
The Effects of Aqueous Extract of Jamaican Cherry (Muntingia
calabura) on D-galactose-induced Liver Damage in
BALB/c Mice (Mus musculus)
Cempaka Jaga Pramudita
1
a
, Dwi Nur Ahsani
2
b
, Ika Fidianingsih
2
c
, Evy Sulistyoningrum
2
d
1
Faculty of Medicine, Universitas Islam Indonesia, Yogyakarta, Indonesia
2
Department of Histology, Faculty of Medicine, Universitas Islam Indonesia, Yogyakarta, Indonesia
Keywords: D-Galactose, Muntingia calabura, Hepatocyte Damage, Jamaican Cherry, Manja Roenigk, Aqueous Extract
Abstract: D-Galactose induces oxidative stress that will damage the hepatocytes. The aqueous extract of Muntingia
calabura leaves (AQMC) is known to have a potential antioxidant to prevent hepatocyte damage caused by
oxidative stress. This study aimed to determine the effects of AQMC leaf extract on liver damage induced by
D-Galactose in BALB/c Mus musculus. This was experimental research with a completely-randomized design
involving 20 hepatic samples (paraffin blocks and HE staining) from 5 groups. The groups included K1
(healthy), K2 (D-galactose-induced), K3 (D-galactose + AQMC 35 mg/kgBW), K4 (D-galactose + AQMC
75mg/kgBW), and K5 (D-galactose + vitamin C 28mg/KgBW). D-galactose was administered for 6 weeks
prior to the therapy (vitamin C or AQMC given for 4 weeks). Liver damage was observed in all fields of view
and described comprehensively. The degree of hepatocyte damage was calculated using the Manja Roenigk
scoring and analyzed using One-way ANOVA with post-hoc Tukey’s HSD test (CI = 95%, α = 0.05). AQMC
leaf extract could reduce D-galactose-induced liver damage in BALB/c Mus musculus. The hepatocyte
damage in the groups given AQMC therapy was less than that in the D-galactose negative control group (K1
= 73.5 + 2.39, K2 = 92.00 + 5.24, K3 = 69.00 + 2.79, K4 = 76.25 + 4.42, K5 = 77.25 + 6.48; p = 0.029).
AQMC at a dose of 35mg/KgBW showed more effective therapeutic potential against D-galactose (K3 =
0.003, K4 = 0.027; post-hoc toward K2). AQMC administration could reduce liver and hepatocyte damage of
BALB/c Mus musculus induced by D-Galactose at a potential dose of 35 mg.
1
INTRODUCTION
Aging can raise the levels of free radicals (ROS) in
the blood and tissues. Excessive ROS in an aging
process is induced by an imbalance between ROS and
antioxidant. Studies show that D-galactose induced in
animal models can describe the process of oxidative
stress in aging. D-galactose increases plasma
Malondialdehyde (MDA) levels (Sulistyoningrum et
al., 2019), raises MDA levels in the liver, and reduces
hepatic antioxidant (SOD) levels (Hadzi-Petrushev et
al., 2015). Induced D-galactose will also activate the
p-53 pathway, thus leading to cell apoptosis, and
a
https://orcid.org/0000-0002-2138-5742
b
https://orcid.org/0000-0003-1344-3997
c
https://orcid.org/0000-0002-1270-4545
d
https://orcid.org/0000-0001-7487-5932
stimulate the p-21 pathway that plays a role in the cell
cycle (Bo-Htay et al., 2018).
Aging reduces the ability of the liver to regenerate
during cellular injury. Research shows that the
hepatocytes in aged rats lose the capability of entering
mitosis (Biondo-Simões et al., 2006), and the cells’
ability to recognize growth factors, such as EGF, also
decreases in aging rats (Schmucker & Sanchez,
2011). The reduced regenerative capability increases
the likelihood of cell damage and apoptosis. Cell
apoptosis in the liver is susceptible not only to
oxidative stress but also to genomic instability and
lipotoxicity (Zhong et al., 2017).
In addition to increasing cell apoptosis, the aging
process in the liver is also histologically marked by
202
Pramudita, C., Ahsani, D., Fidianingsih, I. and Sulistyoningrum, E.
