
Chitosan Nanoparticle as a Delivery System of miRNA 217 for
Suppressing Hepatocellular Carcinoma Progressivity by Targeting
AEG-1/P53
Ulfatun Nisa
1
a
, Indwiani Astuti
2
b
, Ronny Martien
3
c
, Dhani Rinaldi Maulana
4
d
, Ysrafil
5
e
1
Medicinal Plant and Traditional Medicine Research and Development Center, Ministry of Health, Tawangmangu,
Indonesia
2
Departement of Pharmacology and Therapy, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada,
Yogyakarta, Indonesia
3
Department of Pharmaceutics, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta, Indonesia
4
Department of Biotechnology, The Graduate School, Universitas Gadjah Mada, Yogyakarta, Indonesia
5
Department of Pharmacy, Health Polytechnic of Gorontalo, Gorontalo, Indonesia
Keyword: Chitosan, Nanoparticle, miRNA-217, AEG-1, Hepatocellular carcinoma
Abstract: MicroRNA, especially miRNA-217, has important role in development of Hepatocellular Carcinoma (HCC)
through its relation with astrocyte elevated gene 1 (AEG-1) who modulates several signaling arrays. Delivery
systems could be crucial factors for successful gene therapy. We investigated effects of chitosan nanoparticles
as delivery system of miRNA-217 for targeting AEG-1 in HCC. Chitosan nanoparticles were prepared using
ionic gelation methods. Entrapment efficiency was obtained using a NanoDrop spectrophotometer. Mimic
miRNA-217 encapsulated by chitosan nanoparticles were transfected in HCC cell line HepG2. Viability test
was conducted by using MTT Assay. The dosages of miRNA-217 were ¼, ½ and 1 IC50. A real-time
polymerase chain reaction determined miRNA-217 and mRNA relative expressions. Independent T-tests were
used to analyze the parameter differences. Results showed that chitosan nanoparticles could encapsulate miR-
217 with 92.9% entrapment efficiency. miR-217 was successfully delivered and significantly increase the
endogenous expression of miRNA-217 in HepG2 cells compared to controls. It mediated significant cell
inhibition in chitosan nanoparticles group compared to naked miRNA. The expression of mRNA AEG-1 was
decreased significantly compared to controls. The increased expression of miRNA-217 was negatively
correlated to AEG expression. Chitosan nanoparticles of miRNA-217 may suppress cell line progressivity via
targeting AEG-1.
1 INTRODUCTION
Hepatocellular carcinoma causes the third-highest
cancer-related mortalities with the least survival years
worldwide (Jariwala et al., 2015). The life expectancy
is less than six months from diagnosis (Torre et al.,
2015; Xie et al., 2017; Jia et al., 2018). Furthermore,
most patients with HCC are diagnosed late because of
the asymptomatic course of the disease. The
therapeutic effects of several treatments of HCC are
a
https://orcid.org/0000-0001-8743-3121
b
https://orcid.org/0000-0001-7008-9192
c
https://orcid.org/0000-0001-7291-6497
d
https://orcid.org/0000-0002-6080-2174
e
https://orcid.org/0000-0002-5980-7525
still limited. Since the underlying mechanisms of the
formation of HCC are still elusive, novel therapeutic
strategies are needed for this aggressive malignant
tumor (Jia et al., 2018).
MicroRNA (miRNA) is known to have an
important role in the regulation of gene expression.
Lu et al. revealed that all cancers have different
miRNA expression profiles than normal tissues (Lu
et al., 2005; Santos-carballal, 2018). Downregulated
miRNAs serve as a tumor suppressor miRNA
Nisa, U., Astuti, I., Martien, R., Maulana, D. and Ysrafil, .
Chitosan Nanoparticle as a Delivery System of miRNA 217 for Suppressing Hepatocellular Carcinoma Progressivity by Targeting AEG-1/P53.
DOI: 10.5220/0010489001310138
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 131-138
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
131

regarding HCC development and progression (Jia et
al., 2018). One of the tumor suppressor miRNAs is
mir-217, which is downregulated in patients with
HCC (Jariwala et al., 2015).
The research reported several oncogenic
pathways are regulated by astrocyte elevated gene 1
(AEG-1). These are Akt, nuclear factor-κβ (NF-κB),
Wnt/β-catenin, and mitogen-activated protein kinase
(MAPK) pathways. Additionally, the upregulation of
AEG-1 expression can promote cell proliferation and
anchorage-unrestrained growth ability in HCC (Meng
et al., 2012; Shi and Wang, 2015). Other previous
studies showed overexpression of mRNA and protein
AEG-1 levels in most patients with HCC, associated
with cancer progression and aggressive metastatic
stage (Yoo et al., 2009; Sarkar, 2013; Robertson et
al., 2014).
