
Different Contribution of Estrogen Receptors, ET-1/ETBR and
Superoxide Dismutase and in eNOS Availibility based on Sexual
Dimorphism in Early Stage of Kidney Diabetic Rats
Anisa Fatwa
1,2,3 a
, Dwi Cahyani Ratna Sari
1 b
, Wiwit Ananda Wahyu Setyaningsih
1c
Andrew
Nobiantoro
4d
, Nur Arfian
1e
1
Departement of Anatomy, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada,
Yogyakarta,Indonesia
2
Master Program of Biomedical Science, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada,
Yogyakarta,Indonesia
3
Jakarta InVitro Fertilization, YPK MandiriHospital, Jakarta, Indonesia
4
Undergraduate International Program in School of Medicine, faculty of Medicine, Public Health and Nursing, Universitas
Gadjah Mada, Yogyakarta, Indonesia
Keywords: Early Stage Diabetes Mellitus, eNOS, ETBR, Estrogen Receptors, SOD
Abstract: Background Diabetes mellitus is metabolic diseases which is influenced by multifactorial conditions. Many
factors may contribute to DM progression, such as vasoconstrictor and vasodilator balance, as underlying
factor of endothelial dysfunction. Sex difference in male and female phenotype has not been elucidated in
DM progression, especially in eNOS availibility which associate with endothelin/endothelin receptors and
estrogen signaling. This research aims to elucidate the expression of ERα and Erβ, ppET1 via ETBR as
vasodilator properties, then SOD1 and SOD2 as antioxidant in male and female DM rats. Diabetes Mellitus
was induced in male and female rats with Streptozotocin 60mg/Kg.BB single injection intraperitonially.
Control group was injected with NaCl 0.9%. Rats were terminated at the 1st month. Proteinuria score and
histological structure were determined in all groups. Reverse Transcription-PCR was performed to know
mRNA expression of eNOS, ETBR, ERα and Erβ, SOD1 also SOD2 from kidney. All groups showed that
there were no significant differences in proteinuria score and histological structure related to persistent of
eNOS mRNA expression which confirmed by RT-PCR analysis. There were no significant differences
between eNOS mRNA expression in DM to control groups in each sex. Furthermore, ERα and ERβ mRNA
expression in female were significantly higher than male diabetic rats. Nevertheless, ETBR mRNA expression
was significantly higher in male compared to female diabetic rats. Then, female diabetic groups had higher
SOD1 and SOD2 mRNA expression compared to male. ERs and SOD upregulation in early diabetic female
rats and ETBR upregulation in early diabetic male rats might be associated with persistent eNOS expression
in early diabetic condition.
1 INTRODUCTION
The early stage of Diabetes Mellitus (DM) is
characterized by hyperfiltration, caused by
endothelial dysfunction in glomerular afferent
arterioles (Anderson et al., 1993). Endothelial
a
https://orcid.org/0000- 0002-6333-058X
b
https://orcid.org/0000- 0002-1126-4939
c
https://orcid.org/0000- 0002-4334-5012
d
https://orcid.org/0000- 0003-0296-3487
e
https://orcid.org/0000- 0003-1694-2054
dysfunction triggers the diabetic nephropaty which as
a Chronic Kidney Disease (Cheng et al., 2014). There
is a study that classified the stages of diabetic
nephropaty into two groups, early diabetic
nephropaty based on the beginning of GFR
(Glomerular Filtration Rate) enhancement, and the
Fatwa, A., Ratna Sari, D., Wahyu Setyaningsih, W., Nobiantoro, A. and Arfian, N.
Different Contribution of Estrogen Receptors, ET-1/ETBR and Superoxide Dismutase and in eNOS Availibility based on Sexual Dimorphism in Early Stage of Kidney Diabetic Rats.
DOI: 10.5220/0010489701750183
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 175-183
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
175

advanced stage of diabetes nephropaty starting from
the decrease of GFR (Eleftheriadis et a., 2013). This
classification easily determine the patient based on
the differences of vascular function in chronic kidney
disease, which nowadays still have a limited data.
Therefore there is a need for the approval of vascular
function in intial chronic kidney disease (Forbes et al.,
2003).
DM causes the endothelial dysfunction which is
affected by the decrease of Endothelial Nitric Oxide
(eNOS). eNOS helps relaxation of vascular smooth
muscle cells by activating Nitric Oxide directly (NO).
Stadler et al. (Stadler et al., 2003) revealed the
enhancement of eNOS in the early stages. There are
several potential vasoactive to increase NO
production, namely as estrogen hormone through the
estrogen receptors and endothelin-1 through the
ETBR. Increased oxidative stress is recognized as the
major metabolic abnormality involved in the
development of diabetic nephropaty. ROS production
is increased in endothelial and renal cells in
hyperglycemic conditions.