The Effects of Aqueous Extract of Jamaican Cherry (Muntingia calabura) on D-galactose-induced Liver Damage in BALB/c Mice (Mus musculus).
DOI: 10.5220/0010490102020207
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 202-207
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

dilation of blood vessels, increasing number of
Kupffer cells, and escalation of cell degeneration
(Hashish, 2016). Therapy to reduce aging effects on
the liver should therefore be considered in order to
prevent age-related liver diseases, including hepatic
cirrhosis, liver cancer, or non-alcoholic fatty liver
disease (NAFLD)
Muntingia calabura (MC) is among the medicinal
plants with highly potential preventive properties
against oxidative-stress-induced cell damage. The
administration of methanol extract of Muntingia
calabura leaves (MMC) can prevent increased uric
acid levels and damage to the proximal renal tubules
in DM model rats (Safrida & Sabri, 2019). In
addition, MMC leaf extract given as premedication
for hepatotoxic substances (CCl4) has proved to
reduce the levels of parenchymal liver damage
(Zakaria et al., 2019). The potential protective role of
MC has also been observed in non-methanolic
extracts. A number of studies report the
administration of MC ethanol extract that can
suppress an inflammatory process and prevent gastric
ulcers in ethanol-induced rats (Aziz Ibrahim, 2012;
Lin et al., 2017; Sarimanah et al., 2017). Combined
MC-Ficus carica infusion is able to reduce SGOT and
SGPT levels in paracetamol-induced rats (Lalihatu &
Sudharmono, 2019). Furthermore, the administration
of MC as premedication can prevent carbonated-
drink-induced or ethanol-induced liver damage
(Murti et al., 2016).
2 MATERIAL AND METHODS
2.1 Research Design and Subjects
This research was purely experimental with a
completely-randomized design. The experiment was
conducted from April to October 2016 at the
Laboratory of Histology and Anatomical Pathology
of the Faculty of Medicine, Universitas Islam
Indonesia Yogyakarta after passing the ethical review
from the ethics committee of the Faculty of Medicine
of Universitas Islam Indonesia. This study used 20
livers of BALB/c mice (Mus musculus) obtained
from a previous study (Sulistyoningrum et al., 2019).
The inclusion criterion was the hepatic tissue
obtained from a previous research protocol. If
damage to the hepatic tissue was found
(microscopically or macroscopically) and thereby
resulting in difficulty to interpret the results, the tissue
was excluded. The 20 samples were obtained from 5
groups named K1 (healthy normal group), K2
(negative control group, D-galactose-induced), K3
(dose-1 treatment group, D-galactose-induced and
35mg/kgBW AQMC leaf extract), K4 (dose-2
treatment group, D-galactose-induced and
75mg/kgBW AQMC leaf extract), and K5 (positive
control group, D-galactose-induced and
28mg/KgBW vitamin C). D-galactose was
administered for 6 weeks while the treatment
(administration of AQMC or vitamin C) was given
daily for 4 weeks after induction. The hepatic tissue
was then transversely embedded in a paraffin block
and stained with HE staining.
2.2 Observation of Hepatic
Histomorphological Changes
The liver damage was observed descriptively and
semi-quantitatively. The changes observed in all
fields of view consisted of inflammation,
degeneration, necrosis, indistinctive cell boundaries,
and affected size of the central vein (Zulfi et al.,
2013). The levels of liver damage were calculated on
the basis of Manja Roenigk scoring on 50 cells (1:
normal, 2: inflammation, 3: degeneration, 4: necrosis)
in six identical fields of view for each sample (total
magnification of 400x). Inflammation was marked by
lymphocyte invasion, degeneration was manifested as
clear cytoplasm and giant cells in hepatocytes
(Sookoian et al., 2016), and necrosis was indicated by
changes in hepatocyte nuclei (pyknosis, karyorrhexis,
and karyolysis). The final score of liver damage in
each sample was obtained by multiplying the number
of cells by the Manja Roenigk scoring (Zulfi et al.,
2013). In addition, cell boundaries were classified
into distinctive, indistinctive, and fairly distinctive.