Recently, mRNA delivery systems were explored
to verify the most appropriate method to transfect
mRNA into target cells. However, nanoparticles are
the preferred method because of their potential
advantages (Phua et al., 2013). Nanoparticles can
protect the mRNA, which is susceptible to nuclease
degradation and facilitate uptake leading to targeted
genes (Phua et al., 2013; Glackin et al., 2018).
Cationic-based nanoparticles can interact with a
negatively charged nucleic acid to form
nanocomplexes. One of the polycations of
nanoparticles is chitosan. This polymer has been
widely studied due to its biodegradability and low
toxicity (Esquivel et al., 2015). Several methods can
be applied to prepare chitosan-based nanoparticles,
and the simplest one is ionotropic gelation (Gennari
et al., 2019). This method's key lies in the strong
electrostatic interaction between polymers and
crosslinker agents (Esquivel et al., 2015;
Prasetyo et
al., 2019).
As previously known, viral vectors and
lipofectamine play a pivotal role in drug delivery for
gene therapeutics. In contrast, viral vectors'
disadvantages include significant safety issues and
immunogenic responses (Santos-carballal, 2018; Guo
and Huang, 2012). In terms of cytotoxicity,
lipofectamine has serious toxicity in cell viability
(Mukerjee et al., 2011). Herein, we report the
development of a novel miRNA-217 based chitosan
nanoparticles preparation, which can be employed for
drug delivery (Lee et al., 2011; Khan et al., 2019). To
elucidate the intracellular processing of miRNA 217,
we investigated chitosan nanoparticles' effect
delivery system of miRNA-217 for targeting AEG-1
in HCC.
2 MATERIALS AND METHODS
2.1 Materials
Molecular medium-weight chitosans were obtained
from Sigma Aldrich (St. Louis, MO). Sodium
tripolyphosphate was purchased at LPPT Universitas
Gadjah Mada. The human carcinoma cell line HepG2
was obtained from BPPT (Jakarta, Indonesia). It was
maintained in high glucose DMEM supplemented
with 10% fetal bovine serum (Massachusetts, USA),
1% penicillin-streptomycin (Massachusetts, USA),
and 0.5% amphotericin (Massachusetts, USA) at
37
0
C with 5% CO
2
. miRNeasy Mini Kit, miRCURY
LNA
TM
RT kit, SYBR green PCR kit and
SensiFASTTM SYBR
®
were purchased from Qiagen
(USA).
2.2 Chitosan-Nanoparticle based
miRNA-217 Formulation
The chitosan medium molecular weight was
dissolved into 1% acetic acid. The solution was
vigorously stirred using a magnetic stirrer for 4
hours. The pH of the solution had been adjusted to
5.5 while NaOH 1 M was added. The solution 1%
chitosan was added with acetate buffer ph 5 to
generate 0.2% chitosan solution. Preparation of
chitosan nanoparticles was done with ionic gelation
methods. It was obtained by mixing 0.2% chitosan
and sodium tripolyphosphate (5:1) and incubating for
5 minutes at room temperature. Then, 150 μL mimic
miR-217 was conjugated into 150 μL of chitosan
nanoparticle solution and then incubated for 20
minutes at room temperature (Ysrafil et al., 2020).
2.3 Entrapment Efficiency
miR-217, which was formulated by chitosan
nanoparticles, were centrifuged for 15 minutes at a
speed of 13.000 g. The absorbance of supernatants
was measured by using NANO-Quant.
Determination of efficient entrapment was calculated
using the following equation:
𝐸𝐸%
𝐸𝑛𝑐𝑎𝑝𝑠𝑢𝑙𝑎𝑡𝑒𝑑 𝑚𝑖𝑅𝑁𝐴 𝑓𝑟𝑒𝑒 𝑚𝑖𝑅𝑁𝐴
𝐸𝑛𝑐𝑎𝑝𝑠𝑢𝑙𝑎𝑡𝑒𝑑 𝑚𝑖𝑅𝑁𝐴
𝑥100%
(1)
2.4 Determination of Cell Viability
MTT assay method was used to test HepG2 Line cells'
cytotoxic activity as much as 6 x 10
3
HepG2 cell lines
were planted on a 96-well plate and incubated at
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
132
37
0
C, 5% CO
2
for 24 hours. Then, the media was
discharged from the plate and cleaned using PBS. As
much as 100 μL/well of prepared chitosan
nanoparticles mixed with serum DMEM free media
were put into each well and incubated at 37
0
C and 5%
CO
2
for 24 hours. The following day, each well was
discharged and filled with 100 μL MTT 0.5 mg/ml
and then incubated for 4 hours at 37
0
C and 5% CO
2
.
100 μL SDS 10% were added to stop the reaction,
dissolve formazan crystals, and then incubated
overnight. Each well's absorbance was determined by
using a Micro Plate Reader (Bio-Rad Model 680 XR)
(Ysrafil et al., 2020).
2.5 Cell Transfection and mRNA
Expression Assay
Cells (5x10
5
per well) were plated in 6-well plates
and incubated at 37
0
C and 5% CO
2
for 24 hours.