Increased oxidative stress also is recognized as the
major metabolic abnormality involved in the
development of diabetic nephropaty. ROS production
is increased in endothelial and renal cells in
hyperglycemic conditions. ROS can be dishminis by
antioxidant enzyme enzymes as though superoxide
dismutase, SOD1 and SOD2. But, The
overproduction of ROS diminishes expression of the
antioxidant. SOD1 is located in the cytoplasm, SOD2
in the mitochondria (Alejandra et al., 2016).
There is a study that suggests the possibility of
gender differences in the pathophysiology of the
disease. As in line as the studies, found that
nondiabetic premenopausal women have a lower risk
of having CKD and a slower rate of progression to
end-stage kidney disease compared to non-diabetic
men with the same age. There is a study that also
mentioned that sexual dimorphism affected the NO
system. Female rat has a higher NO level than males,
this is because eNOS can be converted by estrogen
into NO large amounts (Pesce et al., 2005).
There is no study about the expression of eNOS,
ET-1 ETBR, SOD1, SOD2, and Estrogen Receptors
based on sex and in the early stages of diabetes
mellitus. Therefore this study was conducted to assess
the differences in vasoprotector aspects namely as
estrogen receptor and ET-1 via ETBR which can
affect the changes of eNOS in female and male
diabetic rats in the early stages of diabetes. And also
SOD1 and SOD2, important antioxidant defense in
endothelial cells.
2 MATERIALS AND METHODS
We strongly encourage authors to use this document
for the preparation of the camera-ready. Please follow
the instructions closely in order to make the volume
look as uniform as possible (Moore and Lopes, 1999).
Please remember that all the papers must be in
English and without orthographic errors.
Do not add any text to the headers (do not set
running heads) and footers, not even page numbers,
because text will be added electronically.
For a best viewing experience the used font must
be Times New Roman, on a Macintosh use the font
named times, except on special occasions, such as
program code (Section 2.3.8).
3 MATERIALS AND METHODS
3.1 Samples
This research was conducted in Animal House
Anatomy Laboratory Universitas Gadjah Mada,
November 2019 – Januari 2020. This study was
approved by the Ethics Commission of the Faculty of
Public Health and Nursing at Universitas Gadjah
Mada with the number KE/FK/1445/EC/2019.
Sprague-Dawley rats (Rattus norvegicus) were used
in this study. The treatment group was divided into 4
groups with 6 rats in each group. This calculation uses
the Frederer formula. The division of these groups are
Control Female (CF), Diabetes Mellitus Female
(DMF), Control Male (CM) and DMM (Diabetes
Mellitus Male).
3.2 Animal Care
In this study, Sprague Dawley rats (3 months, 200-
300 grams) were obtained from the Animal Research
Unit. Rats are kept in plastic cages measuring
30x40x20 cm, at 23-25 ° C, 40-70% humidity, and
dark:light cycle every 12:12 hours. The rats were
acclimated for 1 week by adlibitum, given the
standard feed AIN-76A and boiled drinking water.
Type 1 DM models were prepared by injection of
Streptozotocin (60 mg / kgBB dissolved in 0.1 M
citric acid pH 4.5) with a single dose
intraperitoneally. Whereas the control group was
injected with NaCl. STZ injection was done after
acclimation for 7 days (8th day). Success was seen by
measuring glucose levels over 300 mg/dL. Tissue
collection was carried out after 1 month of animal
care. Right kidney for transcriptomic testing, fixed
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
176
using RNAlater (Ambion) and immediately put in the
-20oC freezer while the left kidney was having a
fixation using formalin neutral buffer for histological
preparation.
3.3 RNA Extraction, cDNA Synthesis,
and Reverse Transcriptase
Polymerase Chain Reaction
Kidney tissue was extracted using Genezol RNA
solution (GENEzol™, Cat. No. GZR100) based on
the protocol from the manufacturer. The RNA
concentration was quantified using a nanodrop.
The RNA was synthesized into cDNA using cDNA
kit (Toyobo, Cat. No. TRT-101) with PCR. Reverse
transcriptase-polymerase chain reaction (RT-PCR)
was carried out to amplify the following specific
cDNAs.
RT-PCR was performed by mixing cDNA and
Taq Master Mix (GoTaq®Green Master Mix, Cat.
