
Iron Status of Newborns in Maternal Inflammation Status
Differences
Qodri Santosa
1
a
, Alfi Muntafiah
2
b
, and Lantip Rujito
3
c
1
Department of Child Health, Faculty of Medicine, Universitas Jenderal Soedirman, Purwokerto, Indonesia
2
Department of Biochemistry, Faculty of Medicine, Universitas Jenderal Soedirman, Purwokerto, Indonesia
3
Department of Molecular Biology, Faculty of Medicine, Universitas Jenderal Soedirman, Purwokerto, Indonesia
Keywords: inflammation, pregnancy, IL-6, CRP, iron status, ferritin, newborn
Abstract: The human nervous system develops rapidly during the last pregnancy period and the beginning of human
life, requiring much iron. Inflammation during pregnancy may interfere with materno-fetal iron transfer. The
study aimed to compare the newborn iron status levels in maternal inflammation status differences. A cross-
sectional study was conducted with subjects of 84 clinically healthy newborns. We used C-reactive protein
(CRP) and interleukin-6 (IL-6) as parameters of maternal inflammation. Maternal IL-6 was classified into two
test groups based on quartile 1 (Q1) and quartile 2-4 (Q2-4), while CRP in the positive and negative groups.
Statistical analysis used a t-test independent or Mann-Whitney test, with 95% confidence intervals and a
significance limit at p <0.05. All newborns and their mothers were in healthy condition. Ferritin newborns'
levels were higher in the positive than negative CRP group (450.6 ± 194.86 vs. 365.1 ± 212.91, with p = 0.02).
Ferritin newborns were also higher in the maternal IL-6 Q2-4 group than in Q1, at levels 299.03 ± 154.98 vs.
492.35 ± 276.25, with p = 0.003. The study concluded that newborns' serum ferritin levels are higher in the
maternal with CRP-positive and higher IL-6 groups. We should be careful in interpreting the elevated serum
ferritin because it is also an acute-phase reactant.
1 INTRODUCTION
Iron sufficiency during late pregnancy and early
human life is essential for the nervous system's rapid
growth (Collard, 2009). Cellular respiration in the
hippocampus and frontal cortex, neurotransmitter
concentrations, fatty acid profiles, and myelination
will be disrupted if an iron deficiency occurs during
this period, potentially disrupting growth and
development (Georgieff, 2007).
An iron status assessment is essential, but no
single laboratory examination can determine the
diagnosis in all compartments, among red blood cells,
transport, functional, and storage (Wu, 2002). Human
iron stores in the body exist primarily in the form of
ferritin. Declining (low) serum ferritin levels reflect
depleted iron stores. However, ferritin is an acute-
phase reactant whereby concentrations increase
during inflammation and no longer reflect the iron
a
https://orcid.org/0000-0001-7712-2549
b
https://orcid.org/0000-0002-1424-6111
c
https://orcid.org/0000-0001-6595-3265
store's size. Interpretation of average or high serum
ferritin values is difficult in areas of widespread
infection or inflammation. Without inflammation or
liver disease, increased serum ferritin concentrations
indicate iron overload (WHO, 2011).
The inflammatory process is generally
characterized by an increase in pro-inflammatory
cytokines and acute-phase reactants levels.
Interleukin-6 (IL-6), a pro-inflammatory cytokine,
and C-reactive protein (CRP) are the primary
mediators of the host response to inflammation, and
both are also early markers of the acute-phase
response (Sorokin et al., 2010). High levels of IL-6 in
pregnancy can induce hepcidin transcription.
Interleukin-6 - hepcidin axis is responsible for
hypoferremia in pregnant women with excessive
inflammation (Zhang & Enns, 2009; Wrighting &
Andrews, 2006), then interferes with a materno-fetal
194
Santosa, Q., Muntafiah, A. and Rujito, L.
Iron Status of Newborns in Maternal Inflammation Status Differences.
DOI: 10.5220/0010490001940201
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 194-201
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

iron transfer (Nemeth et al., 2004), and may affect
neonatal iron status (Yanoff et al., 2007).
Mild acute inflammation did not increase serum
hepcidin in pregnant women with iron deficiency
anemia. Even Abioye et al. confirmed that anemia of
inflammation during human pregnancy did not affect
newborn iron endowment (Abioye et al., 2018). The
underlying mechanisms are still being debated so that
during pregnancy, it seems unclear how the
difference in newborn iron status between maternal
inflammation status differences.
This study aimed to compare the newborn iron
status in maternal inflammation status differences,
using maternal IL-6 and CRP inflammation
parameters.
