
The Effect of Jackfruit (Artocarpus heterophyllus) Leaf Ethanolic
Extract Gel on Superoxide Dismutase and Interleukin-1β Levels
in Wound Healing after Tooth Extraction in Diabetic Rats
Almasyifa Herlingga Rahmasari Amin¹
1
, Yusuf Asto Pamasja
12
,
Christiana Cahyani Prihastuti
23
, Haris Budi Widodo
24
, Hernayanti
35
¹ Dental Medicine Program, Faculty of Medicine, Jenderal Soedirman University, Purwokerto, Indonesia
2
Dental Medicine Program, Department of Oral Biology, Jenderal Soedirman University, Purwokerto, Indonesia
3
Department of Ecotoxicology, Faculty of Biology, Jenderal Soedirman University, Purwokerto, Indonesia
Keywords: Jackfruit, Diabetes Mellitus, Tooth Extraction, Superoxide Dismutase, IL-1β
Abstract: Diabetes mellitus is a metabolic disease characterized by hyperglycemia, which can cause complications, such
as impaired wound healing after tooth extraction. The high level of blood glucose will increase ROS, leading
to the degradation of SOD and elevation of IL-1β. Jackfruit leaf contains flavonoids with antioxidant and anti-
inflammatory activities. This research aimed to study the effect of topical administration of jackfruit leaf
ethanolic extract gel (JLEEG) after tooth extraction on SOD and IL-1β in gingiva tissue near tooth socket in
diabetic rat models. The study was experimental laboratory research with a randomized posttest-only control
group design. Thirty-five male Wistar rats were used as the sample and divided into 5 groups: T1, T2, T3
(diabetic rat groups treated with JLEEG concentrations of 5%, 10%, and 15% respectively), C1 (healthy
control group), and C2 (negative control group). The treated groups showed higher SOD levels and lowered
IL-1β levels in comparison to the negative control group. Statistical analysis using One-Way ANOVA
indicated significant differences (p<0.01) between the treated groups and the negative control group. 15%
was considered the most effective concentration to reduce the inflammation phase and accelerate the healing
process of tooth extraction wounds in diabetic conditions.
1 INTRODUCTION
Diabetes mellitus is a metabolic disease characterized
by hyperglycemia due to disturbances in insulin
secretion, insulin action, or even both
(American
Diabetes Association, 2013). Insulin is a hormone
produced by the pancreas to regulate glucose
metabolism, and when it does not work correctly,
hyperglycemia will occur and cause severe damage to
nerves and blood vessels (Afifah, 2016; World Health
Organization, 2016). The inhibition of oral wound
healing in diabetic patients is caused by leukocyte
dysfunction, increased blood viscosity, and thickened
blood vessel walls. These can result in
microcirculation and changes in the permeability of
1
https://orcid.org/0000-0002-3200-4917
2
https://orcid.org/0000-0001-8222-4574
3
https://orcid.org/0000-0002-0611-7651
4
https://orcid.org/0000-0001-7164-9841
5
https://orcid.org/0000-0002-3468-7563
blood vessels, thus inhibiting wound healing (Kolluru
et al., 2012; Mozzati et al., 2014; Gould et al., 2015).
Tooth extraction can cause a wound around the
socket
(Pedersen, 1996). The acute wound healing
process in normal individuals can be completed
within three weeks with an inflammatory phase (1-4
days), a proliferative phase (4-21 days), and followed
by a remodeling phase until the following year
(Morison, 2011; Stacey, 2016). If the healing process
stops at one of these phases, the wound will become
chronic, which eventually extends the healing time
(Orsted et al., 2011).
The inflammatory phase involves various
proinflammatory agents, e.g., interleukin-1β (IL-1β),
which serves as the body's defense
(Seil et al., 2012).
Hyperglycemic conditions can stimulate
164
Rahmasari Amin, A., Pamasja, Y., Prihastuti, C., Widodo, H. and Hernayanti, .
The Effect of Jackfruit (Artocarpus heterophyllus) Leaf Ethanolic Extract Gel on Superoxide Dismutase and Interleukin-1 Levels in Wound Healing after Tooth Extraction in Diabetic Rats.