No. M7122). The PCR products were analyzed on 2%
agarose gel along with a 100-bp DNA ladder (Bioron,
Germany, Cat. No. 306009). The expression of the
genes was quantified with a densitometry analysis
using the ImageJ software. Β-actin expression was
used to normalize the expression
Table 1. Primers for PCR Analysis
Gen Sequence (5’ -> 3’)
ppET-1
Forwar
d
CTGGCTCTATGTAAGTCATGG
Reverse GCTCCTGCTCCTCCTTGATG
eNOS
Forwar
d
AAATCCACCCGAGCCACAAT
Reverse GGGCTGCCTTTTTCCAGTTG
ET
B
R
Forwar
d
TCTCAGCCTTTTGTCCGAGC
Reverse CGCCGTTTTCAGTCTCGCA
SOD1 Forwar
d
GCGGTGAACCAGTTGTGGTG
Reverse AGCCACATTGCCCAGGTCTC
SOD2 Forwar
d
ATGTTGTGTCGGGCGGCGTGCAGC
Reverse GCGCCTCGTGGTACTTCTCCTCGGTG
ERα
Forwar
d
CACACACGCTCTGCCTTGAT
Reverse GAGCCACCCTGCTGGTTCAA
ERβ Forwar
d
GCCAATCATGTGCACCAGTTCCTT
Reverse AAAGCCAAGAGAAACGGTGGGCAT
β-actin Forwar
d
GCAGATGTGGATCAGCAAGC
Reverse GGTGTAAAACGCAGCTCAGTAA
3.4 Periodic Acid Schiff Staining
Deparaffinization was done by dipping the glass in
xylol and then dipping it in alcohol level 96% to 30%
after organ embedded in paraffin. The preparations
were then washed using distilled water (Aquades).
Then dipped in 1% alcian blue for 5 minutes. Then
dipped it in 1% periodic acid for 5 minutes. Dipped it
in Schiff reagent for 3-5 minutes then washed it using
flow water for 10 minutes. Then the level of alcohol
dehydration was done from 30% to 96%. Mounted
using an entelan.
3.5 Data Analysis
All data from RT-PCR were tested for Saphiro Wilk's
normality and continued with the One-way Anova or
Kruskall Wallis test, comparing CF and DMF, CM
and DMM, DMF and DMM.
4 RESULTS
4.1 Proteunia Score, Blood Gucose
Levels and Kidney Histology
Measurement of protein levels was also carried out to
determine the function of kidney filtration [8]. The
results of blood glucose and protein levels are as
follows:
Blood glucose levels in the DM group both males
and females had levels >300 g / dl and the value was
significant for the control group (p<0.05), which
means this group had hyperglycemic conditions.
While the protein content in the urine in the DM
group was not significantly different compared to the
Different Contribution of Estrogen Receptors, ET-1/ETBR and Superoxide Dismutase and in eNOS Availibility based on Sexual
Dimorphism in Early Stage of Kidney Diabetic Rats
177
control group in each gender. it could be said that the
kidney was considered to be able to carry out its
functions properly. This can be proven with the
following histological preparations:
Table 2. Protein levels in urine (mg / 100mL) and blood sugar (g / dL) mice after being injected with STZ 1 month before
Level CF DMF CM DMM
Blood glucose (g/dL) 106,33 ± 11,5 531,33 ± 99,25
#
231,5 ± 29,13 497 ± 103, 15
#
Urine protein (mg/100mL) 16,67 ± 15,05 25 ± 12,24 15 ± 16,43 25 ± 12,24
# Values in the same line show significant differences (p≤0.05) versus controls of the same gender. Mean ± Standar
deviation.
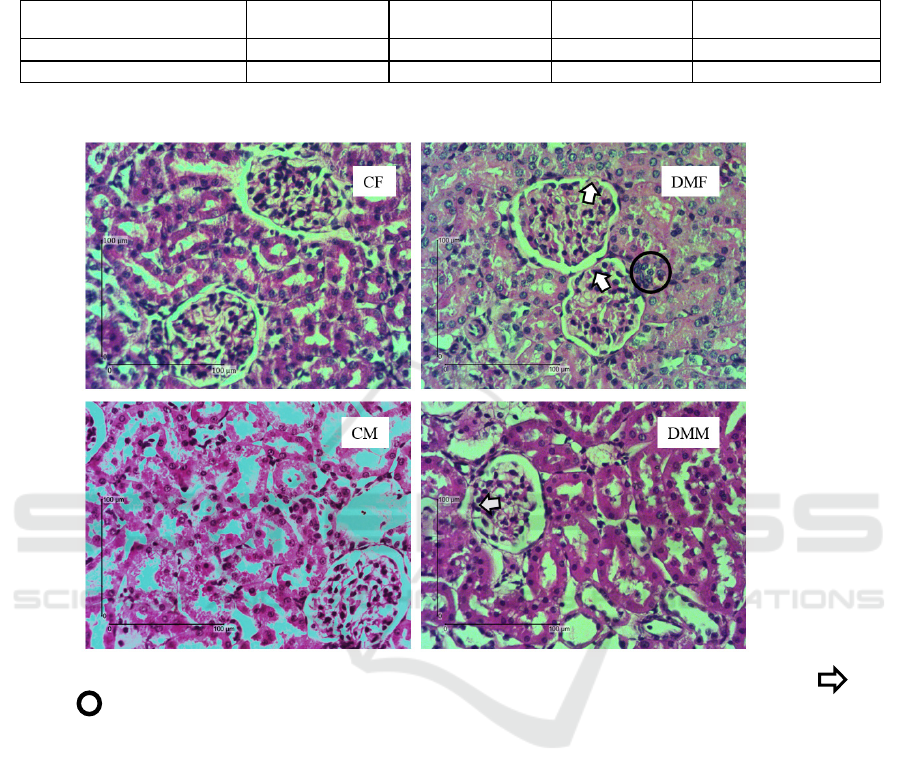
Figure 1. Histological structure of rat kidneys in the early stages of diabetes by PAS staining. The mark of shows
synechiae, shows infiltration of imflamatory cells.