2 MATERIAL AND METHODS
Our study was part of comprehensive research that
assessed various factors associated with neonates'
iron status. A cross-sectional study was conducted in
Purbalingga Regency, Central Java, Indonesia, in
three hospitals, with 84 newborns, from September to
November 2015. The inclusion criteria for newborns
were born spontaneously, from single and term
pregnancy, normal birth weight (≥2.500 to <4.000
grams), with an Apgar score ≥of 7 in the first minute,
and not suffer from significant congenital
abnormalities. We excluded newborns subjects if they
were suffering from severe illness and hematologic-
oncological disease, and the mother had a postpartum
hemorrhage. The Health and Medical Research Ethics
Commission of the Faculty of Medicine, Diponegoro
University/Dr. Kariadi Hospital Semarang provided
ethical approval with No.48/EC/FKRSDK/2015. The
father or mother of the newborn subject signed the
written informed consent before joining the research.
We used CRP and IL-6 as parameters of maternal
inflammation, using maternal venous blood samples.
Maternal IL-6 was classified into two test groups
based on quartile 1 (Q1) and quartile 2-4 (Q2-4),
while CRP in the positive and negative groups.
Newborn iron status parameters included red blood
cell (RBC) count, hemoglobin (Hb), hematocrit (Ht),
and hepcidin using blood samples taken from
umbilical cord blood. In contrast, serum iron (SI) and
serum ferritin (SF) were taken from newborns'
venous blood.
Maternal venous blood samples were taken when
the mother was admitted to the hospital for delivery.
The blood samplings from the umbilical cord blood
were collected immediately after the placenta was
born, whereas the newborn's vein was performed
directly after the baby was born. Parameters of RBC
count, Hb, and Ht of newborns were checked using
Sysmex XN-1000, while hepcidin using the ELISA
method. SI was tested using the IRON Flex® reagent
cartridge, Cat. No. DF85, while SF and maternal IL-
6 using the chemiluminescence immunoassay
(ECLIA) method. Maternal CRP was performed
using the C-Reactive Protein Extended Range
(RCRP) method used on the Dimension® clinical
chemistry.
The statistical analysis to compare newborn
iron status between maternal inflammation status
differences was tested with the independent t-test or
Mann-Whitney test. In groups (positive and
negative), maternal CRP, hepcidin variables were
analyzed using the Mann-Whitney test, while other
variables used the independent t-test. In the two
maternal IL-6 quartile groups (Q1 and Q2-4), variable
RBC, hematocrit, and hepcidin variables were
analyzed using the Mann-Whitney test, while other
variables were tested using an independent t-test. The
statistical test used 95% confidence intervals, with a
limit of significance at p <0.05.
3 RESULTS
A total of 84 newborns participated in our cross-
sectional study. We interviewed as many as 108
pregnant women/parents of prospective subjects in
the initial process. Seven pregnant women refused
because they were afraid or worried about the blood
collection process. Two babies with clinical features
of Down's syndrome, four babies born with
respiratory problems, and 11 babies failed blood
sampling or laboratory techniques, so they could not
continue the study process.
All newborn subjects were at term babies,
born spontaneously, from singleton pregnancies,
Apgar scores ≥ seven at the first minute, average birth
weight (≥ 2,500 to <4,000 grams), and not suffering
from significant congenital abnormalities. The CRP
of all newborn subjects was negative. (Table 1). Table
2 shows that all maternal subjects were pregnant at
term, did not suffer from diabetes mellitus, pre-
eclampsia/eclampsia, and antepartum hemorrhage,
came from Javanese, and with Hb >8 g/dL.