The size of central vein was then compared with that
of the healthy control group to obtain three categories
of size change named normal, slightly enlarged (>1.5-
2-fold), and enlarged (>3-fold).
2.3 Statistical Analysis
An analysis was performed on the semi-quantitative
data of liver damage scores. The normality was
examined using the Saphiro Wilk test while the
significance test involved One-way ANOVA
followed by Post-Hoc Tukey’s HSD test. All of the
statistical tests were done at a confidence level of
95% (α = 0.05)
3 RESULTS
The hepatic histomorphological changes during the
aging process (D-galactose-induced) include
The Effects of Aqueous Extract of Jamaican Cherry (Muntingia calabura) on D-galactose-induced Liver Damage in BALB/c Mice (Mus
musculus)
203
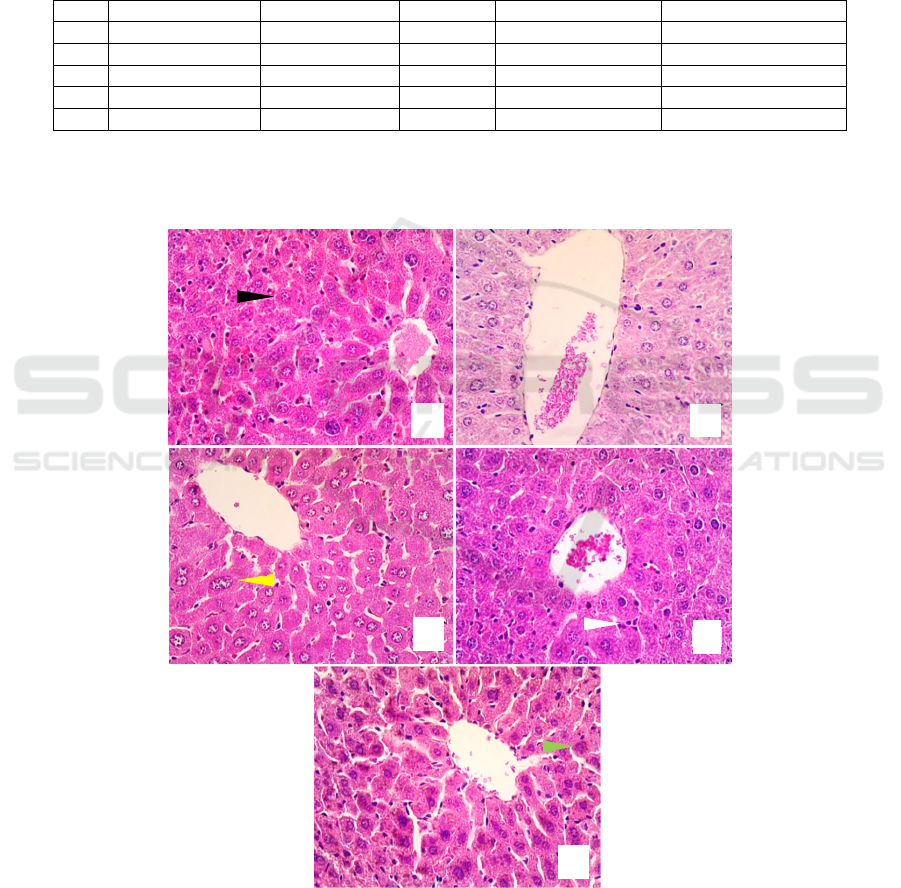
inflammation, degeneration, necrosis and a change in
the central vein size (Figure 1). The hepatic
histological changes were evident in the negative
control group (K2), and inflammation was found in
all of the study groups. The administration of AQMC
leaf extract improved the histopathological features
of D-galactose-induced liver damage in mice.
Meanwhile, the administration of vitamin C resulted
in histopathological features resembling those of
AQMC leaf extract at a dose of 75mg/kgBW (Table
1). The hepatocyte damage found in this study
showed a significant difference (p = 0.029, Table 2),
which was mainly found between the AQMC leaf
extract treatment group and the K2 group (dose of
35mg/KgBW: 0.003, dose of 75mg/KgBW: 0.027,
Table 3).
Table 1: Histological features of liver damage in different groups.