4.2 eNOS mRNA Expression
The picture above implies that there have not been
any noticeable changes in the glomerulus and renal
tubules in the diabetes group when compared to the
control group in both males and females. There was
no visible loss of brush border in proximal tubule and
intraluminal cast which is a marker of tubular injury.
However, in DMB there were accumulation of
inflammatory celss in the insterstitial area. It did not
occur in DMJ. In addition, although the glomerulus
of the DM group had synechiae, there was no visible
thickening of the basement membrane (Setyaningsih
et al., 2017; Haryono et al., 2018).
The results obtained from eNOS gene
amplification showed that there was no significant
difference in the STZ injection treatment compared
with controls in each gender (p> 0.05) as well as
between the male DM rat group and the female DM
group (p> 0.05).
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
178
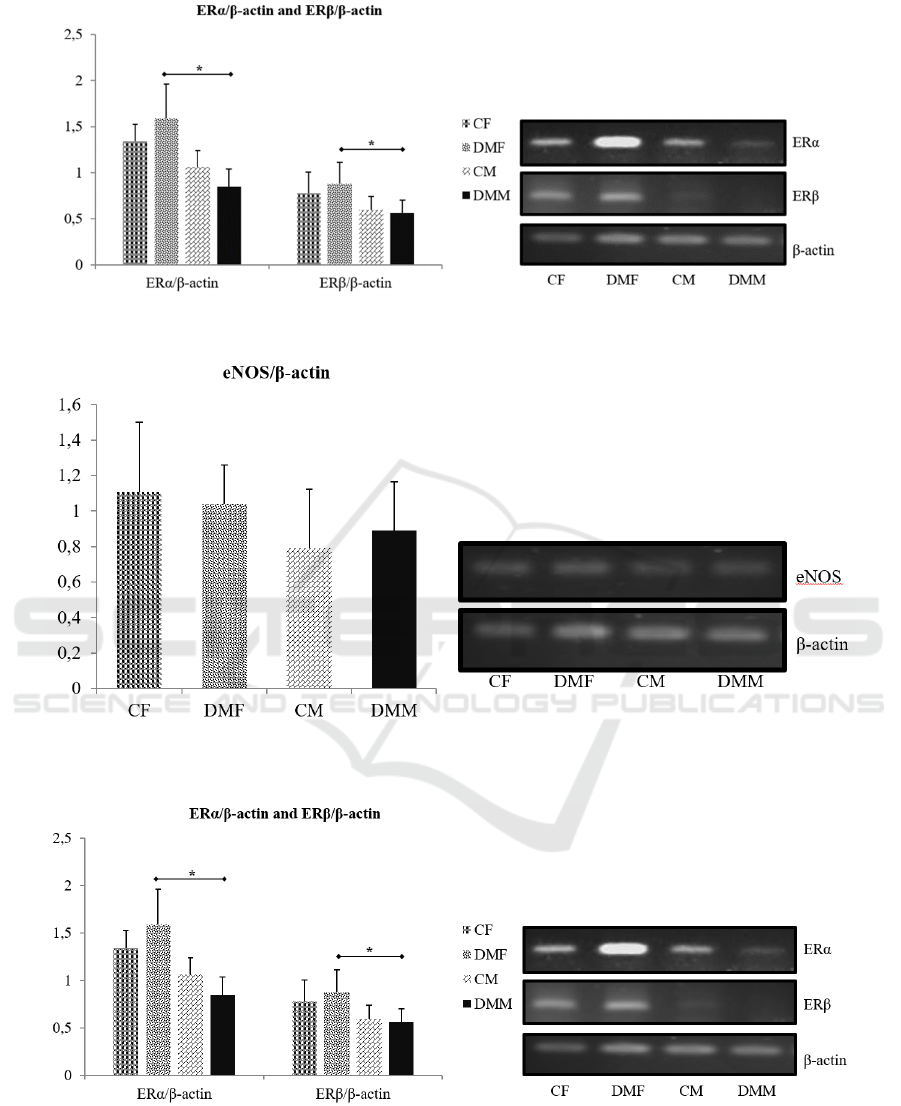
Figure 2. eNOS mRNA Expression in Rat Kidney in Early Stag Diabetes Models
Figure 3. Estrogen receptor mRNA expression in rat kidney in early stage diabetes models. * shows a significant difference
(p≤0.05).