DOI: 10.5220/0010489501640169
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 164-169
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

proinflammatory cytokine production by increasing
free radicals, therefore prolonging the inflammatory
phase and inhibiting the wound healing process
(Gonzalez et al., 2012). Previous research proved that
the inflammatory phase in rat models of diabetes
mellitus lasted longer, with proinflammatory
cytokine levels reaching their peaks on Day-5 and
starting to decrease on Day-10, rather than on Days
1-4 (Mirza et al., 2014).
An antioxidant enzyme can inhibit free radicals in
the body, namely Superoxide Dismutase (SOD). SOD
can prevent cell damage caused by oxidative stress
compounds, usually known as Reactive Oxygen
Species (ROS). In hyperglycemic conditions, the
forming of ROS can take 3-4 times faster than the
dismutation process by SOD, thus allowing SOD
levels to decrease. Consequently, additional
antioxidants from outside the body are needed in this
situation
(Mittal et al., 2014).
Flavonoids are known as one of the external
sources of antioxidants to reduce ROS. They can
neutralize free radicals and stimulate the production
of antioxidant enzymes in the body
(Panche et al.,
2016; Dewanto and Isnaeni, 2017). They can also act
as anti-inflammatory agents by inhibiting
inflammatory cytokines' production to accelerate the
wound healing process
(Leyva-López et al., 2016).
Jackfruit (Artocarpus heterophyllus) leaf can be used
as a natural antioxidant and anti-inflammatory
because they contain flavonoids, saponins, and
tannins
(Hamzah et al., 2013; Asmaliani and Iwo,
2016)
A previous study showed that the treatment of
male albino rats with incision wounds in
subcutaneous skin tissue with 5% jackfruit leaf
methanolic extract ointment led to a significant
reduction in epithelialization period, an increase in
epithelialization process, and an acceleration in
wound contraction when compared to the control
group
(Gupta et al., 2009). Another study also
revealed that 5%, 10%, and 15% concentrations of
jackfruit leaf ethanolic extract ointment could
accelerate incision wounds' healing process in
subcutaneous skin of rabbit models
(Hamzah et al.,
2013).
Based on this background, the purpose of this
study was to compare the effects of 5%, 10%, and
15% concentrations of JLEEG on SOD and IL-1β
levels in wound healing after tooth extraction in
diabetic models of Wistar rats. Rats with diabetes
mellitus condition would be induced with
streptozotocin (Wang and Wang, 2017). The levels of
SOD and IL-1β in diabetic rats after tooth extraction
were measured in the inflammatory phase, on Day-6
after they were treated with the jackfruit leaf extract
for 5 days.
2 MATERIALS AND METHODS
The Ethics Commission of the Faculty of Medicine
Jenderal Soedirman University approved the content
and execution of this study with the letter
042/KEPK/II/2019 and 1009/KEPK/II/2019. The
study was experimental laboratory research with a
randomized posttest-only control group design. The
samples were 35 male Wistar rats, aged 2-3 months,
with a 200-250 bodyweight. The rats were divided
into 5 groups: three treated groups, namely T1, T2,
and T3 (diabetic rat groups treated with JLEEG
concentrations of 5%, 10%, and 15% respectively)
and two control groups, namely C1 (healthy control
group without diabetic condition) and C2 (negative
diabetic control group), both of which were treated
with 2% concentration of Na-CMC after tooth
extraction.
2.1 Jackfruit Leaf Ethanolic Extract
Gel Processing
2 grams of Na-CMC base gel was sprinkled over 100
ml of heated distilled water and waited at least 24
hours until the entire powder was dissolved to obtain
a concentration of 2% (w/v). 0.5 grams, 1 gram, and
1.5 grams of jackfruit leaf extract were then added to
9.5 ml of 2% Na-CMC solution to obtain a 10 ml gel
with different concentrations of 5%, 10%, and 15%
(w/v), respectively. Subsequently, the solution was
stirred until it was homogeneous and cooled down
until it turned into a gel
(Nofikasari et al., 2016).