After removing the media, each well plate was filled
with 750 μL prepared chitosan nanoparticles (mixed
with free media serum) and incubated at 370C and
5% CO2 for 24 hours. According to the kit's
instruction manual, total RNA was isolated using the
miRNeasy Mini Kit (Qiagen). A nanodrop
determined the concentration of RNA. Then, cDNA
was synthesized using the miRCURY LNA
TM
RT kit
(Qiagen). Quantification of miR-217 was determined
by quantitative polymerase chain reaction (PCR)
using miRCURY LNA
TM
and SYBR green PCR kit.
The sequence of miRNA and references were:
5'UACUGCAUCAGGAACUGAUUGGA3'and
5'UAGCAGCACGUAAAUAUUGGCG'3,
respectively. Meanwhile, the quantification of AEG-
1 mRNA expression was measured by qPCR using
SensiFASTTM SYBR
®
. The primers for AEG-1 and
beta-actin mRNA were as follows for forward:
5’TGACTTCAACAGCGACACCCA3’; reverse:
5’CACCCTGTTGCTGTAGCCAAA-3’ and
forward: 5’GGGAATTCAAAACTGGAACGGT
GAAGG3’; and reverse: 5’GGAAGCTTATCAA
AGTCCTCGGCCACA-3, respectively. The Biorad
CFX 96 C.1000 quantitative PCR machine was used
to quantify measurements of all gene transcriptions.
The experiments were performed in triplicate.
Relative gene expressions were analyzed using the 2
-
ΔΔCT
methods, and the results were expressed as the
fold change.
2.6 Statistical Analysis
All measurements were presented in mean ± standard
deviation (SD). To determine the significant
difference between groups, independent t-tests were
performed in each group. Spearman/ Pearson
correlation analysis was also done to find the
relationship between miRNA-217 and AEG-1
mRNA. All data were analyzed using SPSS 22
software (IBM Corp., Chicago), and graphics were
presented by GraphPad Prism 7. Statistical
significance was set at P < 0.05.
3 RESULTS
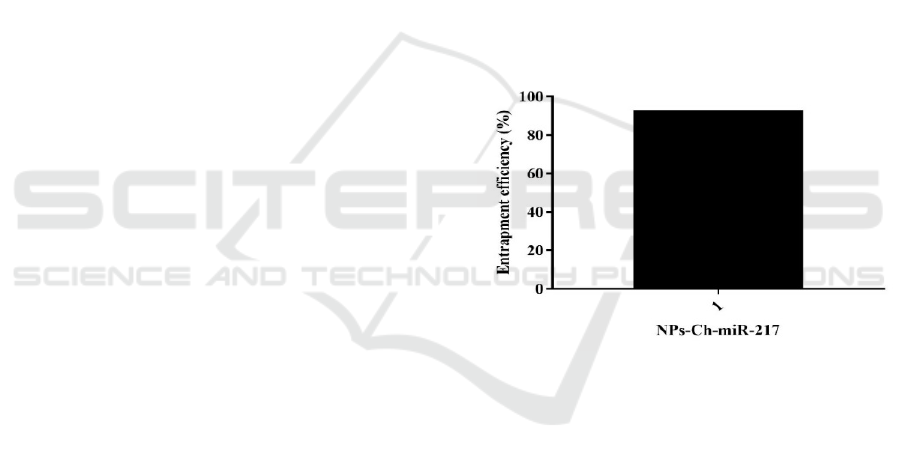
The Entrapment efficiency NPs-Ch-miR-17 was
presented in fig.1. The absorption efficiency of NPs-
Ch-miR-17 was 92.19%. This percentage showed the
amount of miRNA 217, which is in the nanochitosan
matrix.
Figure 1: Entrapment efficiency of NPs-Ch-miR-17
3.1 Viability Cells
The cytotoxicity effect of nanoparticles mimic
miRNA 217 in the HepG2 -HCC cell line was
measured to determine viability cells test. Variety of
dosage concentrations of NPCs mimic miRNA 217
had were correlated to the presentation of cancer cell
inhibition up to IC50 value. The IC50 value of this
study was 120 nM. The result of the viability cells
assay had been shown in figure 2.