Figure 4. Estrogen receptor mRNA expression in rat kidney in early stage diabetes models. * shows a significant difference
(p≤0.05).
Different Contribution of Estrogen Receptors, ET-1/ETBR and Superoxide Dismutase and in eNOS Availibility based on Sexual
Dimorphism in Early Stage of Kidney Diabetic Rats
179
4.3 Estrogen Receptors mRNA
Expression
The result showed that there was no significant
differences between the control group and the DM
group in the ERα and ERβ genes in both male and
female gender (p> 0.05). In female, estrogen receptor
gene amplification in the DM group showed an
upward trend, whereas in male species tended to
decrease.
4.4 ppET-1/ ETBR mRNA Expression
The ppET-1 mRNA expression showed that there was
no significant differences between the control groups
of female and female DM, male and male DM control
and between female DM and male DM (p> 0.05).
Whereas in the ETBR gene the results obtained
showed significance in the male DM group compared
with the female DM group. Male DM group had
higher gene expression than female DM groups
(p≤0.05). These results can be chosen from the
following histogram:
4.5 SOD1 and SOD2 mRNA
Expression
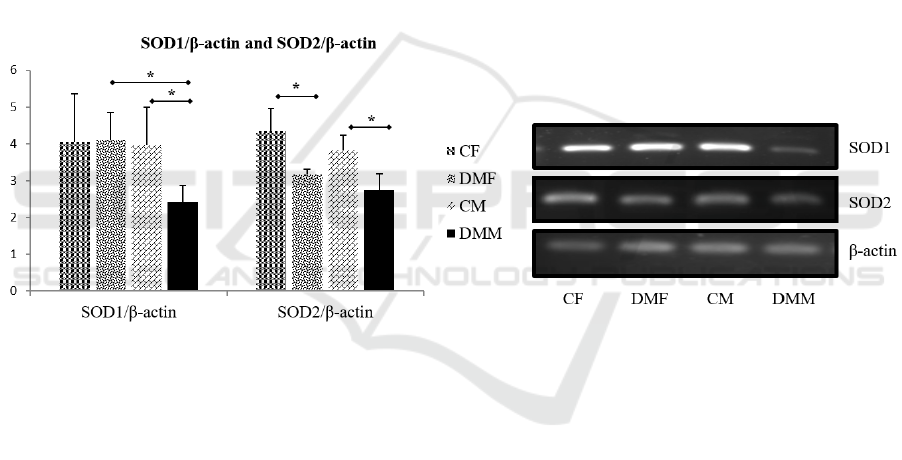
From the picture above, there was no significant
difference in SOD1 mRNA expression in the female
groups, whereas the DMM was lower than their
control. The figure also showed that DMF mRNA
expression was higher than DMM. Meanwhile, the
expression of SOD2 mRNA in the DM group was
higher than the control group in each sex. While the
male and female DM groups did not have significant
differences, but had a tendency for DMF to be higher
than DMJ.
Figure 5. Expression of SOD1 and SOD2 mRNA in rat kidney in early stage diabetes models. * shows a
significant difference (p≤0.05)
5 DISCUSSIONS
In the early stages of diabetes, levels of Nitric Oxide
(NO) increase while in the advanced stages of
diabetic nephropaty, the NO levels decrease
(Nakagawa et al., 2008). The study was confirmed by
the research of Stadler et al. (2003) which stated that
an increase in eNOS works as a vasodilator of blood
vessels.