2.2 Diabetic Rat Models
Rat models of diabetes mellitus were induced by the
injection of streptozotocin (STZ). STZ was injected
into fasting rats intraperitoneally with a concentration
of 2.5 ml (45 mg/kg BW) after being diluted in 0.05
M citrate buffer with 4.5 pH. Three days after the STZ
injection, the average blood glucose levels were 265
mg/dL (≥200 mg/dL) (Daniel et al., 2015).
2.3 Tooth Extraction and Treatment
Administration
Rats were injected with ketamine intraperitoneally at
a dose of 85 mg/kg BW. The mandibular left incisors
in rats were extracted. The treatment after tooth
The Effect of Jackfruit (Artocarpus heterophyllus) Leaf Ethanolic Extract Gel on Superoxide Dismutase and Interleukin-1 Levels in Wound
Healing after Tooth Extraction in Diabetic Rats
165
extraction was given topically using a cotton swab for
five consecutive days. Na-CMC with a concentration
of 2% was given for the control groups (C1 and C2),
and JLEEG with different concentrations was given
for the treated groups (T1, T2, and T3).
2.4 Tissue Retrieval and Isolation
On Day-6, the rats were terminated using ether, and ±
25 mg gingival tissue near the tooth socket was taken.
The tissue sample was then frozen with liquid
nitrogen and mashed with a mortar and pestle. Every
5 mg of tissue was added with 1 ml of lysis buffer.
The tissue was smoothed, then the sonication was
performed (10
11
x 3 with 30-second intervals). The
tissue lysate was incubated for 45 minutes, then
centrifuged at 12,000 rpm for 20 minutes. The
supernatant was separated from the pellets, put in a
new tube, and stored at -80
0
C.
2.5 Measurement of SOD and IL-1β
Levels
Each group's SOD enzyme levels were measured with
a UV-Vis spectrophotometer at a wavelength of 505
nm and a temperature of 25
0
C.
The formula to calculate SOD levels applies as
follows:
sample absorbance
absorbance standard
30.65 U/ml
The IL-1β level of each group was measured using
the Rat IL-1β ELISA Elabscience® kit.
2.6 Statistical Data Analysis
The results were analyzed statistically for data
normality with Shapiro-Wilk and data homogeneity
with Levene's test. The data were then analyzed using
a parametric statistical test (One-Way ANOVA) and
Post Hoc analysis (Least Significant Difference) to
figure out the significant differences between the
groups with a confidence level of 95% (p<0.05) or
99% (p<0.01).
3 RESULTS
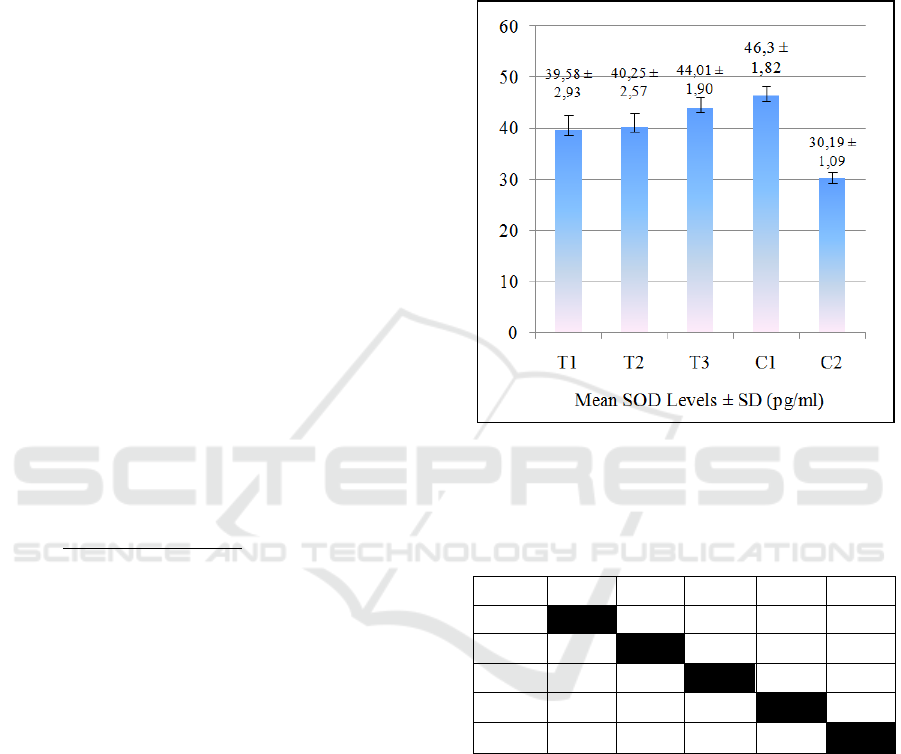
The results of the mean SOD levels of all groups are
provided in Figure 1. Figure 1 shows that in the
treated groups, SOD levels increased over JLEEG
concentrations. The lowest SOD level was in the
negative control group (C2), while the highest SOD
level in the healthy control group (C1). The One-way
ANOVA hypothesis test showed p-value = 0.000
(p<0.01). This result means that there was a very
significant effect of JLEEG concentration on the SOD
level. The data was then tested on Post Hoc LSD, and
the results are given in Table 1.
