life.
Throughout the journey of life, infancy, childhood,
adolescence, and women of childbearing age,
especially during pregnancy, they are the ID risk
period due to their high iron requirements. Children
(women) who grow up to be anaemic adolescents will
become anaemic pregnant women someday. For the
success of optimal children's brain development, all
ID problems, including chronic inflammation
problems, must be cut off and overcome in all human
stage life cycles. We must break this vicious cycle of
ID.
5 PLACENTAL TRANSFUSION:
IMPORTANT BUT
OVERLOOKED IN ID
PREVENTION
The general prevention strategies of ID (including
iron supplementation) can be practised from infants
aged six months until pregnant women. However, in
infants under six months is quite complicated, and
there is still a controversy regarding supplementation
in exclusive breastfeeding infants. Then, what is the
ID/ IDA preventive solution for children aged 0-6
months?
An inexpensive and easy preventive ID/ IDA
solution for infants 0-6 months has been provided by
delaying cord clamping at birth. In the first few
minutes after the baby is born, there is still circulation
from the newborn's placenta. Blood flow from the
neonate to the placenta (through the umbilical artery)
only occurs during the first 20-25 seconds after the
baby is born. Otherwise, the closure of blood flow in
the umbilical vein (from the placenta to neonate) can
last up to the first 3 minutes, and after that, the blood
flow is minimal and meaningless (Dewey &
Chaparro, 2007).
The debate about umbilical cord clamping time
has occurred for more than two centuries (Philip &
Saigal, 2004). The Pan American Health
Organization believes that the optimal timing of
umbilical cord clamping for all babies (regardless of
gestational age or weight) is when the cord circulation
stops, usually about 3 minutes or more after the baby
is born (Chaparro et al., 2007). The American College
of Obstetricians and Gynecologists (ACOG) argues
that delayed clamping for the mother does not affect
postpartum haemorrhage incidence. In contrast, for
term infants, there is not enough evidence of benefit
for them, and the risk of hyperbilirubinemia should be
considered (Committee on Obstetric Practice ACOG,
2012).
WHO recommend clamping the umbilical cord at
1-3 minutes after birth for all deliveries in 2012
(WHO, 2012a). They did not suggest an early
clamping (<1 minute) unless the neonate is
Iron for Human Brain Development: A Fulfill Strategy in the First 1,000 Days of Life
219

asphyxiated and requires immediate resuscitation
(WHO 2012a; WHO, 2012b). Delayed umbilical cord
clamping (1-3 minutes) is recommended for
improved maternal and infant health and nutrition
outcomes (WHO, 2014). The care protocol for
expected delivery in Indonesia, Jaringan Nasional
Pelatihan Klinik-Kesehatan Reproduksi (JNPK-KR,
2017), recommends clamping of the umbilical cord 2-
3 minutes after birth if there is no need for
resuscitation of the baby (JNPK-KR, 2017). Due to
the importance of delayed cord clamping, WHO gives
remarks that for basic neonatal resuscitation, if the
baby rescue team in the delivery process has
experience providing adequate positive-pressure
ventilation without cutting the umbilical cord,
actually ventilation can be initiated before cutting the
cord (WHO, 2014).
Delaying to clamp the umbilical cord for 2–3 min,
or until cord pulsations cease, facilitates a
physiological blood transfer of placental blood to the
infant (called "placental transfusion"), the majority of
which occurs within 3 min. The placental transfusion
provides sufficient iron reserves for the growth and
development of the baby's brain in the first 6–8
months of human life and prevents or delays the
development of ID until other interventions – such as
the use of iron-fortified foods– can be implemented
after exclusive breastfeeding period (WHO, 2016).
6 SUMMARY
This article describes brain development and the
crucial role played by iron. As an essential
micronutrient in human brain development, fulfil iron
needs in the first 1,000 days of life is a fundamental
step to achieve optimal child development in the
future. However, both deficiency and iron overload
harm maternal and neonatal outcomes. Iron
deficiency in the first two years of life impairs the
child's long-term development (possibly irreversible),
even though ID has been corrected. ID prevention is
essential—general ID prevention strategies approach,
including food-based approaches, infection disease
control program, and iron supplementation.