The results obtained from eNOS gene
amplification showed that there was no significant
difference in the STZ injection treatment compared
with controls in each gender as well as between the
male DM rat group and the female DM group. The
results obtained are not in accordance with the theory,
it is possible because the kidneys used in this study
was the entire kidney, which consist of the cortex and
medulla while according to the study of Han et al.,
(Han et al., 2005) stated that there are a differences in
the concentration of eNOS expression in the renal
cortex and medulla. The expression of eNOS in the
renal medulla is higher than the cortex, especially in
the arcuate and interlobular arteries. This can be taken
into consideration because there may be the NO
donors between the cortex and medulla (Nakagawa et
al, 2008). Kidney Vascular Tree protein isolation also
proves that there is a significant increase in eNOS in
the early stage of diabetic rats rather than isolation of
all parts of the kidney by immunoblotting methods
(De Vriese et al., 2001).
In diabetic kidney, there are hemodynamic
changes characterized by hyperfiltration (Hadi et al.,
2007). The enhancement of GFR is associated with
glomerular hyperfusion caused by the reduction of
intrarenal vascular resistance and hypertension from
glomerular capillaries resulting in the decreased
blood flow of preglomerular resistant blood vessels
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
180

compared to postglomerular. Although renal (kidney)
vasodilation experiments in diabetes are still under
debate, some researchers suggest that an
enhancement in NO associated with hyperfiltration in
the initial diabetes is supported by the administration
of L-arginine which is an endogenous inihibitor
eNOS and the administration of NOS blockers that
prevent excessive hypefiltration and decrease normal
GFR in the early stages of animals testing the of
diabetes (Diederich et al., 1994).
Another consideration of the causes of eNOS
results that are not significant between treatments is
the evaluation of other factors that may be responsible
for the changing response (both increasing and
decreasing) in the early stages of diabetes. One
example of an experiment that caused no change in
eNOS in the early stages of diabetes in the study was
the inability of daltobran, which is an antagonist of
the Tromboxan A2 receptor, in blood vessel
relaxation in the 8th week. This happens because
vasoconstrictor agents are still stronger than
daltobran (Wells et al., 2005).
In this study, gender also affects the differences in
eNOS concentrations. Estrogen through estrogen
receptors modulating eNOS to relax blood vessels is
also one of the factors that can be evaluated, whereas
in the male sex, estrogen is not found in abundant
quantities. The result showed that there was no
significant differences between the control group and
the DM group in the ERα and ERβ genes in both male
and female gender. In female, estrogen receptor gene
amplification in the DM group showed an upward
trend, whereas in male species tended to decrease.
However, there were significant differences between
female DM groups and male DM groups. The male
DM group was lower than the female DM group on
the amplification results of the two estrogen receptor
genes. This is possible due to the mention that sexual
dimorphism affects the NO system. Female mice
have a higher NO level than males (Stadler et al.,
2003)..
Wells et al., (Diederich et al., 1994) confirmed
this study by stating that the condition of
hyperglycemia in animal models was followed by a
tendency for increased expression of ERα protein in
the female DM group and a decreased tendency in the
DM group with male. Similarly, the expression ERβ.
The study also mentions that there has been a
decrease in estrogen levels in blood in male and
female DM rats that are not directly caused due to
hyperglycaemia, but are directly caused by the
absence of insulin which can reduce the ability to
convert androgens to estrogen or decrease aromatase
activity (Barkhem et al., 1998). The relations between
DM and estrogen can be proven by the presence of
menstrual disorder that often occurs in diabetics who
cause abnormal ovarian hormone synthesis. This
implies that the regulation of estrogen receptors is not
directly caused by hyperglycemia but is caused by the
hormone estrogen itself. In addition, the increase in
estrogen receptors in female DM groups may be due
to estrogen through estrogen receptors which are
known to regulate glucose uptake and increase insulin
sensitivity (Weels et al., 2005).
Endothelin-1 is also one factor that must be
considered because Endothelin-1 is a powerful
vasoconstrictor with mitogenic, prooxidative and
proinflammatory abilities of vascular function that is
significant to vascular function. Excessive production
and functional improvement of ET-1 are reported to
be agents of development of diabetic nephropaty
(Pernow et al., 2012). Whereas ET-1 regulation
through ETBR will cause relaxation of blood vessels.
The ppET-1 mRNA expression showed that there was
no significant differences between the control groups
of female and female DM, male and male DM control
and between female DM and male DM. Whereas in
the ETBR gene the results obtained showed
significance in the male DM group compared with the
female DM group. Male DM group had higher gene
expression than female DM groups.
There was no differences in ppET-1 mRNA
expression in the early stages of diabetes mellitus in
each treatment group. This is different from the
theory that gender affects the expression of ET-1.
Pesce et al. mentions female rats have higher NO
levels than males. This is proven by the
administration of testosterone in transsexuals causing
increased levels of ET-1 (Polderman et al., 1993).