The results showed that hematocrit levels, SF,
and hepcidin newborns in the maternal CRP group
had abnormal data distribution. The hepcidin variable
was still not standard after being transformed, so it
was analyzed using the Mann-Whitney test, while
other variables used the independent t-test. (Table 3)
Iron Status of Newborns in Maternal Inflammation Status Differences
195

Table 1. Characteristics of newborn
Characteristics of newborn Value
Sex (gender):
- Male, n (%)
- Female, n (%)
38 (45.2)
46 (54.8)
Gestation, week [median
(ran
g
e)
39.22
(37
–
41)
Apgar score (AS)
- 1 min, [median (ran
g
e) 8.04 (7
–
9)
- 5 min, [median (ran
g
e) 9 (8
–
10)
Heart rate,
b
eats/min [median (ran
g
e)
129.88
(110
–
148)
Birth weight,
g
ram [median (ran
g
e)
3190.06
(2600
–
3900)
CRP:
- Positive, n (%)
-
N
e
g
ative, n (%)
84 (100)
0 (0)
Table 2. Characteristics of the mother
Characteristics of mother Value
Age of mother, year (𝑥 ± SD) 27.43 ± 5.38
Gravida:
- Gravida ≤ 2, n (%)
- Gravida ≥ 3, n (%)
68 (81.0)
16 (19.0)
Education levels:
- > Senior High School, (%)
- ≤ Senior Hi
g
h School, (%)
20 (23.8)
64 (76.2)
Systolic, mmHg (𝑥±SD)
119.85 ± 9.19
Diastolic, mmHg (𝑥±SD) 73.33 ± 6.47
Fe tablet, n (%) 84 (100)
Ante-natal care ≥ fou
r
times 84 (100)
Smoking during pregnancy:
- Yes, (%)
-
N
o, (%)
1 (1.2)
83 (98.8)
Table 3. Iron Status Parameters based on Maternal CRP Groups
Iron Status Newborns Parameters
Maternal CRP Groups
p
CRP positive
(n=48)
*)
CRP negative
(n=36)
RBC (umbilical cord), 10
6
/mm
3
(𝑥±SD) 4.2±0.44 4.3±0.46 0.209
a
Hb (umbilical cord), g/dL (𝑥±SD)
15.2±1.47
15.2±1.49
0.857
a
Ht (umbilical cord), % (𝑥±SD) 45.0±5.12 44.9±4.98 0.986
a
Serum Iron, μg/dL (𝑥±SB) 114.3±51.30 111.3±54.96 0.799
a
Serum Ferritin ng/
m
L (𝑥±SD)
450.6±194.86 365.1±212.91 0.023
a
Hepcidin (umbilical cord), ng/mL
(𝑥
±SD)
[median (min-max)]
4.2±1.62
4.8(1.66-6.90)
3.8±1.73
3.3(1.58-6.85)
0.426
b
Remarks: a t-test independent; b, Mann-Whitney; *, ferritin, n=45
The results showed that SF newborn levels were
higher in the CRP positive mothers group than the
opposing group, with a mean of 450.6 ± 194.86 vs.
365.1 ± 212.91 ng/mL with p = 0.023. Other
parameters of iron status for newborns were not
significantly different in these maternal CRP groups.
We divided newborns subjects into the two quartile
groups of maternal IL-6 (Q1 and Q2-4). Parameters
RBC, hematocrit, and hepcidin levels have abnormal
data distribution. After transforming the data, the data
distribution of hematocrit and hepcidin were not
expected, so they were analyzed using the Mann-
Whitney test. Meanwhile, other variables were tested
using an independent t-test. Table 4 shows that the
mean SF newborns in the quartile group (Q1 vs. Q2-
4) were higher in the maternal IL-6 group Q2-4, with
a mean of 299.03 ± 154.98 v.s 492.35 ± 276.25 ng/mL
and statistically significant with p = 0.003. In Table
5, we divided newborn hepcidin and SF into maternal
IL-6 gradations Q1, Q2-3, and Q4. Among the
maternal IL-6 quartile group, we found that the
median hepcidin cord blood was not different (p:
0,610), while the mean of newborn SF was
significantly different (p: 0.006). However, by post
hoc of LSD analysis, we only found differences in SF
newborns' standard in the IL-6 Q1 vs. Q2-3 (p:
0.002). Meanwhile, the newborn SF levels in the IL-
6 maternal Q1 group were not different from the Q4