Figure 1: The mean ± Standard Deviation of SOD Levels.
T1= 5% JLEEG treated group; T2=10% JLEEG treated
group; T3= 15% JLEEG treated group; C1= healthy control
group; C2= negative control group.
Table 1: Results of Post-Hoc LSD test on SOD Levels.
Group T1 T2 T3 C1 C2
T1 0.565
0.001
**
0.000
**
0.000
**
T2 0.565 0.003
**
0.000
**
0.000
**
T3 0.001
**
0.003
**
0.056 0.000
**
C1 0.000
**
0.000
**
0.056
0.000
**
C2 0.000
**
0.000
**
0.000
**
0.000
**
Note: T1= 5% JLEEG treated group; T2=10% JLEEG
treated group; T3= 15% JLEEG treated group; C1= healthy
control group; C2= negative control group.
*
= a significant difference (p<0.05)
**
= a very significant difference (p<0.01)
The results of the Post-Hoc LSD test revealed that
there was a very significant difference between the
treated groups (T1, T2, and T3) and the negative
control group (C2) (p≤0.01). However, there was no
significant difference between T3 and C1 in SOD
levels (p>0.05). The results of the mean IL-1β levels
of the groups are presented in Figure 2.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
166
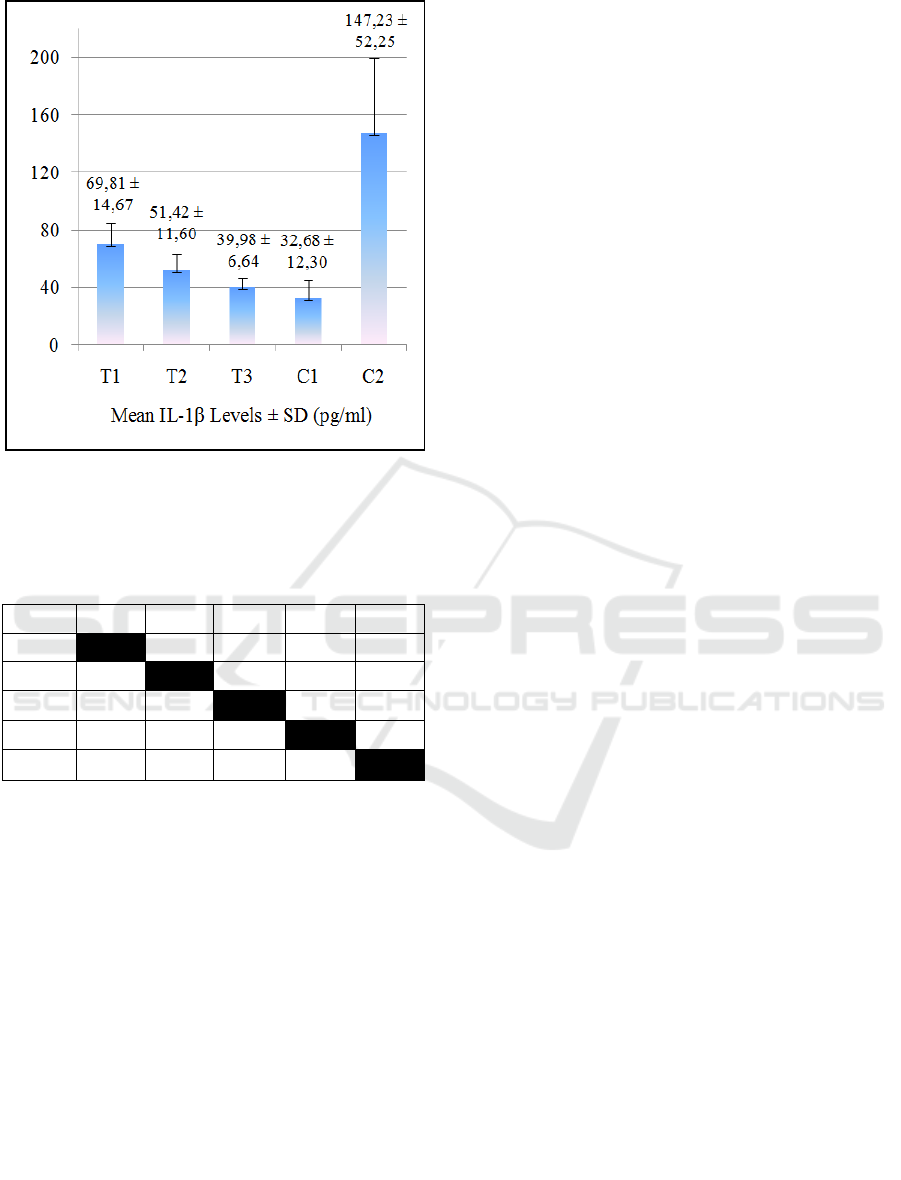
Figure 2: The mean ± Standard Deviation of IL-1β Levels.
T1= 5% JLEEG treated group; T2= 10% JLEEG treated
group; T3= 15% JLEEG treated group; C1= healthy control
group; C2= negative control group.
Table 2: Results of Post Hoc LSD test on IL-1β levels.
Group T1 T2 T3 C1 C2
T1 0.049
*
0.001
**
0.000
**
0.000
**
T2 0.049
**
0.115 0.002
**
0.000
**
T3 0.001
**
0.115 0.102 0.000
**
C1 0.000
**
0.002
**
0.102
0.000
**
C2 0.000
**
0.000
**
0.000
**
0.000
**
Figure 2 shows that the higher the JLEEG
concentrations in the treated groups, the lower the IL-
1β levels. The highest IL-1β level appeared in the
negative control group (C2), while the lowest in the
healthy control group (C1). The One-way ANOVA