Chitosan Nanoparticle as a Delivery System of miRNA 217 for Suppressing Hepatocellular Carcinoma Progressivity by Targeting
AEG-1/P53
133
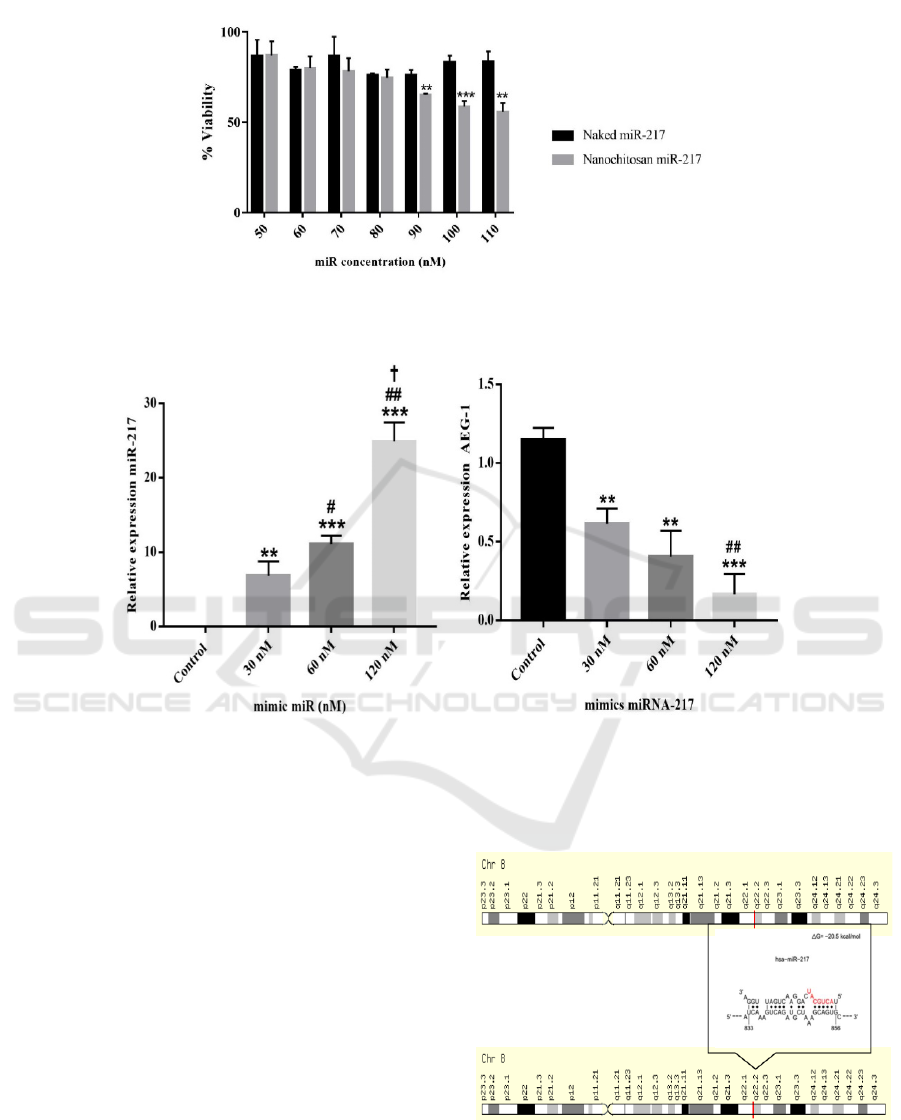
3.2 In Silico Target Prediction
Figure 3 showed the results of in silico target
prediction of miRNA 217. It showed AEG 1 had
been a target of miRNA 217 at 833-856 base with ΔG
= 20.5 kcal/mol. Based on the result, miRNA217 is a
tumor suppressor miRNA regulating oncogenic post
transcription gene AEG-1.
The independent t-test was conducted to whether
there were significant differences between the 30 nM
group (1/4 IC50), the 60 nM group (1/2 IC50), the
120 nM group (IC50) and the control group. These
findings resulted in a significant difference between
treatment groups compared to the control group with
a p-value <0.005.
Figure 3: In silico prediction of hsa-miRNA-217
recognized AEG-1 in the cytoplasm of HepG2 cells using
STARMirDB.
Figure 2: Viability assays to analyze inhibition of Proliferation of chitosan nanoparticle of mimic MiR-217 to HepG2
cell line (n=3). It is presented in mean ± SD. *P < 0.05.
(A) (B)
Figure 4: Relative of expression endogenous miRNA 217 (A) and mRNA AEG-1 (B) HepG2 cell line post treatment
nanoparticles mimic miRNA 217 in several doses which were measured by qPCR
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
134
Meanwhile, the highest level of difference was
found in the group with a dose of 120 nM with a p-
value <0.001. To determine whether there was a
correlation between miRNA-217 expression and
AEG-1 in each treatment group, the Pearson test was
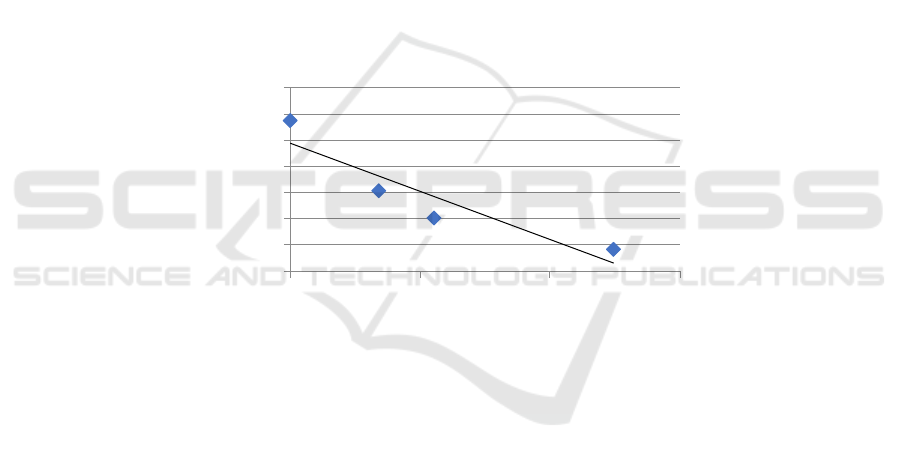
performed and obtained r and p values of -0.870 and
0.0001, respectively, which showed there was a very
strong negative correlation between relative
expressions of miRNA 217 and AEG-1 mRNA (p
<0.05).