group (p: 0.068). Likewise, the mean SF newborns at
Q2-3 and Q4 did not differ (p: 0.267).
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
196
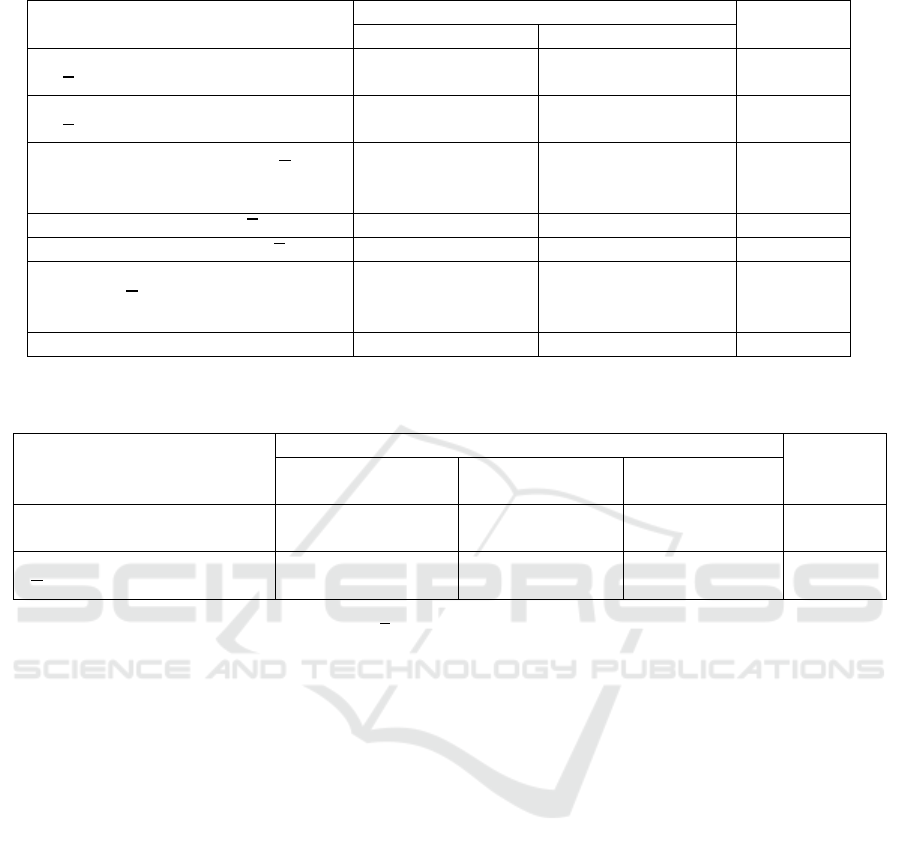
Table 4. Iron status parameters based on maternal IL-6 quartile (Q1 and Q2-4) groups
Iron Status Newborns Parameters
Maternal IL-6 Quartile Groups
p
Q1 (n=21)
Q2-4 (n=63)
*)
RBC (umbilical cord), 10
6
/mm
3
(𝑥
±SD)
4.3±0.47 4.2±0.45 0.432
a
Hb (umbilical cord), g/dL
(𝑥
±SD)
15.4±1.80
15.1±1.35
0.358
a
Ht (umbilical cord), % (𝑥±SD)
[median (min-max)]
45.7±5.88
44.5(38.10-
59.70)
44.7±4.74
44.3(34.80-
56.90)
0.877
b
Serum Iron, μg/dL (𝑥±SB) 121.7±50.34 110.1±53.40 0.386
a
Serum Ferritin ng/
m
L (𝑥±SD)
299.03±154.98 492.35±276.25 0.003
a
Hepcidin (umbilical cord),
ng/mL (𝑥
±SD)
[median (min-max)]
4.2±1.75
3.6(1.58-6.85)
4.0±1.65
3.9(1.66-6.90)
0.345
b
Remarks: a t-test independent; b, Mann-Whitney test; *, ferritin neonatal (n=61)
Table 5. Serum ferritin and hepcidin of newborn based on maternal IL-6 quartile (Q1, Q2-3, and Q4) groups
Newborns Parameters
Maternal IL-6 Quartile Groups
p
Quartile 1
(n=21)
Quartile 2-3
(n=42)
Quartile 4
(n=21)
Hepsidin cord blood,
[median (min-max)]
3.6 (1.58-
6.85)
4.4 (1.66-
6.90)
3.9 (1.83-
5.89)
0.610
b
Serum ferritin, ng/mL,
(𝑥
±SB)
299.0 ±
154.98
472.3 ±
206.75
412.3 ±
210.61
0.006
a
Remarks:
Statistical tests used 95% confidence intervals (𝑥
±SB), mean ± standard deviations; a one-way Anova test; Post hoc analysis
of LSD: IL-6 Mother quartile I vs. II-III p = 0.002; I vs. IV p = 0.068; II-III and IV p = 0.267
4 DISCUSSION
The study aimed to compare the newborn iron status
levels in maternal inflammation status differences.
The newborns' iron status using the RBC count, Hb,
Ht, SI, and hepcidin did not differ between the
maternal CRP group (positive vs. negative) and the
IL-6 maternal quartile group (Q1 vs. Q2-4). In
contrast above, the mean SF newborn was
significantly higher in the CRP positive group.
Likewise, the mean SF newborn was higher in the
maternal IL-6 Q2-4 group (inflammatory mothers), p
<0.05. However, after dividing the maternal IL-6 into
Q1, Q2-3, and Q4, the newborn SF levels in the IL-6
Q4 group tended to decrease compared to Q2-3,
although the two groups did not differ significantly.
These results indicate that iron status was
generally unaffected. Still, SF newborns as a
parameter of iron storage will increase or be higher in
newborns born to mothers with positive CRP and
more elevated IL-6 (inflammatory mothers). We
assume that maternal inflammatory status in the last
trimester of pregnancy tends to increase the iron
stores (SF) of babies born under normal pregnancy
conditions. We considered that the maternal
inflammatory disease is a "physiological strategy" to
increase iron storage (SF levels) of the fetus for the
third trimesters' rapid growth.
Research on inflammation in pregnancy (such as
pregnancy with obesity) has different effects. Dao et
al. found no statistically significant differences in
CRP, IL-6, or hepcidin levels in cord blood between
the obese and non-obese maternal groups (Dao et al.,
2013). Still, serum iron and transferrin saturation in
cord blood were lower in neonates born to obese
women than those of average weight. Furthermore,
Cao et al. also concluded that the prepregnancy body
mass index (BMI) has no negative impact on maternal
or neonatal iron status (Cao et al., 2016). Jones et al.