Complementary foods should provide animal food
sources that are rich in heme-iron, which is easily
absorbed. Children (girl) who grow up to be anaemic
adolescents will become anaemic pregnant women
someday, so all ID problems must be cut off and
overcome in all human life cycles. We must break this
vicious cycle of ID. Especially for exclusively
breastfed babies in the 0-6 month period, the delay of
umbilical cord clamping (about 2-3 minutes) after
birth provides sufficient iron reserves for the baby's
life for 6-8 months.
REFERENCES
Aisen P, Enns C, and Wessling-Resnick M. (2001)
'Chemistry and biology of eukaryotic iron metabolism'.
Int J Biochem Cell Biol., 33(10):940-959. DOI:
10.1016/s1357-2725(01)00063-2.
Barks A, SJB F, Georgieff MK, and Tran PV. (2018) 'Early-
life neuronal-specific iron deficiency alters the adult
mouse hippocampal transcriptome". J Nutr.
148(10):1521–1528. DOI: 10.1093/jn/nxy125.
Bastian TW, Rao R, Tran PV and Georgieff MK. (2020)
'The effects of early-life iron deficiency on brain energy
metabolism'. Neurosci Insights., 15:1-12. DOI:
10.1177/2633105520935104.
Bastian TW, von Hohenberg WC, Mickelson DJ, Lanier
LM, and Georgieff MK. (2016) 'Iron deficiency impairs
developing hippocampal neuron gene expression,
energy metabolism and dendrite complexity'. Dev.
Neurosci., 38(4):264–276. DOI: 10.1159/000448514.
Beard JL and Connor JR. (2003) 'Iron status and neural
functioning'. Annu. Rev. Nutr., 23:41–58. DOI:
10.1146/annurev.nutr.23.020102.075739.
Bellieni CV. (2016) 'The Golden 1,000 Days'. J Gen
Practice. 4(2): 250. DOI:10.4172/2329-9126.1000250.
Brannon PM, and Taylor CL. (2017) 'Iron supplementation
during pregnancy and infancy: uncertainties and
implications for research and policy'. Nutrients.
9(12):1327. DOI: 10.3390/nu9121327.
Breymann C. (2015) 'Iron deficiency anemia in pregnancy'.
Semin. Hematol. 52(4):339–347. DOI:
10.1053/j.seminhematol.2015.07.003.
Cappellini MD, Musallam KM, and Taher AT. (2020) 'Iron
deficiency anaemia revisited". J Intern Med.,
287(2):153–170. DOI: 10.1111/joim.13004.
Chaparro C, Lutter C, and Hubner AVC. (2007) 'Essential
delivery care practices for maternal and newborn health
and nutrition'. Pan American Health Organization,
Reginal office of the Word Health Organization and
USAID.
Committee on Obstetric Practice American College of
Obstetricians and Gynecologists. (2012) 'Committee
Opinion No.543: Timing of umbilical cord clamping
after birth'. Obstet Gynecol. 120(6): 1522-1526.
Dewey KG, and Chaparro CM. (2007) 'Mineral metabolism
and body composition iron status of breastfed infants'.
Proc Nutr Soc., 66(3):412–422. DOI:
10.1017/S002966510700568X.
Dewey KG, and Oaks BM. (2017) 'U-shaped curve for risk
associated with maternal iron status or
supplementation'. Am J Clin Nutr. 106(Suppl
6):1694S–1702S. DOI: 10.3945/ajcn.117.156075.
Ferreira A, Neves P and Gozzelino R. (2019) 'Multilevel
impacts of iron in the brain: the cross talk between
neurophysiological mechanisms, cognition, and social
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
220

behavior'. Pharmaceuticals, 12(3):126;
DOI:10.3390/ph12030126.
Fox SE, Levitt P, and Nelson CA. (2010) 'How the timing
and quality of early experiences influence the
development of brain architecture'. Child Dev.,
81(1):28–40. DOI: 10.1111/j.1467-8624.2009.01380.x.
Halterman JS, Kaczorowski C, Aligne A, Auinger P, and
Szilagyi PG. (2001) 'Iron deficiency and cognitive
achievement among school-aged children and
adolescents in the United States'. Pediatrics.;107:1381-
1386.
Georgieff MK. (2007) 'Nutrition and the developing brain:
nutrient priorities and measurement'. Am J Clin Nutr.,
85(2):614S–620S. DOI: 10.1093/ajcn/85.2.614S.