4 DISCUSSION
Mechanism of microRNA-217 encapsulation
through the ionic interaction between negative
charge of the (-NH3+) group miRNA and positive
charge of tripolyphosphate of crosslinker. Indeed, the
concentration of chitosan affects entrapment
efficiency. The low chitosan concentration's low
viscosity might cause effectively penetrating miRNA
into the polymer matrix (Csaba et al., 2009; Ysrafil
et al., 2020). In this study concentration of chitosan
was 0.2%. Like previous research, this result showed
that drug ecapsulation efficiency prepared by the
ionic interaction method was more than 90%
(Agnihotri et al.,2004).
The mitochondrial dehydrogenase assay was
used to determine cellular metabolic activity as an
indicator of cell viability, proliferation and
cytotoxicity. Indication of mimic microRNA has
effectively entered the HepG2 cell is the percentage
of cell growth in the treated group led to initiate
cancer cell death (Ysrafil et al., 2020). Furthermore,
the expression of miRNA 217 was upregulated in the
treated group compared to controls.
The addition of a positive charge from the
cationic polymers into miRNA causes agarose cells'
oligonucleotide migration ability. As a delivery
system, chitosan nanoparticles will protect miRNA
from the degradation of nucleases in HepG2 cells.
Inside these cells, chitosan nanoparticles will interact
with lysozyme, an enzyme that can degrade chitosan
after cellular uptake occurs. The enzyme hydrolyzes
the glycoside bonds in chitosan's chemical structure,
causing the release of miRNA in the cytosol leading
to diminishing the targeted gene's expression (Freier
et al., 2005).
The study conducted by Zhang et al. in 2017 used
lipofectamine 2000 as a miRNA 217 mimic
transfection agent. The study used a dose of 100 nM
with an incubation period of 36 days resulting in
upregulation of miRNA-217 with a relative
expression of 2.6-2.7 times higher compared to
controls and downregulation of MTDH with
expression values which were relatively 0.3-0.4 times
lower compared to controls (Zhang et al., 2017)
.
These results are almost the same as in this study
using the chitosan nanoparticle transfection agent.
However, using a lipofectamine drug delivery system
certainly has a negative effect, namely high
cytotoxicity (Wang et al., 2018). Cytotoxicity studies
are one of the main concerns for the selection of
transfection reagents. Generally, transfection reagent
toxicity has a positive correlation with transfection
efficacy. However, both are influenced by the type of
cell (Wang et al., 2018).
One of the target genes of miRNA 217 is AEG-1.
This study follows previous research conducted by
Zhang et al. in 2016, which explains that miRNA 217
can inhibit HCC cancer cell proliferation by targeting
MTDH. The finding showed miRNA 217 could
suppress AEG-1 of mRNA and protein expression
(Zhang et al., 2017). Another study stated that mir-
30a-5p could reduce mRNA expression and MTDH
protein (Li et al., 2015).
Figure 5: Correlation test between relative expression of miRNA 217 and AEG-1 mRNA
0,000
0,200
0,400
0,600
0,800
1,000
1,200
1,400
0 102030
RelativeexpressionofmiRNA
217
RelativeexpressionofmRNAMTDH
Chitosan Nanoparticle as a Delivery System of miRNA 217 for Suppressing Hepatocellular Carcinoma Progressivity by Targeting
AEG-1/P53
135

Also, the activation of various signaling
pathways, such as PI3K/Akt, NF-κB, and Wnt/β-
catenin pathways, is regulated by AEG-1 (Yang et al.,
2018). Increased expression of AEG-1 causes
increased expression of genes that support
malignancy (He et al., 2015). Hence, it interacts with
NFκB resulting in growth, survival and invasion of
cancer cells. Simultaneously, tumor progression and
proangiogenesis through the PI3K/AKT pathway and
the Wnt/β-catenin pathway MAPK cause changes to
epithelial-mesenchymal transition for metastasis
(Dhiman et al., 2019). The NFκB pathway also
becomes active in the presence of AEG-1
phosphorylation. Increased AEG-1 expression will
activate inflammation regulated by NFκB, supporting
tumor development in HCC (Sarkar et al., 2008;
Emdad et al., 2016). Another study also states that
AEG-1 overexpression can suppress PTEN protein
expression (Li et al., 2015). Meanwhile, Wen fang li
et al. revealed that apoptosis-related protein
expression, notably PTEN and p53, had been
regulated by AEG-1 (Li et al., 2015).