Inflammation Degeneration Necrosis Cell boundaries Central vein size
K1 + - - distinctive normal
K2 ++ +++ ++ indistinctive enlar
g
e
d
K3 + + + distinctive normal
K4 + ++ + fairly distinctive slightly enlarge
d
K5 ++ ++ + fairly distinctive slightly enlarge
d
Note: - normal, + mild, ++ moderate, +++ severe. K1: healthy normal group, K2: negative control group (D-galactose-induced), K3:
dose-1 treatment group (D-galactose-induced and 35 mg/kgBW AQMC leaf extract), K4: dose-2 treatment group (D-galactose-
induced and 75 mg/kgBW AQMC leaf extract), K5: positive control group (D-galactose-induced and 28 mg/kgBW vitamin C).
Figure 1. Hepatic histological features with HE staining and 40x objective magnification in all groups (in alphabetical order
for K1, K2, K3, K4, K5). Black arrow: normal liver, white arrow: inflammation, yellow arrow: degeneration, green arrow:
necrosis, VS: central vein.
V
A
B
C
D
E
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
204
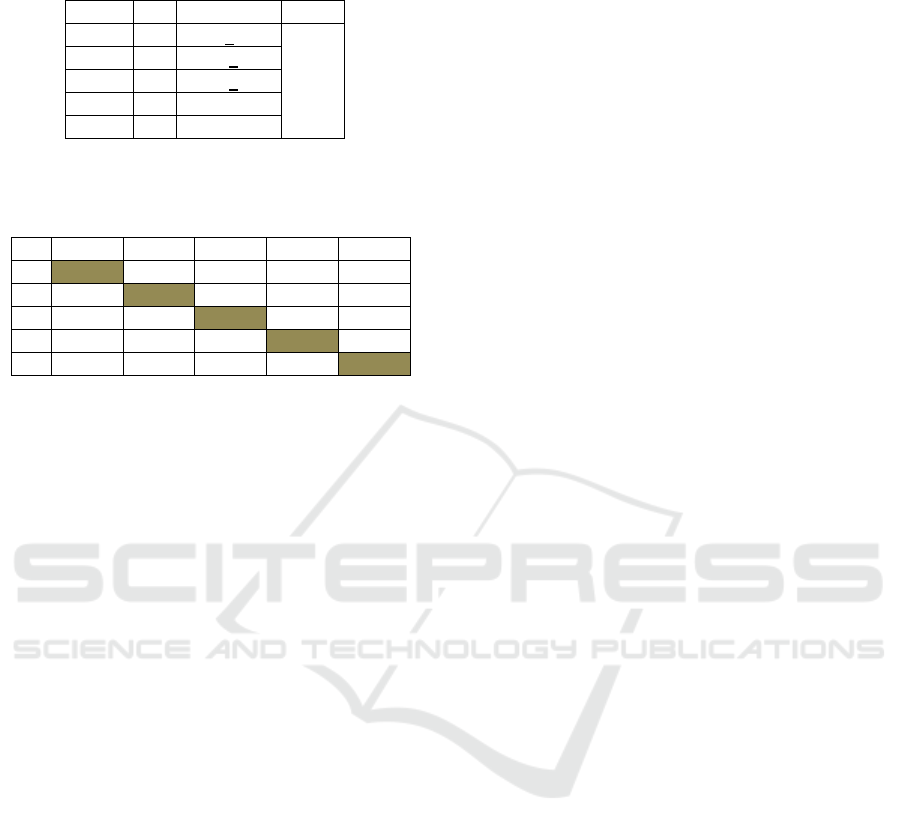
Table 2: Mean Anova of hepatocyte damage in different
groups.
Group n Mean+SD p
K1 4 73.5+2.39 0.029
K2 4 92.00+5.24
K3 4 69.00+2.79
K4 4 76.25+4.42
K5 4 77.25+6.48
*: (p<0.05)
Table 3: Total score of hepatocyte damage from Post-Hoc
test.