Georgieff MK, Krebs NF and Cusick SE. (2019) 'The
benefits and risks of iron supplementation in pregnancy
and childhood'. Annu. Rev. Nutr. 39:121-146. DOI:
10.1146/annurev-nutr-082018-124213.
Harvey L J, Berti C, Casgrain A, Cetin I, Collings R,
Gurinovic M, Hermoso M, Hooper L, Hurst R,
Koletzko B, Ngo J, Vinas BR, Vollhardt C, Vucic V,
and Fairweather-Tait SJ. (2013) 'Estimating iron
requirements for deriving dietary reference values'. Crit
Rev Food Sci Nutr. 53(10), pp.1064-1076. DOI:
10.1080/10408398.2012.742860.
ILO Convention No. 182. (1999). Concerning the
Prohibition and Immediate Action for the Elimination
of The Worst Forms of Child Labour.
JNPK-KR. (2017) Asuhan Persalinan Normal. Asuhan
esensial bagi ibu bersalin dan bayi baru lahir serta
penatalaksanaan komplikasi segera pasca persalinan
dan nifas. Buku Acuan.
Jorgenson LA, Wobken JD, and Georgieff MK. (2003)
'Perinatal iron deficiency alters apical dendritic growth
in hippocampal CA1 pyramidal neurons'. Dev
Neurosci., 25(6), pp.412–420. DOI:
10.1159/000075667.
Kerber KJ, de Graft-Johnson JE, qar A Bhutta Z, Okong P,
Starrs A, and Lawn JE. (2007) 'Continuum of care for
maternal, newborn, and child health: from slogan to
service delivery'. Lancet.;370(9595):1358–1369. DOI:
10.1016/S0140-6736(07)61578-5.
Kuzawa CW. (1998) 'Adipose tissue in human infancy and
childhood: an evolutionary perspective'. Am. J. Phys.
Anthropol; 107(Suppl. 27), pp.177–209. DOI:
10.1002/(sici)1096-8644(1998)107:27+<177::aid-
ajpa7>3.0.co;2-b
Lönnerdal B. (2017) Excess iron intake as a factor in
growth, infections and development of infants and
young children. Am J Clin Nutr. 106(Suppl 6):1681S–
1687S. DOI: 10.3945/ajcn.117.156042.
Lozoff B, Jimenez E, and Smith JB. (2006) 'Double burden
of iron deficiency in infancy and low Socioeconomic
status: a longitudinal analysis of cognitive test scores to
age 19 years'. Arch Pediatr Adolesc Med.
160(11):1108-1113. DOI:
10.1001/archpedi.160.11.1108.
Martorell R. (2017) 'Improved nutrition in the first 1000
days and adult human capital and health'. Am J Hum
Biol., 29(2). DOI:10.1002/ajhb.22952.
Mattei D and Pietrobelli A. (2019 ) 'Micronutrients and
brain development'. Curr Nutr Rep., 8(2), pp.99-107.
DOI: 10.1007/s13668-019-0268-z.
McCann A, Amadó MP and Moore SE. (2020) 'The role of
iron in brain development: a systematic review'.
Nutrients,12(7), 2001. DOI: 10.3390/nu12072001.
Musallam KM, and Taher AT. (2018) 'Iron deficiency
beyond erythropoiesis: should we be concerned?' Curr
Med Res Opin., 34(1), pp. 81-93. DOI:
10.1080/03007995.2017.1394833.
Nnah IC and Wessling-Resnick M. (2018) 'Brain iron
homeostasis: a focus on microglialiron'.
Pharmaceuticals, 11(4):129. DOI:
10.3390/ph11040129.
Paganini D and Zimmermann MB. (2017) 'The effects of
iron fortification and supplementation on the gut
microbiome and diarrhea in infants and children: A
review'. Am J Clin Nutr. 106(Suppl 6):1688S–1693S.
DOI: 10.3945/ajcn.117.156067.
Pasricha SR, Hayes E, Kalumba K, and Biggs BA. (2013)
'Effect of daily iron supplementation on health in
children aged 4–23 months: A systematic review and
meta-analysis of randomized controlled trials'. Lancet
Glob. Health., 1(2):e77–e86. DOI: 10.1016/S2214-
109X(13)70046-9.
Pietrobelli A, Agosti M, and MeNu Group. (2017)
'Nutrition in the first 1000 days: ten practices to
minimize obesity emerging from published science'. Int
J Environ Res Public Health. 14(12):1491. DOI:
10.3390/ijerph14121491.