test results indicated the value of p = 0.000 (p<0.01).
This result means that there was a very significant
effect of JLEEG concentration on IL-1β level. The
data was then tested on Post Hoc LSD, and the results
are given in Table 2.
The results of the LSD Post Hoc test indicated that
there was a very significant difference between the
treated groups. In addition, there was no significant
difference between T3 and C1 in terms of IL-1β
levels.
4 DISCUSSION
The SOD levels of the diabetic rats in the negative
control group (C2) were lower than those of the
healthy control group (C1), while the IL-1β levels of
C2 were higher than those of C1. This result means
that diabetes mellitus in rats affects both SOD and IL-
1β levels after tooth extraction.
An excessive amount of ROS resulting from the
condition of diabetes mellitus will decrease the
antioxidant levels of the body, i.e., superoxide
dismutase (SOD). The amount of antioxidant
enzymes is lower in pancreatic beta cells than in any
other organs with only a limited amount of SOD,
making it more sensitive to ROS's attack. This
condition will drastically reduce SOD levels in
wounded diabetic rats
21
. On the other hand, a high
amount of ROS can lead to the accumulation of
advanced glycosylation products (AGEs), which will
bind to the receptor for AGE (RAGE), thereby
activating NF-κB and stimulating proinflammatory
cytokines such as IL-1β. An enormous amount of
ROS will enable IL-1β to be secreted in high levels,
thus prolonging the inflammation time (Graves and
Kayal, 2008; Shita, 2015).
The results showed a significant effect of JLEEG
administration on post-tooth extraction wounds in the
treated groups with 5%, 10%, and 15% JLEEG
compared to the negative control group. The SOD
levels were higher in the treated groups, while the IL-
1β levels were lower than the negative control group.