These findings suggested that deletion of AEG-1
might increase p53 expression by upregulating
PTEN. As a tumor suppressor, p53 can initiate cell
death and suppress cell proliferation (Li et al., 2015;
Kruiswijk, Labuschagne and Vousden, 2015).
Another study revealed that the absence of AEG-1
induces apoptosis of hepatocytes, hinder mutated
transformation. Overexpression of AEG-1
encourages the tumorigenic process to maintain
various stress (Robertson et al., 2018). Increased
cytoplasmic AEG-1 expression acts as an RNA
binding protein that can induce chemoresistance. It is
of clinical significance to restore anti-cancer therapy's
sensitivity (Meng et al., 2012). This study strongly
suggested developing AEG-1-targeted as a promising
therapeutic strategy. Indeed it deserves further
investigation.
5 CONCLUSION
We concluded that nanoparticle chitosan could be
used as a delivery system for targeted therapy. In
addition, miRNA 217 can suppress hepatocellular
carcinoma progressivity by targeting AEG-1/p53.
ACKNOWLEDGMENT
This research was supported by the DAMAS project
of the Faculty of Medicine, Public Health and
Nursing UGM Yogyakarta. We thank the LRT,
LPPT, Biochemistry technician of Gadjah Mada
University for this research.
CONFLICT OF INTEREST
There is no conflict of interest in this research.
REFERENCES
Agnihotri, S. A., Mallikarjuna, N. N. and Aminabhavi, T.
M. (2004) 'Recent advances on chitosan-based micro-
and nanoparticles in drug delivery B', Journal of
Controlled Release, 100, pp. 5–28.
doi:10.1016/j.jconrel.2004.08.010.
Csaba, N., Köping-höggård, M. and Alonso, M. J.(2009)
'Ionically crosslinked chitosan/tripolyphosphate
nanoparticles for oligonucleotide and plasmid DNA
delivery', International Journal of Pharmaceutics, 382,
pp. 205–214. doi: 10.1016/j.ijpharm.2009.07.028.
Csaba, N., Köping-Höggård, M. and Alonso, M. J. (2009)
'Ionically crosslinked chitosan/tripolyphosphate
nanoparticles for oligonucleotide and plasmid DNA
delivery.', International journal of pharmaceutics.
Netherlands, 382(1–2), pp. 205–214. doi:
10.1016/j.ijpharm.2009.07.028.
Dhiman G, Srivastava N, Goyal M, Rakha E, Lothion-Roy
J, Mongan NP, Miftakhova RR, Khaiboullina SF,
Rizvanov AA, Baranwal M. (2019) 'Metadherin : A
Therapeutic Target in Multiple Cancers', Frontiets in
Oncology, 9, pp. 1–8. doi: 10.3389/fonc.2019.00349.
Emdad L, Das SK, Hu B, Kegelman T, Kang DC, Lee SG,
Sarkar D, Fisher PB. (2016) AEG-1 / MTDH / LYRIC :
A Promiscuous Protein Partner Critical in Cancer ,
Obesity , and CNS Diseases, Advances in Cancer
Research. doi: 10.1016/bs.acr.2016.05.002.
Esquivel R, Juárez J, Almada M, Ibarra J, and Valdez1 MA.
(2015) 'Synthesis and Characterization of New
Thiolated Chitosan Nanoparticles Obtained by Ionic
Gelation Method', International Journal of Polymer
Science, 2015, pp. 1–18. doi: 10.1155/2015/502058.
Freier T, Koh HS, Kazazian K, Shoichet MS (2005)
'Controlling cell adhesion and degradation of chitosan
films by N -acetylation', Biomaterials, 26, pp. 5872–
5878. doi: 10.1016/j.biomaterials.2005.02.033.
Gennari, A., Rios de la Rosa, J. M., Hohn, E., Pelliccia, M.,
Lallana, E., Donno, R., Tirella, A., & Tirelli, N. (2019).
The different ways to chitosan/hyaluronic acid
nanoparticles: templated vs direct complexation.
Influence of particle preparation on morphology, cell
uptake and silencing efficiency. Beilstein journal of
nanotechnology, 10, 2594–2608.
https://doi.org/10.3762/bjnano.10.250.