K1 K2 K3 K4 K5
K1 0.011* 0.493 0.674 0.567
K2 0.011* 0.003* 0.027* 0.036*
K3 0.493 0.003* 0.276 0.218
K4 0.674 0.027* 0.276 0.878
K5 0.567 0.036* 0.218 0.878
4 DISCUSSION
This research shows that D-Galactose induces cell
death in the liver. The highest degree of liver damage
was found in the D-Galactose-induced group with no
treatment (neither AQMC leaf extract nor vitamin C).
The administration of AQMC leaf extract provides
improved features of hepatic architecture in that the
dose of 35mg/kgBW mirrored the normal features
while the dose of 70 mg/KgBW approximated the
features in the group given vitamin C. The total score
of liver damage was significantly different in all study
groups. A significant difference was particularly
evident between the group with AQMC leaf extract at
a dose of 35 mg/kg BW and the positive control
group. There was an insignificant difference between
the hepatic histological features of the mice receiving
AQMC leaf extract and those of the healthy group.
Therefore, it indicates that the administration of
AQMC leaf extract (especially at a dose of
35mg/kgBW) can improve the histological features of
aging liver (D-galactose-induced). Such histological
changes have the same features with those of the liver
of healthy mice (not induced by D-galactose).
When induced to the body, D-Galactose increases
the levels of galactose, and galactose will reduce to
galactitol, a compound that cannot be further
metabolized, thereby accumulating intracellularly
and increasing the cell osmotic pressure. This process
will eventually lead to swollen cells.
Histologically, this feature is recognized as
ballooning degeneration/cell swelling with clear
cytoplasm (Ye et al., 2014). D-Galactose will also
reduce free amine groups in amino acids or proteins,
thus leading to AGEs formation through glycation as
well as ROS formation (Parameshwaran et al., 2010).
The administration of D-galactose for 6 weeks
correspondingly led to an elevated level of free
radicals (plasma MDA levels) by approximately 3-
fold of that of the healthy group (Sulistyoningrum et
al., 2019).
Muntingia calabura has a hepatoprotective effect.
A study shows that the administration of methanol
extract of Muntingia calabura leaves (MMC leaf
extract) followed by soft drink administration can
significantly prevent increases in SGOT and SGPT
levels (Siddiq et al., 2019). MMC leaf extract
administered for 7 days followed by the
administration of hepatotoxic substances (CCL4 or
paracetamol) can prevent further liver damage
(Zakaria et al., 2019) (Mahmood et al., 2014). MMC
leaf extract as premedication for CCl4 administration
is able to prevent elevated ALT levels, increased pro-
inflammatory cytokines (NO, TNF-α, IL-β, IL-6),
and higher ratio of the liver weight to the body
weight. MMC leaf extract administered to rats at
therapeutic doses of 250 mg/kgBW and 500
mg/KgBW results in a liver weight ratio closer to that
of the healthy group. ALT levels in the 500
mg/KgBW treatment group are similar to those of the
positive control group (receiving N-acetyl-cysteine
therapy and induced by CCl4). MMC leaf extract
(doses of 50.250 and 50 mg/kgBW) as premedication
before induction by CCl4 is also able to significantly
increase the antioxidant levels (SOD and CAT) in the
body. Histopathological features of liver damage
become minimal in the 500 mg/kgBW treatment
group (Zakaria et al., 2019). MMC leaf extract as
premedication is also able to prevent liver damage
induced by paracetamol. In line with the report by
Zakaria et al., (2019), MMC leaf extract as
premedication is able to prevent an increase in the
relative weight of the liver but unable to prevent
increased levels of liver enzymes (ALT, AST, and
ALP). Minimal necrosis and inflammation are found
in the group receiving MMC leaf extract as
premedication (Mahmood et al., 2014).