This condition can be affected by flavonoid content
in jackfruit leaf extract known to have anti-
inflammatory abilities to accelerate the wound
healing process by suppressing excessive
inflammatory mediator activity
(Asmaliani and Iwo,
2016). According to previous studies, there was an
increase in SOD activity in diabetic rats after they
were given ginger extract and cardamom leaf. There
was a decrease in the IL-1β levels of diabetic rats after
they were administered with Moringa oleifera
extract, known to contain flavonoids (Morakinyo et
al., 2011; Sari et al., 2014; Muhammad et al., 2016).
The content of flavonoids in jackfruit leaf also
plays a vital role as an antioxidant agent by increasing
SOD levels. Research with plant extracts containing
flavonoids has been proven to increase the SOD
activity of the wounded diabetic rats because they are
associated with reducing lipid peroxidation and
ROS's decrease, e.g., superoxide anion (Rahmawati et
al., 2014). Furthermore, jackfruit leaf also contains
Cu and Zn, which can function as SOD cofactors to
form Cu, Zn-SOD and increase SOD levels in the
body. A high amount of SOD can suppress
The Effect of Jackfruit (Artocarpus heterophyllus) Leaf Ethanolic Extract Gel on Superoxide Dismutase and Interleukin-1 Levels in Wound
Healing after Tooth Extraction in Diabetic Rats
167

superoxide anion in a diabetic rat, thereby
accelerating the healing process by reducing the
inflammatory period (Fattah et al., 2012; Sun et al.,
2015).
Moreover, flavonoids can serve as extreme metal
chelating (Fe2 + and Cu2 +), so free radicals are not
formed through the Fenton reaction, and the
proinflammatory cytokines decrease (Birben et al.,
2012). The existence of saponins in jackfruit leaf can
reduce blood glucose by increasing the small
intestine's permeability and increase substance uptake
to inhibit the absorption of smaller substance
molecules that should be absorbed more quickly, i.e.,
glucose (Fiana and Oktaria, 2016).
Flavonoids can prevent ROS formation by
donating H
+
atoms, allowing them to become neutral
and their levels to decrease. The decreased levels of
ROS will affect the transcription factor of NF-κB, so
there will be a decrease in the production of IL-1β.
The decreasing levels of IL-1β can affect the
phospholipase A2 enzyme in degrading the
phospholipid enzyme, which can prevent the
production of arachidonic acid from being excessive.
A decrease in arachidonic acid itself can reduce the
production of prostaglandin-2 (PGE-2) through the
cyclooxygenase-2 (COX-2) pathway. A small
amount of PGE-2, which acts as an inflammatory
mediator, will reduce inflammatory responses to
vasodilation, edema, and pain, allowing the wound
healing process to run normally (Panche et al., 2016;
Leyva-López et al., 2016).
This study indicated that the increased
concentrations of JLEEG could decrease IL-1β levels
and increase SOD levels in the treated groups. The
post-hoc LSD test results also revealed that the
administration of JLEEG with a concentration of 15%
to the diabetic rats with tooth extraction wounds
could increase SOD levels and reduce IL-1β levels
closer to healthy rats without diabetes mellitus. This
result implies that the inflammatory process in this
group decreased and continued to the proliferation
phase. Therefore, the topical administration of
JLEEG at 15% is considered the most effective
compared to the lower concentrations to enhance the
wound healing process after tooth extraction in
diabetic conditions.
The results of this study indicated a potential role
of the jackfruit leaf ethanolic extract topical gel as an
adjuvant treatment to enhance the wound healing
process after tooth extraction in diabetic patients.
Advanced research is needed to determine the optimal
dose and lethal dose of the ethanol extract of jackfruit
leaf.
5 CONCLUSION
The administration of jackfruit leaf ethanolic extract
gel increases the SOD level. It decreases IL-1β level
on post-extraction tooth socket tissue in diabetes
mellitus rat models, suggesting the acceleration of the
healing process after tooth extraction. The 15% gel
concentration is considered the most effective
compared to 5% and 10% gel concentrations.