Glackin, C. A. (2018) Nanoparticle Delivery of TWIST
Small Interfering RNA and Anticancer Drugs : A
Therapeutic Approach for Combating Cancer. 1st edn,
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
136

Mesoporous Silica-based Nanomaterials and
Biomedical Applications - Part B. 1st edn. Elsevier Inc.
doi: 10.1016/bs.enz.2018.08.004.
Guo, X. I. A. and Huang, L. (2012) 'Recent Advances in
Nonviral Vectors for Gene Delivery', Accounts of
Chemical Research, 45(7), pp. 971–979.
He, R., Yang, L., Lin, X., Chen, X., Lin, X., Wei, F., Liang,
X., Luo, Y., Wu, Y., Gan, T., Dang, Y., & Chen, G.
(2015). MiR-30a-5p suppresses cell growth and
enhances apoptosis of hepatocellular carcinoma cells
via targeting AEG-1. International journal of clinical
and experimental pathology, 8(12), 15632–15641.
(2015) 'MiR-30a-5p suppresses cell growth and
enhances apoptosis of hepatocellular carcinoma cells
via targeting AEG-1', International Journal Clinical
Experiment Pathology, 8(12), pp. 15632–15641.
Rajasekaran D, Srivastava J, Ebeid K, Gredler R, Akiel M,
Jariwala N, Robertson CL, Shen XN, Siddiq A, Fisher
PB, Salem AK, Sarkar D (2015) 'Combination of
Nanoparticle-Delivered siRNA for Astrocyte Elevated
Gene-1 (AEG-1) and All-trans Retinoic Acid (ATRA):
An Effective Therapeutic Strategy for Hepatocellular
Carcinoma (HCC)', Bioconjugate Chemistry. American
Chemical Society, 26(8), pp. 1651–1661. doi:
10.1021/acs.bioconjchem.5b00254.
Jia C, Tang D, Sun C, Yao L, Li F, Hu Y, Zhang X, Wu D.
(2018) 'MicroRNA ‑ 466 inhibits the aggressive
behaviors of hepatocellular carcinoma by directly
targeting metadherin', Oncology Reports, 40, pp. 3890–
3898. doi: 10.3892/or.2018.6763.
Khan, Ibrahim, Saeed, K. and Khan, Idrees (2019)
'Nanoparticles : Properties , applications and toxicities',
Arabian Journal of Chemistry. The Authors, 12(7), pp.
908–931. doi: 10.1016/j.arabjc.2017.05.011.
Kruiswijk, F., Labuschagne, C. F. and Vousden, K. H.
(2015) 'p53 in survival, death and metabolic health: a
lifeguard with a licence to kill.', Nature reviews.
Molecular cell biology. England, 16(7), pp. 393–405.
doi: 10.1038/nrm4007.
ee JE, Lee N, Kim T, Kim J, Hyeon T (2011)
'Multifunctional mesoporous silica nanocomposite
nanoparticles for theranostic applications', Accounts of
Chemical Research, 44(10), pp. 893–902. doi:
10.1021/ar2000259.
Li WF, Ou Q, Dai H, Liu CA. (2015) 'Lentiviral-Mediated
Short Hairpin RNA Knockdown of MTDH Inhibits Cell
Growth and Induces Apoptosis by Regulating the
PTEN / AKT Pathway in Hepatocellular Carcinoma',
International Journal of Molecular Sciences, 16, pp.
19419–19432. doi: 10.3390/ijms160819419.
Li WF, Dai H, Ou Q, Zuo GQ, Liu CA. (2015)
'Overexpression of microRNA-30a-5p inhibits liver
cancer cell proliferation and induces apoptosis by
targeting MTDH / PTEN / AKT pathway', Tumor
Biology. doi: 10.1007/s13277-015-4456-1.
Li, W., Zhou, J. and Xu, Y. (2015) 'Study of the in vitro
cytotoxicity testing of medical devices ( Review )',
Biomedical Reports, 3, pp. 617–620. doi:
10.3892/br.2015.481.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck
D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
Downing JR, Jacks T, Horvitz HR, Golub TR (2005)
'MicroRNA expression profiles classify human
cancers.', Nature. England, 435(7043), pp. 834–838.
doi: 10.1038/nature03702.
Meng, X., Zhu, D., Yang, S., Wang, X., Xiong, Z., Zhang,
Y., Brachova, P. and Leslie, Kimberly K (2012)
'Cytoplasmic Metadherin ( MTDH ) Provides Survival',
The Journal of Biological Chemistry, 287(7), pp. 4485–
4491. doi: 10.1074/jbc.C111.291518.
Mukerjee, A. et al. (2011) 'Efficient nanoparticle mediated
sustained {RNA} interference in human primary
endothelial cells', Nanotechnology. {IOP} Publishing,
22(44), p. 445101. doi: 10.1088/0957-
4484/22/44/445101.