The doses of 35 mg/KgBW and 70 mg/KgBW for
the mice in this study are equivalent to the doses of
250 mg/KgBW and 500 mg/KgBW in experimental
rats. This indicates that the findings of this study are
in accordance with previous studies in which the
potency of MMC leaf extract is found at both doses
(Mahmood et al., 2014; Siddiq et al., 2019; Zakaria et
al., 2019). In contrast to the potential dose of MMC
leaf extract at 500 mg/kgBW, that of AQMC leaf
extract in this study is found at 250 mg/kgBW. This
The Effects of Aqueous Extract of Jamaican Cherry (Muntingia calabura) on D-galactose-induced Liver Damage in BALB/c Mice (Mus
musculus)
205

difference is likely caused by the different treatment
given to the experimental animals. The main
objective of this study was to examine the therapeutic
effects of AQMC leaf extract on damaged liver while
the study using MMC leaf extract aimed to
investigate the protective effects on the induction of
liver damage. Another reason for the difference is the
compound concentrations in MC due to the extraction
process. Kolar, Kamble and Dixit, (2011) report that
AQMC leaf extract has a total flavonoid content
exceeding that of MMC leaf extract but with a lower
phenolic content. The antioxidant activity of MMC
leaf extract is apparently higher than that of AQMC
leaf extract.
AQMC leaf extract has more benefits than other
types of extract. Compared to petroleum ether and
ethyl acetate extracts, the total phenolic content of
AQMC leaf extract is twice higher with a better
hepatoprotective effect (indicating only minimal
inflammation). In addition, premedication using
AQMC leaf extract is able to suppress increasing
hepatic enzyme levels (ALT, AST, and ALP) in rats
induced by paracetamol. By comparison with the
doses of 50 and 250 mg/KgBW, the dose of 500
mg/KgBW in rats shows the best potency (in terms of
the liver weight ratio, hepatic histological features,
antioxidant levels, and liver enzyme levels) (Zakaria
et al., 2018).
The protective effect of MC is associated with its
antioxidant activities in the flavonoid compounds,
such as catechin, gallocatechin, epigallocatechin
narigenin, and quercetin in MC (Pereira et al., 2018).
The hydroxyl complex in the phenol compounds in
MC can inhibit proton donation in ROS formation
(Balakrishnan et al., 2011). The polyphenol
compounds in MC inhibit glycosidation reactions and
have an anti-glycation activity by inhibiting RAGEs
signaling (Sadowska-Bartosz & Bartosz, 2015). The
saponins in MC also slow down aging through
activation of the AKT FOXO3a pathway and the
Nuclear factor-erythroid 2-related factor-2 pathway.
This process will improve the expression and
functions of antioxidant enzymes, such as superoxide
dismutase-2 (SOD-2), catalase, glutathione
reductase, glutamate-cysteine ligase, and heme
oxygenase-1 (Khan Y et al., 2015). In addition to the
antioxidant potential, research by Rofiee et al., (2015)
shows that the protective effect of MC is manifested
through the bile acid biosynthesis and arachidonic
acid metabolism.
This study has a limitation in that the effects of
AQMC leaf extract on impaired liver function (levels
of ALT, AST and ALP enzymes) were not
investigated. However, the findings of this study have
been able to illustrate that MMC leaf extract has a
therapeutic effect on liver damage, particularly on an
aging liver. Further research with reference to the
therapeutic effects of AQMC leaf extract is required
by taking into account the various stimuli of both
acute and chronic tissue damage.
5 CONCLUSION
The administration of AQMC leaf extract can reduce
liver and hepatocyte damage of BALB/c Mus
musculus induced by D-Galactose. The potential dose
of AQMC leaf extract is 35 mg.
REFERENCES
Aziz Ibrahim, I. A. (2012). Leaves Extract of Muntingia
Calabura Protects Against Gastric Ulcer Induced by
Ethanol in Sprague-Dawley Rats. Clinical and
Experimental Pharmacology, 01(S5).
https://doi.org/10.4172/2161-1459.s5-004
Balakrishnan, K. P., Narayanaswamy, N., & Duraisamy, A.
(2011). Tyrosinase inhibition and anti-oxidant
properties of Muntingia calabura extracts: In vitro
studies. International Journal of Pharma and Bio
Sciences, 2(1), 294–303.
Biondo-Simões, M. D. L. P., Matias, J. E. F., Montibeller,
G. R., Siqueira, L. C. D., Nunes, E. D. S., & Grassi, C.
A. (2006). Effect of aging on liver regeneration in rats.
Acta Cirurgica Brasileira, 21(4), 197–202.
https://doi.org/10.1590/S0102-86502006000400002
Bo-Htay, C., Palee, S., Apaijai, N., Chattipakorn, S. C., &
Chattipakorn, N. (2018). Effects of d-galactose-induced
ageing on the heart and its potential interventions.