REFERENCES
American Diabetes Association, 2013. Standards of
medical care in diabetes—2013. Diabetes
care, 36(Supplement 1), pp.S11-S66.
Afifah, H.N., 2016. Mengenal Jenis-Jenis Insulin Terbaru
untuk Pengobatan Diabetes. Majalah
Farmasetika, 1(4), pp.1-4.
World Health Organization, 2016. Global report on
diabetes. WHO Library Cataloguing in Publication
Data. World Health Organization.
Kolluru, G.K., Bir, S.C. and Kevil, C.G., 2012. Endothelial
dysfunction and diabetes: effects on angiogenesis,
vascular remodeling, and wound healing. International
Journal of Vascular Medicine, 2012.
Mozzati, M., Gallesio, G., di Romana, S., Bergamasco, L.
and Pol, R., 2014. Efficacy of plasma-rich growth factor
in the healing of postextraction sockets in patients
affected by insulin-dependent diabetes
mellitus. Journal of Oral and Maxillofacial
Surgery, 72(3), pp.456-462.
Gould, L., Abadir, P., Brem, H., Carter, M., Conner‐Kerr,
T., Davidson, J., DiPietro, L., Falanga, V., Fife, C.,
Gardner, S. and Grice, E., 2015. Chronic wound repair
and healing in older adults: current status and future
research. Wound Repair and Regeneration, 23(1), pp.1-
13.
Pedersen, G.W., 1996. Buku Ajar Praktis Bedah Mulut,
terj. EGC, Jakarta.
Morison, M. J. 2011. Manajemen Luka. EGC, Jakarta
Stacey, M. 2016. Why Does Wound Heal? Wounds
International, 7(1), pp.16-21.
Orsted, H.L., Keast, D., Forest-Lalande, L. and Megie,
M.F., 2011. Basic principles of wound healing. Wound
Care Canada, 9(2), pp.4-12.
Seil, M., Ouaaliti, M.E., Abdou Foumekoye, S., Pochet, S.
and Dehaye, J.P., 2012. Distinct regulation by
lipopolysaccharides of the expression of interleukin-1β
by murine macrophages and salivary glands. Innate
Immunity, 18(1), pp.14-24.
Gonzalez, Y., Herrera, M.T., Soldevila, G., Garcia-Garcia,
L., Fabián, G., Pérez-Armendariz, E.M., Bobadilla, K.,
Guzmán-Beltrán, S., Sada, E. and Torres, M., 2012.
High glucose concentrations induce TNF-α production
through the down-regulation of CD33 in primary
human monocytes. BMC immunology, 13(1), p.19.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
168

Mirza, R.E., Fang, M.M., Weinheimer-Haus, E.M., Ennis,
W.J. and Koh, T.J., 2014. Sustained inflammasome
activity in macrophages impairs wound healing in type
2 diabetic humans and mice. Diabetes, 63(3), pp.1103-
1114.
Mittal, M., Siddiqui, M.R., Tran, K., Reddy, S.P. and
Malik, A.B., 2014. Reactive oxygen species in
inflammation and tissue injury. Antioxidants & redox
signaling, 20(7), pp.1126-1167.
Panche, A.N., Diwan, A.D. and Chandra, S.R., 2016.
Flavonoids: an overview. Journal of nutritional
science, 5.
Dewanto, H.N. and Isnaeni, W., 2017. Pengaruh Ekstrak
Kulit Buah Rambutan terhadap Kualitas Sperma Tikus
yang Terpapar Asap Rokok. Life Science, 6(2), pp.62-
68.
Leyva-López, N., Gutierrez-Grijalva, E.P., Ambriz-Perez,
D.L. and Heredia, J.B., 2016. Flavonoids as cytokine
modulators: a possible therapy for inflammation-related
diseases. International journal of molecular
sciences, 17(6), p.921.
Asmaliani, I., Iwo, M. I. 2016. Uji Aktivitas Antiinflamasi
dari Ekstrak Metanol Daun Nangka terhadap Tikus
yang Diinduksi Karagenan. As-Syifaa Jurnal
Farmasi, 8(2), pp.28-32.