(2016) reported that maternal obesity during
pregnancy is negatively associated with maternal and
neonatal iron status (Jones et al., 2016). Research in
316 newborns said that compared to non-obese
pregnant women (BMI <30 kg/m2), obese women
Iron Status of Newborns in Maternal Inflammation Status Differences
197

delivered offspring with lower iron status, as assessed
using SF and zinc protoporphyrin/heme (Phillips et
al., 2014).
Inflammation is the necessary process as a
response to injury and also central to reproductive
success. Such as ovulation, menstruation,
implantation, and parturition are all inflammatory
processes. A physiologic systemic inflammatory
response also characterizes pregnancy (Romero et al.,
2007). Concentrations of CRP and IL-6 in obese
women compared to normal-weight women indicated
an inflammatory response (Buss et al., 2012). CRP
level has been reported to be elevated in pregnant
women without pregnancy complications than in non-
pregnant women (Fink et al., 2019; Watts et al.,
1991). Reproductive success appears to be influenced
by cytokine activity's strict regulation (Austgulen et
al., 1994). So, if not exaggerated/not excessive,
inflammation has essential roles in reproductive
physiology.
Another review of the inflammatory process, in
which chronic and excessive inflammation of
pregnancy can lead to poor pregnancy outcomes.
Generally, research on IL-6 in pregnancy has been
associated with poor outcomes in mothers and their
babies. IL-6 has been implicated as a mediating factor
in maternal inflammation processes to alterations in
fetal brain development (Buss et al., 2012; Rudolph
et al., 2018; Estes and McAllister, 2016).
The main factor responsible for altered iron
metabolism in inflammatory conditions is hepcidin
(Wessling-Resnick, 2010). Hepcidin regulated iron
homeostasis by controls iron absorption and recycling
(Ganz, 2013).
Transcription of hepcidin is induced
when systemic iron levels are high and down-
regulates its receptor, ferroportin (FPN), preventing
iron export to blood plasma (Ganz, 2013). The
abnormality of raised hepcidin causes intracellular
sequestration and decreased intestinal iron absorption
due to the downregulation of FPN expression in
macrophages and enterocytes (Cherayil, 2015).
Furthermore, changes in hepcidin levels can rapidly
modulate and control plasma iron concentrations.
Commonly, in non-pregnant obese women,
hepcidin is up-regulated (Tussing-Humphreys et al.,
2012), otherwise during a healthy pregnancy,
hepcidin is reduced and enabling increased materno-
fetal iron transfer (Fisher & Nemeth, 2017). Maternal
hepcidin is suppressed during the second and third
trimesters, which increases iron availability for
materno-fetal transfer (Fisher & Nemeth, 2017). The
mechanism of maternal hepcidin suppression is
unclear (Sangkhae et al., 2020). The interpretation of
hepcidin levels, such as ferritin, should also be
considered concurrently with inflammation markers
(Sanni et al., 2020).
The inflammatory markers of CRP and IL-6 have
long been known. As a pro-inflammatory cytokine,
IL-6 is frequently elevated in obese pregnant women.
It has been shown to induce hepcidin expression, a
negative regulator of intestinal iron absorption,
macrophage iron efflux, and hepatic iron stores (Ganz
& Nemeth, 2006). Inflammation, such as obesity in
pregnant women, may lead to hepcidin excess and
decreased iron transfer to the fetus (Flynn et al.,
2018), affecting newborn iron status.
In low-grade inflammation in non-pregnant
women, obesity is associated with increased hepcidin,
induced iron sequestration, and decreased circulating
iron (Tussing-Humphreys et al., 2012). The CRP and
IL-6 were more remarkable in obese than normal-
weight pregnant women (Fisher & Nemeth, 2017),
and inflammatory conditions in pregnancy are lower
and more visualized in obese pregnancies (Dosch et
al., 2016). However, Flynn et al. found no
relationship between CRP or IL-6 and hepcidin in
obese or normal-weight women. It might indicate that
the association between inflammatory mediators and
hepcidin is not extant in pregnancy (Fisher and
Nemeth, 2017). Other pathways may play an essential
iron regulatory role in pregnancy.
Why does maternal inflammatory condition
increase in SF newborns (iron stores) while all
newborn subjects with CRP are negative? A recent
study confirmed that mild acute inflammation did not
increase serum hepcidin in women with IDA,
suggesting that low iron status and erythropoiesis
drive offset the inflammatory stimulus on hepcidin
expression. In non-anemic women, inflammation
increased serum hepcidin and produced mild
hypoferremia. However, it did not reduce dietary iron
absorption, suggesting that iron-recycling
macrophages are more sensitive than the enterocyte
high serum hepcidin during inflammation (Stoffel et
al., 2019). Abioye et al. confirmed that anemia of
inflammation during human pregnancy did not affect
newborn iron endowment (Abioye et al., 2018).