Expression of ppET-1 gene that was not significantly
different between DM groups in each gender was
possible because estrogen did not affect ppET-1
levels at mRNA levels. This was evidenced by
expression of ECE (Endhotelin Converting Enzyme)
which did not differ between treatments (Nuedling et
al., 2003).
ETBR expression in the male DM group was
higher than females. It might be said that the stable
state of eNOS in the DM group compared to the
control in the male group was due to high ETBR
expression. So that it can activate eNOS and produce
NO. ETBR is expressed in endothelial cell muscle,
ETBR is responsive to all ET isoforms that cause
vasodilation by releasing NO and PGI2 (Soe et al.,
1994). This evidence allows that ETBR is used as a
vasodilator for blood vessel protection in male rats. In
addition, estrogen has the ability to derive ETBR
regulation through structures known from the ERE
Different Contribution of Estrogen Receptors, ET-1/ETBR and Superoxide Dismutase and in eNOS Availibility based on Sexual
Dimorphism in Early Stage of Kidney Diabetic Rats
181

(estrogen response element) found in the 5 'upstream
region of the ETBR gene (Cheng et al., 1993).
Diabetes can increased oxidative stresses that
occurs when reactive oxygen species (ROS) leviated.
Then, antioxidant was formed as defence system. 4,
25. Enzyme superoxide dismutase can formed
hydrogen peroxide as a stable product of ROS from
free radicals in response to inflammatory conditions
32. Based on the results, mRNA expression SOD1
and SOD2. in DMF groups was higher than DMM. It
might be associated with estrogen can act as potensial
antioxidant which could contribute to eNOS
persistent 22, even SOD1 in DMF is noteworthy than
CF. Less of SOD is needed in women compared to
men who will not benefit from the antioxidant
properties of estrogen. Hamed et al. found that SOD
can restores NO production and ability of glucose-
stressed endothelial progenitor cells.
6 CONCLUSIONS
Estrogen receptors and superoxide dismutase
upregulated in early diabetic female rats. They can
provide signal for good function of endothelial cell.
Moreover, ETBR upregulated in early diabetic male
rats might be associated with persistent eNOS
expression in early diabetic condition. ETBR
knockout in diabetics rats can be used to demonstrate
ppET-1/ ETBR signaling for vascular relaxation via
eNOS in males.
REFERENCES
Anderson, S., Jung, F.F., and Ingelfinger J.R., 1993. Renal
renin-angiotensin system in diabetes: functional,
immunohistochemical, and molecular biological
correlations. Am J Physiol. 265(4), pp. 477-86.
Cheng, H and Harris, R C, 2014. Renal endothelial
dysfunction in diabetic nephropathy. Cardiovasc
Hematol Disord Drug Targets. 14(1), pp. 22-33.
Eleftheriadis, E., Antoniadi, G., Pissas, G., Liakopoulos,
V., and Stephanidis, I., 2013. The renal endothelium in
diabetic nephropathy. Ren Fail. 35(4), pp. 592-9.
Forbes, J.M., Cooper, M.E., Oldfield, M.D, and Thomas,
M.C, 2003. Role of advanced glycation end products
in diabetic nephropathy. J Am Soc Nephrol, 14, pp.
254–8.
Stadler, K., Jenei, V., Azy, G.V., Somogyi, A and Jakus, J.,
2003. Increased nitric oxide levels as an early sign of
premature aging in diabetes. Free Radic Biol Med,
35(10), pp. 1240-51.
Alejandra, G.M., Leonardo, P., Francisco, G.Y and Jorge,
A., 2016. Oxidative stress in diabetic nephropathy
with early chronic kidney disease. J Diabetes Res,
2016, pp. 1-7.
Pesce, J.H.C., Zheng, W., Kim, J., Kim, J., Zhang, Y.,
sMenini, S, et al., 2005. Sex differences in renal injury
and nitric oxide production in renal wrap hypertension.
Am J Physiol Heart Circ Physiol, 288, pp. 43-7.
Verdiansyah., 2016. Kidney function diagnose. CDK-
237.Elsevier, 43(2), pp. 148-54.
Setyaningsih, W., Arfian, N., Suryadi, E., Romi, M.M.,
Tranggono, U. and Sari, D.C.R., 2017. Hyperuricemia
induces Wnt5a / Ror2 gene expression, epithelial –
mesenchymal transition (EMT) and kidney tubular
injury in mice. Iran J Med Sci, 41(2), pp. 1–10.
Haryono, A., Nugrahaningsih, D.A., Sari, D.C.R., Romi,
M.M and Arfian, N., 2018. Reduction of serum uric
acid associated with attenuation of renal injury,
inflammation and macrophages M1/M2 ratio in
hyperuricemic mice model. Kobe J Med Sci, 64(3),
pp. 107-14.