Hamzah, H., Fatimawali, F., Yamlean, P.V. and Mongi, J.,
2013. Formulasi salep ekstrak etanol daun nangka
(Artocarpus heterophyllus Lam.) dan uji efektivitas
terhadap penyembuhan luka terbuka pada
kelinci. PHARMACON, 2(3).
20. Gupta, N., Jain, U.K. and Pathak, A.K., 2009. Wound
healing properties of Artocarpus heterophyllus
Lam. Ancient science of life, 28(4), p.36.
Wang, J. and Wang, H., 2017. Oxidative stress in pancreatic
beta cell regeneration. Oxidative Medicine and Cellular
Longevity, 2017.
Nofikasari, I., Rufaida, A., Aqmarina, C.D., Failasofia, F.,
Fauzia, A.R. and Handajani, J., 2016. Efek aplikasi
topikal gel ekstrak pandan wangi terhadap
penyembuhan luka gingiva. Majalah Kedokteran Gigi
Indonesia, 2(2), pp.53-59.
Daniel, E.E., Mohammed, A., Tanko, Y., Ahmed, A.,
Adams, M.D. and Atsukwei, D., 2015. Effects of
lycopene on thyroid profile in streptozotocin-induced
diabetic wistar rats. European Journal of
Biotechnology and Bioscience, 3(1), pp.21-28.
Shita, A. D. P. 2015. Perubahan Level TNF-A dan IL-1
pada Kondisi Diabetes Melitus. Proceeding of
Dentistry Scientific Meeting II. I-V:1-7.
Graves, D.T. and Kayal, R.A., 2008. Diabetic
complications and dysregulated innate
immunity. Frontiers in bioscience: a journal and
virtual library, 13, p.1227.
Morakinyo, A.O., Akindele, A.J. and Ahmed, Z., 2011.
Modulation of antioxidant enzymes and inflammatory
cytokines: possible mechanism of anti-diabetic effect of
ginger extracts. African Journal of Biomedical
Research, 14(3), pp.195-202.
Sari, P.E., Simanjuntak, S.B.I. and Winarsi, H., 2014.
Aktivitas Enzim Superoksida Dismutase Tikus
Diabetes yang Diberi Ekstrak Daun Kapulaga
Amomum Cardamomum. Scripta Biologica, 1(3),
pp.196-196.
Muhammad, A.A., Arulselvan, P., Cheah, P.S., Abas, F.
and Fakurazi, S., 2016. Evaluation of wound healing
properties of bioactive aqueous fraction from Moringa
oleifera Lam on experimentally induced diabetic
animal model. Drug design, development and
therapy, 10, p.1715.
Rahmawati, G., Rachmawati, F.N. and Winarsi, H., 2014.
Aktivitas superoksida dismutase tikus diabetes yang
diberi ekstrak batang kapulaga dan
glibenklamid. Scripta Biologica, 1(3), pp.197-201.
Sun, Y., Yang, J., Wang, H., Zu, C., Tan, L. and Wu, G.,
2015. Standardization of leaf sampling technique in
jackfruit nutrient status diagnosis. Agricultural
Sciences, 6(02), p.232.
Fattah, M., Anggraeni, S.R., Alfian, S.D., Levita, J. and
Diantini, A., 2012. Stabilitas Sampel SOD-Eritrosit dan
GPx-Blood dalam Masa Penyimpanan Tujuh
Hari. Indonesian Journal of Clinical Pharmacy, 1(4),
pp.162-170.
Birben, E., Sahiner, U.M., Sackesen, C., Erzurum, S. and
Kalayci, O., 2012. Oxidative stress and antioxidant
defense. World Allergy Organization Journal, 5(1),
pp.9-19.
Fiana, N. and Oktaria, D., 2016. Pengaruh kandungan
saponin dalam daging buah mahkota dewa (Phaleria
macrocarpa) terhadap penurunan kadar glukosa
darah. Jurnal Majority, 5(4), pp.128-132.
The Effect of Jackfruit (Artocarpus heterophyllus) Leaf Ethanolic Extract Gel on Superoxide Dismutase and Interleukin-1 Levels in Wound
Healing after Tooth Extraction in Diabetic Rats
169