Philip AGS, and Saigal S. (2004) 'When should we clamp
the umbilical cord?' Neo Reviews. 5(4):e142-e154.
DOI: https://doi.org/10.1542/neo.5-4-e142.
Rouault TA. (2013) 'Iron metabolism in the CNS:
implications for neurodegenerative diseases'. Nat. Rev.
Neurosci. 14(8):551–564. DOI: 10.1038/nrn3453.
Prado EL, and Dewey KG. (2014) 'Nutrition and brain
development in early life'. Nutr Rev. 72(4):267–284.
DOI: 10.1111/nure.12102.
Seriki SA, Adebayo OF, and Odetola AO. (2017) 'Iron:
From dietary sources to utilization in the body'. Glob J
Nanomed. 3(3), pp. 85-91. doi:
10.19080/GJN.2017.03.555615.
Stiles J, and Jernigan TL. (2010) 'The basics of brain
development'. Neuropsychol Rev. 20(4), pp.327–348.
DOI: 10.1007/s11065-010-9148-4.
Thompson RA, and Nelson CA. (2001) 'Developmental
science and the media: early brain development'. Am
Psychol., 56(1), pp.5-15. DOI: 10.1037/0003-
066x.56.1.5.
Tyagi E, Zhuang Y, Agrawal R, Ying Z, and Gomez-Pinilla
F. (2015) 'Interactive actions of Bdnf methylation and
cell metabolism for building neural resilience under the
influence of diet'. Neurobiol Dis. 73:307–18. DOI:
10.1016/j.nbd.2014.09.014.
Undang-Undang Republik Indonesia 23. (2002)
‘Perlindungan Anak’
WHO. (2001) 'Iron deficiency anaemia assessment,
prevention, and control. A guide for programme
managers'.
Iron for Human Brain Development: A Fulfill Strategy in the First 1,000 Days of Life
221

https://www.who.int/nutrition/publications/en/ida_asse
ssment_prevention_control.pdf
WHO. (2004) 'Focusing on anaemia: Towards an integrated
approach for effective anaemia control'.
https://www.who.int/medical_devices/publications/en/
WHO_UNICEF-anaemiastatement.pdf?ua=1
WHO. (2012a) 'WHO recommendations for the prevention
and treatment of postpartum haemorrhage'.
https://www.who.int/iris/bitstream/10665/75411/1/978
9241548502_eng.pdf?ua=1.
WHO. (2012b) 'Guidelines on basic newborn resuscitation.
Genewa'.
http://apps.who.int/iris/bitstream/10665/75157/1/9789
241503693_eng.pdf?ua=1
WHO. (2014) 'Guideline: Delayed umbilical cord clamping
for improved maternal and infant health and nutrition
outcomes.
https://www.who.int/nutrition/publications/guidelines/
cord_clamping/en/.
WHO. (2016) 'Guideline: daily iron supplementation in
infants and children'.
http://apps.who.int/iris/bitstream/10665/204712/1/978
9241549523_eng.pdf?ua=1&ua=1
WHO. (2018a) 'Guideline: Fortification of rice with
vitamins and minerals as a public health strategy'.
Geneva: World Health Organization,
https://apps.who.int/iris/bitstream/handle/10665/27253
5/9789241550291-eng.pdf?sequence=1&isAllowed=y
WHO. (2018b) 'WHO recommendation on intermittent oral
iron and folic acid supplementation'.
https://extranet.who.int/rhl/topics/preconception-
pregnancy-childbirth-and-postpartum-care/antenatal-
care/who-recommendation-intermittent-oral-iron-and-
folic-acid-supplementation
WHO. (2018c) 'WHO recommendation on daily oral iron
and folic acid supplementation'.
https://extranet.who.int/rhl/topics/preconception-
pregnancy-childbirth-and-postpartum-care/antenatal-
care/who-recommendation-daily-oral-iron-and-folic-
acid-supplementation
WHO, and FAO. (2006) 'Guidelines on food fortification
with micronutrients'.
https://www.who.int/nutrition/publications/guide_food
_fortification_micronutrients.pdf
Zhang C, and Rawal S. (2017) Dietary iron intake, iron
status and gestational diabetes. Am J Clin Nutr.,
106(Suppl 6): 1672S–1680S. DOI:
10.3945/ajcn.117.156034.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
222