Phua, K., Leong, K. and Nair, S. (2013) 'Transfection
Efficiency and Transgene Expression Kinetics of
mRNA Delivered in Naked and Nanoparticle Format',
Journal of controlled release : official journal of the
Controlled Release Society, 166. doi:
10.1016/j.jconrel.2012.12.029.
Prasetyo, Y. A., Abdassah, M. and Rusdiana, T. (2019)
'Preparation and Characterization of Glucosamine
Nanoparticle by Ionic Gelation Method Using Chitosan
and Alginate', Indonesian Journal of Pharmaceuics,
1(1), pp. 1–10.
Robertson CL, Srivastava J, Siddiq A, Gredler R, Emdad L,
Rajasekaran D, Akiel M, Shen XN, Guo C, Giashuddin
S, Wang XY, Ghosh S, Subler MA, Windle JJ, Fisher
PB, Sarkar D. (2014) 'Genetic Deletion of AEG-1
Prevents Hepatocarcinogenesis', Molecular and
Cellular Pathobiology, 74(21), pp. 6184–6194.
Robertson CL, Mendoza RG, Jariwala N, Dozmorov M,
Mukhopadhyay ND, Subler MA, Windle JJ, Lai Z,
Fisher PB, Ghosh S, Sarkar D (2018) 'Astrocyte
elevated gene-1 regulates macrophage activation in
hepatocellular carcinogenesis', Cancer Research,
78(22), pp. 6436–6446. doi: 10.1158/0008-5472.CAN-
18-0659.
Santos-carballal, B. (2018) 'Chitosan in Non-Viral Gene
Delivery : Role of Structure , Characterization Methods
, and Insights in Cancer and Rare Diseases Therapies',
Polymers, 10(444), pp. 1–51. doi:
10.3390/polym10040444.
Sarkar D, Park ES, Emdad L, Lee SG, Su ZZ, Fisher PB.
(2008) 'Molecular basis of nuclear factor-κB activation
by astrocyte elevated gene-1', Cancer Research, 68(5),
pp. 1478–1484. doi: 10.1158/0008-5472.CAN-07-
6164.
Sarkar, D. (2013) 'AEG-1/MTDH/LYRIC in Liver Cancer
Devanand', Advanced Cancer Research. 1st edn.
Elsevier Inc., 120, pp. 1–21. doi: 10.1016/B978-0-12-
401676-7.00007-3.
Shi, X. and Wang, X. (2015) 'The role of MTDH / AEG-1
in the progression of cancer', International Journal
Clinical Experiment Medicine, 8(4), pp. 4795–4807.
orre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J,
Jemal A (2015) 'Global cancer statistics, 2012.', CA: a
Chitosan Nanoparticle as a Delivery System of miRNA 217 for Suppressing Hepatocellular Carcinoma Progressivity by Targeting
AEG-1/P53
137

cancer journal for clinicians. United States, 65(2), pp.
87–108. doi: 10.3322/caac.21262.
Wang T, Larcher LM, Ma L, Veedu RN (2018) 'Systematic
Screening of Commonly Used Commercial
Transfection Reagents towards Efficient Transfection',
Molecules, 23(2564), pp. 1–15. doi:
10.3390/molecules23102564.
Xie G, Liang Ou H, Wang J, Wang G, Wang YF (2017)
'Investigation of expression level of MTDH ,
CEACAM1 and HBx in HBV- associated
hepatocarcinoma tissues and its clinical significance .',
Biomedical Research, 28(18), pp. 8038–8043.
Yang L, Tian Y, Leong WS, Song H, Yang W, Meiqi
Wang, Wang X, Kong J, Shan B, Song Z (2018)
'Efficient and tumor-specific knockdown of MTDH
gene attenuates paclitaxel resistance of breast cancer
cells both in vivo and in vitro', Breast Cancer Research.
Breast Cancer Research, 20(113), pp. 1–13.
Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY,
Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA,
Llovet JM, Fisher PB, Sarkar D (2009) 'Astrocyte
elevated gene-1 regulates hepatocellular carcinoma
development and progression Find the latest version :
Astrocyte elevated gene-1 regulates hepatocellular
carcinoma development and progression', The Journal
of Clinical Investigation, 119(3), pp. 465–477. doi:
10.1172/JCI36460.the.
Ysrafil Y, Astuti I, Anwar SL, Martien R, Sumadi FAN,
Wardhana T, Haryana SM (2020) 'MicroRNA-155-5p
Diminishes in Vitro Ovarian Cancer Cell Viability by
Targeting HIF1α Expression', Advanced
Pharmaceutical Bulletin, 10(4), pp. 630–637. doi:
10.15171/jcvtr.2015.24.
Zhang M, Li M, Li N, Zhang Z, Liu N, Han X, Liu Q, Liao
C (2017) 'miR-217 suppresses proliferation , migration
, and invasion promoting apoptosis via targeting
MTDH in hepatocellular carcinoma', Oncology
Reports, 37, pp. 1772–1778. doi:
10.3892/or.2017.5401.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
138