Journal of Cellular and Molecular Medicine, 22(3),
1392–1410. https://doi.org/10.1111/jcmm.13472
Hadzi-Petrushev, N., Stojkovski, V., Mitrov, D., &
Mladenov, M. (2015). D-galactose induced changes in
enzymatic antioxidant status in rats of different ages.
Physiological Research, 64(1), 61–70.
https://doi.org/10.33549/physiolres.932786
Hashish, H. A. (2016). Effect of age on the sildenafil impact
on the histological and ultra-structure of the liver in
male albino rat. Journal of Histology and
Histopathology, 3(1), 5. https://doi.org/10.7243/2055-
091x-3-5
Khan Y, M. A., Mundasada, S. C., & Ramadas, D. (2015).
Antioxidant Activity : Root, Leaves and Fruits Aqueous
Extracts of MuntingiaCalabura. Journal of Innovations
in Pharmaceuticals and Biological Sciences, 2(4), 363–
368.
Kolar, F. R., Kamble, V. S., & Dixit, G. B. (2011).
Phytochemical constituents and antioxidant potential of
some underused fruits. African Journal of Pharmacy
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
206

and Pharmacology, 5(18), 2067–2072.
https://doi.org/10.5897/AJPP11.475
Lalihatu, M. R., & Sudharmono, U. (2019). Effectiveness
of Boiled Cherry Leaf (muntingia calabura L) and Figs
Leaves (Ficus Carica) Toward SGOT SGPT Serum of
Male Wistar Strain Rats with Acute Hepatitis Models.
Abstract Proceedings International Scholars
Conference, 7(1), 716–726.
https://doi.org/10.35974/isc.v7i1.2076
Lin, J. T., Chang, Y. Y., Chen, Y. C., Shen, B. Y., & Yang,
D. J. (2017). Molecular mechanisms of the effects of
the ethanolic extract of Muntingia calabura Linn. fruit
on lipopolysaccharide-induced pro-inflammatory
mediators in macrophages. Food and Function, 8(3),
1245–1253. https://doi.org/10.1039/c6fo01735e
Mahmood, N. D., Mamat, S. S., Kamisan, F. H., Yahya, F.,
Kamarolzaman, M. F. F., Nasir, N., Mohtarrudin, N.,
Tohid, S. F. M., & Zakaria, Z. A. (2014). Amelioration
of paracetamol-induced hepatotoxicity in rat by the
administration of methanol extract of Muntingia
calabura L. Leaves. BioMed Research International,
2014. https://doi.org/10.1155/2014/695678
Murti, F. K., Amarwati, S., & Wijayahadi, N. (2016).
Terhadap Gambaran Mikroskopis Hepar Tikus Wistar.
Kedokteran Diponegoro, 5(4), 871–883.
Parameshwaran, K., Irwin, M. H., Steliou, K., & Pinkert, C.
A. (2010). D-galactose effectiveness in modeling aging
and therapeutic antioxidant treatment in mice.
Rejuvenation Research, 13(6), 729–735.
https://doi.org/10.1089/rej.2010.1020
Pereira, G. A., Arruda, H. S., de Morais, D. R., Eberlin, M.
N., & Pastore, G. M. (2018). Carbohydrates, volatile
and phenolic compounds composition, and antioxidant
activity of calabura (Muntingia calabura L.) fruit. Food
Research International, 108(March), 264–273.
https://doi.org/10.1016/j.foodres.2018.03.046
Rofiee, M. S., Yusof, M. I. M., Abdul Hisam, E. E., Bannur,
Z., Zakaria, Z. A., Somchit, M. N., Teh, L. K., & Salleh,
M. Z. (2015). Isolating the metabolic pathways
involved in the hepatoprotective effect of Muntingia
calabura against CCl4-induced liver injury using
LC/MS Q-TOF. Journal of Ethnopharmacology, 166,
109–118. https://doi.org/10.1016/j.jep.2015.03.016
Sadowska-Bartosz, I., & Bartosz, G. (2015). Prevention of
protein glycation by natural compounds. Molecules,
20(2), 3309–3334.