Nakagawa, T., Tanabe, K., Croker, B.P., Johnson, R.J.,
Grant, M.B., Kosugi, T, et al., 2008. Endothelial
dysfunction as a potential contributor in diabetic
nephropathy. Nat Rev Nephrol, 7, pp. 36–44.
Han, K.H., Lim, J.M., Kim, W.Y., Kim, H., Madsen, K.M
and Kim, J., 2005. Expression of endothelial nitric
oxide synthase in developing rat kidney. Am J Physiol
Renal Physiol, 288(4), pp. 694–702
Nakagawa, T., Sato, W., Glushakova, O., Heinig, M.,
Clarke, T., Campbell- Thompson, M., et al., 2008.
Diabetic endothelial nitric oxide synthase knockout
mice develop advanced diabetic nephropathy. J Am
Soc Nephrol, 18, pp. 539-50.
De Vriese, A.S., Stoenoiu, M.S., Elger, M., Devuyst, O.,
Vanholder, R., Kriz, W, et al., 2001. Diabetes-induced
microvascular dysfunction in the hydronephrotic
kidney: role of nitric oxide. Kidney Int, 60(1), pp. 202-
10.
Hadi, H.A and Suwaidi, J.A., 2007. Endothelial dysfunction
in diabetes mellitus. Vasc Health Risk Manag, 3(6)
pp., 853-76.
Diederich, D., Skopec, J., Diederich, A and Dai, F.X., 1994.
Endothelial dysfunction in mesenteric arteries of
diabetic rat: role of free radicals. Am J Physiol,
266(35), pp. 1153-61.
Wells, C.C., Riazi, S., Mankhey, R.W., Bhatti, F.,
Ecelbarger, C. and Maric. C., 2005. Diabetic
nephropathy is Associated with decrease circulating
estradiol levels and imbalanced in the expression of
renal Eetrogen receptors. Gend med, 2(4), pp. 227-37.
Barkhem, T., Carlsson, B., Nilsson, Y., Enmark, E.,
Gustafsson, J and Nilsson, S., 1998. Differential
response of estrogen receptor alpha and estrogen
receptor beta to partial estrogen agonists/ antagonists.
Mol Pharmacol, 54, pp. 105-12.
Pernow, J., Shemyakin, A and Bohm, F., 2012. New
perspectives on endothelin-1 in atherosclerosis and
diabetes mellitus. Life Sci, 91, pp. 507-16.
Polderman, K.H., Stehouwer, C.D., Van Kamp, G.J.,
Dekker, G.A., Verheugt, F.W and Gooren, L.J., 1993.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
182

Influence of sex hormones on plasma endothelin
levels. Ann Intern Med, 118, pp. 429-32.
Nuedling, S., Van Eickles, M., Alle’ra, A., Doevendans, P.,
Meyer, R., Vetter, H., 2003. 17β-Estradiol regulates
the expression of endothelin receptor typeB in the
heart. Br J Pharmaco, 40(1), pp. 195-201.
Soe, B., Oemar, B.S, Siebenmann, R., Von Segesser, L. and
Lüscher, T.F., 1994. Both ETA and ETB receptors
mediate contraction to endothelin-1 in human blood
vessels. Circulation, 89, pp.1203-8.
Cheng, H.F., Su, Y.M., Yeh, J.R and Chang, K.J., 1993.
Alternative transcript of the nonselective-type
endothelin receptor from rat brain. Mol Pharmacol,
44, pp. 533-8.
Haidara MA, Yassin HZ, Rateb M, et al. Role of oxidative
stress in development of cardiovascular complications
in diabetes mellitus. Curr Vasc Pharmacol. 2006; 4:
215–27.
Yang, D., Li, J., Yuan, Z and Liu, X., 2013. Effect of
hormone replacement therapy on cardiovascular
outcomes: a meta-analysis of randomized controlled
trials. PLoS One, 8, pp. e62329.
Bell, J.R., Bernasochi, G.B., Varma, U., Raaijmakers,
A.J.A and Delbridge, L.M.D., 2013. Sex and sex
hormones in cardiac stress mechanistic insights. J
Steroid Biochem Mol Biol, 137, pp. 124–35.
Hamid, S., Brenner, B., Aharom, A., Daoud, D and Roguin,
A., 2009. Nitric oxide and superoxide dismutase
modulate endothelial progenitor cell function in type 2
diabetes mellitus. Cardiovas. Diabetol, 8(56), pp. 1-
12.
Different Contribution of Estrogen Receptors, ET-1/ETBR and Superoxide Dismutase and in eNOS Availibility based on Sexual
Dimorphism in Early Stage of Kidney Diabetic Rats
183