Previous concepts still understand that cytokines
(e.g., IL-6) can cross the placenta when the placental
barrier was damaged. An animal experiment by
Dahlgren J et al. proved that maternal IL-6 could
cross the placental border to the fetus, both in a
condition where the placental barrier is impaired or
normal (Dahlgren et al., 2006). In previous research,
Zaretsky et al. confirmed that fetal IL-6 could also
penetrate the placenta into the maternal circulation
(Zaretsky et al., 2004). So, IL-6 can pass bidirectional
transfer from maternal to fetus and vice versa.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
198

Maternal IL-6 passes to the fetus, and subsequently,
IL-6 in the fetus can affect iron status.
Our data (Table 5) showed, in maternal IL-6
gradations Q1, Q2-3, and Q4, we found that the mean
of newborn SF was significantly different among
those groups. The Post Hoc of LSD analysis stated
that SF newborns' standard was higher in the IL-6
maternal Q2-3 (middle quartile) group than in the Q1
(lowest quartile) group with p: 0.002. We also noted
that the newborn SF levels in the IL-6 Q4 (highest
quartile) group were not different from the Q1 and
Q2-3 groups. The hepcidin and SF newborn values
increased IL-6 maternal Q1 to Q2-3, then decreased
in the IL-6 Q4 group. These results may indicate an
inverted U-shaped association between IL-6 maternal
and neonatal iron status (SF and hepcidin).
Maternal iron status (including serum ferritin) has
a U-shaped association with adverse pregnancy
outcomes (Dewey & Oaks, 2017). We must be
careful because SF is also an acute-phase reactant.
However, elevated ferritin is a marker of increased
iron stores and inflammation, and the specific
contribution of excess iron has not been resolved. It
is poorly understood how to regulate iron homeostasis
between maternal and fetal during pregnancy,
including maternal, placental, and fetal signals
(Sangkhae et al., 2020). Like ferritin, IL-6 maternal,
as an acute-phase response, so we must be careful to
interpret the meaning of increased SF in newborns.
The implication of this study, because there is an
indication of an inverted U-shaped association
between maternal inflammation (levels of maternal
IL-6) and neonatal iron status. Hence less or
excessive inflammation may decrease the iron status
of newborns. We must be careful to interpret the
newborn SF level because it is also an acute reaction
protein.
5 CONCLUSION
Our study compared neonate iron status levels in
maternal inflammation status differences. Our
findings indicated that inflammation is the necessary
process for reproductive success. We conclude that
(not excessive) inflammation in pregnancy does not
affect iron status (based on RBC count, Hb, Ht, SI,
hepcidin parameters) but increases the SF (iron
storage) of the newborns. The SF levels of newborns
are higher in the maternal with CRP-positive and
higher IL-6 groups. There is an indication of an
inverted U-shaped association between maternal
inflammation and neonatal iron status. Hence less or
excessive inflammation during pregnancy may
decrease the iron status of newborns. We must be
careful to interpret the newborn SF level because it is
also an acute reaction protein. This study's limitation
could not conclude a cause and effect between
maternal inflammation and the iron status of
newborns because it is only an observational study.
Future studies need to involve broader factors,
especially IL-6, hepcidin, and other variables
affecting neonate iron status in maternal, placenta,
and cord blood. It is necessary to carry out research
that can describe how orchestral iron metabolism in
the fetus.
ACKNOWLEDGMENTS
Our gratitude goes to all parties involved in the
research, from Ummu Hani Hospital, Harapan Ibu
Hospital, and Panti Nugroho Hospital, Purbalingga.
REFERENCES
Abioye AI, Park S, Ripp K, McDonald E A, Kurtis J D, Wu
H, Pond-Tor S, Sharma S, Ernerudh J, Baltazar P,
Olveda RM, Tallo V, Friedman JF. (2018) 'Anemia of
inflammation during human pregnancy does not affect
newborn iron endowment.' J Nutr., 148 (3), pp. 427–
436. DOI: 10.1093/jn/nxx052.
Austgulen R, Lien E, Liabakk N-B, Jacobsen G, and
Arntzen KJ. (1994) 'Increased levels of cytokines and
cytokine activity modifiers in normal pregnancy.' Eur J
Obstet. Gynecol. Reprod. Biol., 57(3), pp. 149-155.
DOI: 10.1016/0028-2243(94)90291-7.