https://doi.org/10.3390/molecules20023309
Safrida, S., & Sabri, M. (2019). Effect of Muntingia
calabura l. Stem bark extracts on uric acid concentration
and renal histopathology in diabetic rats. Medicina
(Lithuania), 55(10).
https://doi.org/10.3390/medicina55100695
Sarimanah, J., Ketut Adnyana, I., Sukandar, E. Y., &
Kurniati, N. F. (2017). The antirheumatic activity of
Muntingia calabura L. Leaves ethanol extract and its
fraction. Asian Journal of Pharmaceutical and Clinical
Research,
10(1), 84–86.
https://doi.org/10.22159/ajpcr.2017.v10i1.14102
Schmucker, D. L., & Sanchez, H. (2011). Liver
regeneration and aging: A current perspective. Current
Gerontology and Geriatrics Research, 2011.
https://doi.org/10.1155/2011/526379
Siddiq, M. N. A. A., Marliyati, S. A., Riyadi, H., &
Winarsih, W. (2019). Effects of Kersen leaves extract
(Muntingia calabura L.) on SGOT and SGPT levels of
soft drink induced mice. Jurnal Gizi Dan Pangan,
14(2), 69–76.
https://doi.org/10.25182/jgp.2019.14.2.69-76
Sookoian, S., Castaño, G. O., Scian, R., San Martino, J., &
Pirola, C. J. (2016). Heat Shock Protein 27 is down-
regulated in Ballooned Hepatocytes of Patients with
Nonalcoholic Steatohepatitis (NASH). Scientific
Reports, 6. https://doi.org/10.1038/srep22528
Sulistyoningrum, E., Rosmelia, R., Hamid, M. K., &
Nuraini, W. S. T. (2019). Anti-aging effects of
Muntingia calabura leaves extract in D-galactose-
induced skin aging mouse model. Journal of Applied
Pharmaceutical Science, 9(9), 23–29.
https://doi.org/10.7324/JAPS.2019.90904
Ye, Y., Jia, R. R., Tang, L., & Chen, F. (2014). In vivo
antioxidant and anti-skin-aging activities of ethyl
acetate extraction from idesia polycarpa defatted fruit
residue in aging mice induced by D-galactose.
Evidence-Based Complementary and Alternative
Medicine, 2014. https://doi.org/10.1155/2014/185716
Zakaria, Z. A., Mahmood, N. D., Mamat, S. S., Nasir, N.,
& Omar, M. H. (2018). Endogenous antioxidant and
LOX-mediated systems contribute to the
hepatoprotective activity of aqueous partition of
methanol extract of Muntingia calabura L. leaves
against paracetamol intoxication. Frontiers in
Pharmacology, 8(FEB), 1–14.
https://doi.org/10.3389/fphar.2017.00982
Zakaria, Z. A., Mahmood, N. D., Omar, M. H., Taher, M.,
& Basir, R. (2019). Methanol extract of Muntingia
calabura leaves attenuates CCl4-induced liver injury:
possible synergistic action of flavonoids and volatile
bioactive compounds on endogenous defence system.
Pharmaceutical Biology, 57(1), 335–344.
https://doi.org/10.1080/13880209.2019.1606836
Zhong, H. H., Hu, S. J., Yu, B., Jiang, S. S., Zhang, J., Luo,
D., Yang, M. W., Su, W. Y., Shao, Y. L., Deng, H. L.,
Hong, F. F., & Yang, S. L. (2017). Apoptosis in the
aging liver. Oncotarget, 8(60), 102640–102652.
https://doi.org/10.18632/oncotarget.21123
Zulfi, Z., Ilyas, S., & Hutahaean, S. (2013). Pengaruh
Pemberian Vitamin C Dan E Terhadap Gambaran
Histologis Ginjal Mencit (Mus Musculus L.) Yang
Dipajankan Monosodium Glutamat (Msg). Saintia
Biologi, 1(3), 1–6.
The Effects of Aqueous Extract of Jamaican Cherry (Muntingia calabura) on D-galactose-induced Liver Damage in BALB/c Mice (Mus
musculus)
207