Buss C, Entringer S, and Wadhwa PD. (2012) 'Fetal
programming of brain development: intrauterine stress
and susceptibility to psychopathology.' Sci Signal.,
5(245):pt7. DOI: 10.1126/signal.2003406.
Cao C, Pressman EK, Cooper EM, Guillet R, Westerman
M, and O'Brien KO. (2016) 'Prepregnancy body mass
index and gestational weight gain have no negative
impact on maternal or neonatal iron status.' Reprod Sci.,
23(5), pp. 613–622. DOI: 10.1177/1933719115607976.
Cherayil BI. (2015) 'Pathophysiology of iron homeostasis
during inflammatory states.' J Pediatr., 167(0): S15–
S19. DOI: 10.1016/j.jpeds.2015.07.015.
Collard KJ. (2009) 'Iron homeostasis in the neonate.'
Pediatrics. 123(4), pp.1208-1216. DOI:
10.1542/peds.2008-1047.
Dao MC, Sen S, Iyer C, Klebenov D, and Meydani SN.
(2013) 'Obesity during pregnancy and fetal iron status:
is hepcidin the link?' J Perinatol., 33(3), pp.177–181.
DOI: 10.1038/jp.2012.81.
Dahlgren J, Samuelsson A, Jansson T, and Holmȁng AA.
(2006) 'Interleukin-6 in the maternal circulation reaches
the rat fetus in mid-gestation'. Pediatr Res., 60 (2), pp.
147–151. DOI: 10.1203/01.pdr.0000230026.74139.18.
Iron Status of Newborns in Maternal Inflammation Status Differences
199

Dewey KG and Oaks BM. (2017) 'U-shaped curve for risk
associated with maternal hemoglobin, iron status, or
iron supplementation.' Am J Clin Nutr., 106(Suppl
6):1694S–1702S. DOI: 10.3945/ajcn.117.156075.
Dosch NC, Guslits EF, Weber MB, Murray SE, Ha B, and
Coe CL. (2016) 'Maternal obesity affects inflammatory
and iron indices in umbilical cord blood.' J Pediatr.,
172: 20–28. DOI: 10.1016/j.jpeds.2016.02.023.
Estes ML & McAllister AK. (2016) 'Maternal immune
activation: implications for neuropsychiatric disorders'.
Science. 353(6301), pp.772–777. DOI:
10.1126/science.aag3194.
Fink NR, Chawes B, Bønnelykke K, Thorsen J, Stokholm
J, Rasmussen MA. (2019) 'Levels of low-grade
systemic inflammation in pregnant mothers and their
offspring are correlated.' Scientific Reports. 9:3043.
Doi: 10.1038/s41598-019-39620-5.
Fisher AL and Nemeth E. (2017) 'Iron homeostasis during
pregnancy.' Am. J. Clin. Nutr., 106(Suppl 6): 1567s–
1574s. DOI: 10.3945/ajcn.117.155812.
Flynn AC, Begum S, White SL, Dalrymple K, Gill C, and
Alwan NA. (2018) 'Relationships between maternal
obesity and maternal and neonatal iron status.'
Nutrients. 10(8):1000. DOI: 10.3390/nu10081000.
Ganz T and Nemeth E. (2006) 'Iron imports IV Hepcidin
and regulation of body iron metabolism.' Am J Physiol
Gastrointest Liver Physiol., 290(2): G199-203. DOI:
10.1152/ajpgi.00412.2005.
Ganz T. (2013) 'Systemic iron homeostasis.' Physiol Rev.,
93(4), pp.1721–1741. DOI:
10.1152/physrev.00008.2013.
Georgieff MK. (2007) 'Nutrition and the developing brain:
nutrient priorities and measurement.' Am J Clin Nutr.,
85(2):614S–620S. DOI: 10.1093/ajcn/85.2.614S.
Jones AD, Zhao G, Jiang YP, Zhou M, Xu G, and Kaciroti
N. (2016) 'Maternal obesity during pregnancy is
negatively associated with maternal and neonatal iron
status.' Eur. J. Clin. Nutr., 70(8), pp. 918-924. DOI:
10.1038/ejcn.2015.229.
Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S,
Pedersen BK, Ganz T. (2004) 'IL-6 mediates
hypoferremia of inflammation by inducing the
synthesis of the iron regulatory hormone hepcidin'. J
Clin Invest., 113(9), pp. 1271–1276. DOI:
10.1172/JCI20945.
Phillips AK, Roy SC, Lundberg R, Guilbert TW, Auger AP,
Blohowiak SE, Coe CL, Kling PJ. (2014) 'Neonatal iron
status is impaired by maternal obesity and excessive
weight gain during pregnancy.' J Perinatol., 34(7),
pp.513–518. DOI: 10.1038/jp.2014.42.
Rudolph MD, Graham AM, Feczko E, Miranda-
Dominguez O, Rasmussen JM, Nardos R, Wadhwa PD,
Buss C, Fair DA. (2018) 'Maternal IL-6 during
pregnancy can be estimated from newborn brain
connectivity and predicts future working memory in
offspring'. Nature Neuroscience. 21(5), pp.765-772.
DOI: 10.1038/s41593-018-0128-y.
Romero R, Gotsch F, Pineles B, and Kusanovic JP. (2007)
'Inflammation in pregnancy: its roles in reproductive
physiology, obstetrical complications, and fetal injury.'
Nutrition Reviews. 65(12 Pt 2): S194–S202. DOI:
10.1111/j.1753-4887.2007.tb00362.x.
Sangkhae V, Fisher AL, Wong S, Koenig MD, Tussing-
Humphreys L, Chu A, Lelić M, Ganz T, Nemeth E.
(2020) 'Effects of maternal iron status on placental and
fetal iron homeostasis.'
J Clin Invest., 130(2):625-640.
DOI: 10.1172/JCI127341.
Sanni OB, Chambers T, Li JH, Rowe S, Woodman AG,
Ospina MB, and Bourque SL. (2020) 'A systematic
review and meta-analysis of the correlation between
maternal and neonatal iron status and haematologic
indices.' clinical medicine. 27:100555. DOI:
10.1016/j.eclinm.2020.100555.
Sorokin Y, Romero R, Mele L, Wapner RJ, Iams JD,
Dudley DJ, Spong CY, Peaceman AM, Leveno KJ,
Harper M, Caritis SN, Miodovnik M, Mercer BM,
Thorp JM, O'Sullivan MJ, Ramin SM, Carpenter MW,
Rouse DJ, Sibai B. (2010) 'Maternal serum interleukin-
6, c-reactive protein, and matrix metalloproteinase-9
concentrations as risk factors for preterm birth < 32
weeks and adverse neonatal outcomes'. Am J Perinatol.,
27(8):631–640. DOI: 10.1055/s-0030-1249366.
Stoffel NU, Lazrak M, Bellitir S, Mir NE, Hamdouchi AE,
Barkat A, Zeder C, Moretti D, Aguenaou H,
Zimmermann MB. (2019) 'The opposing effects of
acute inflammation and iron deficiency anemia on
serum hepcidin and iron absorption in young women.'
Haematologica. 104(6):1143-1149. DOI:
10.3324/Haematol.2018.208645.
Tussing-Humphreys L, Pusatcioglu C, Nemeth E, and
Braunschweig C. (2012) 'Rethinking iron regulation
and assessment in iron deficiency, anemia of chronic
disease, and obesity: Introducing hepcidin.' J Acad Nutr
Diet., 112(3), pp.391–400. DOI:
10.1016/j.jada.2011.08.038.
Watts DG, Krohn MA, Wener MH, and Eschenbach DA.
(1991) 'C-reactive protein in normal pregnancy.' Obstet.
Gynecol., 77(2), pp.176–180. DOI: 10.1097/00006250-
199102000-00002.
Wessling-Resnick M. (2010) 'Iron homeostasis and the
inflammatory response.' Annu Rev Nutr., 30:105-122.
DOI: 10.1146/annurev.nutr.012809.104804.
Wrighting DM and Andrews NC. (2006) 'Interleukin-6
induces hepcidin expression through STAT3'. Blood.
108(9), pp.3204-3209. DOI: 10.1182/blood-2006-06-
027631.
Wu AC, Lesperance L, Bernstein H. (2002) 'Screening for
iron deficiency.' Pediatrics in Review. 23(5), pp. 171-
178. DOI: 10.1542/pir.23-5-171.
WHO. (2011) 'Serum ferritin concentrations for the
assessment of iron status and iron deficiency in
populations.' Available at:
https://www.who.int/vmnis/indicators/serum_ferritin.p
df
Yanoff LB, Menzie CM, Denkinger B, Sebring NG,
McHugh T, Remaley AT, Yanovski JA. (2007)
'Inflammation and iron deficiency in the hypoferremia
of obesity.' Int J Obes., 31(9), pp.1412–1419. DOI:
10.1038/sj.ijo.0803625.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
200

Zhang A and Enns CA. (2009) 'Molecular mechanisms of
normal iron homeostasis.' Hematology Am Soc Hematol
Educ Program., 207-214. DOI: 10.1182/asheducation-
2009.1.207.
Zaretsky MV, Alexander JM, Byrd W, and Bawdon RE.
(2004) 'Transfer of inflammatory cytokines across the
placenta.' Obstet Gynecol., 103(3),pp. 546-550. DOI:
10.1097/01.AOG.0000114980.40445.83.
Iron Status of Newborns in Maternal Inflammation Status Differences
201
