
The Activities of Streptomyces W-5A as Antibacterial and
Antibiofilm towards Methicillin-resistant Staphylococcus aureus 2983
Annisa Permata Dinda
1a
, Ari Asnani
1b
and Dwi Utami Anjarwati
2c
1
Department of Chemistry, Faculty of Mathematics and Sciences, Universitas Jenderal Soedirman, Purwokerto, Indonesia
2
Department of Microbiology, Faculty of Medicine, Universitas Jenderal Soedirman, Purwokerto, Indonesia
Keywords: MRSA, Streptomyces, antibiofilm, antibacterial
Abstract: Methicillin-resistant Staphylococcus aureus (MRSA) causes nosocomial infection worldwide. MRSA can
defend itself by forming a biofilm layer, thereby increasing the virulence factor. WHO categorizes MRSA as
high risk on the Priority Pathogen List for searching for new antibiotics. Recently, we have reported the
potency of Streptomyces W-5A as anti-MRSA based on qualitative screening. Thus, this research aimed to
analyze the potency of Streptomyces W-5A as antibacterial and antibiofilm towards MRSA 2983. Cultivation
of Streptomyces W-5A used Starch Casein Nitrate (SCN) agar, and anti-MRSA extract production used SCN
broth. Samples were taken at incubation times of 0, 3, 6, 9, 12, and 15 days. Each sample was tested for
antibacterial activity with the Kirby-Bauer method, whereas inhibition of biofilm formation and biofilm
degradation with microtiter method. The results showed that the optimum antibacterial activity was achieved
after nine days of incubation with an inhibition zone of 9.589 ± 0.521. The optimum % inhibition of biofilm
formation was 77.806 ± 13.595% after 12 days of incubation. The optimum % degradation of biofilm was
80.465 ± 7.586% after nine days of incubation. These findings suggest that Streptomyces W-5A has the
potency to produce antibacterial and antibiofilm compounds against MRSA 2983.
1 INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA)
is an S. aureus bacterium resistant to methicillin and
several other beta-lactam antibiotics (Boucher &
Corey, 2008). MRSA is an infection-causing
bacterium that can defend itself by forming a
protective layer called a biofilm. S. aureus biofilms
develop rapidly and form colonies on moist and
nutrient-rich surfaces (Tarver, 2009). The ability to
form biofilms is one of S. aureus' virulence factors,
which causes antibiotic resistance to bacteria (Høiby
et al., 2010; Lee et al., 2013).
Infection by microbes is estimated to be 80%
related to the formation of biofilms, which contribute
to the nature of antibiotic resistance (Archer et al.,
2011). Biofilms consist of microbial cells and a
matrix of extracellular polymeric substance (EPS). As
much as 50-90% of the EPS matrix's main ingredients
are organic carbon consisting of polysaccharides,
a
https://orcid.org/0000-0002-1992-6685
b
https://orcid.org/0000-0002-8569-2565
c
https://orcid.org/0000-0001-8394-2543
proteins, nucleic acids, lipids, phospholipids, and
humic substances, and 15% are bacterial cells
(Deshpande & Joshi, 2011). These biofilms cause
antibiotics cannot reach the target, bacterial cells;
hence the bacteria that cause infection cannot be
inhibited or destroyed using antibiotics. Therefore,
exploration of antibiofilm compounds becomes
necessary. The antibiofilm compounds are expected
to inhibit biofilm formation and degrade biofilms
(Konai & Haldar, 2017). The inhibitory activity of
biofilms occurs because these compounds have
antibacterial activity, while biofilms' degradation
activity occurs because they can depolymerize
complex compounds in the biofilm matrix.
Actinobacteria, particularly the genus
Streptomyces, has been reported to produce bioactive
compounds with anti-MRSA activity. Streptomyces
albus, granaticin B from Streptomyces violaceoruber,
and streptorubin B from Streptomyces sp. MC11204
has been reported to inhibit biofilm formation and
Dinda, A., Asnani, A. and Anjarwati, D.
The Activities of Streptomyces W-5A as Antibacterial and Antibiofilm towards Methicillin-resistant Staphylococcus aureus 2983.
DOI: 10.5220/0010488601090115
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 109-115
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
109

damage the S. aureus biofilm (Oja et al., 2015; Suzuki
et al., 2015). Bhakyashree and Krishnan (2018)
reported that Streptomyces sp. Strain VITBKA3
showed anti-MRSA activity. Balasubramanian et al.
(2017) also reported that ethyl acetate extract from
Streptomyces sp. strain SBT343 could reduce biofilm
formation of several Staphylococcal species,
including MRSA USA300.
Recently, Asnani et al. (2020) have reported the
potency of Streptomyces W-5A as anti-MRSA based
on qualitative screening. Streptomyces W-5A was
isolated from mangrove areas in Segara Anakan
Cilacap, a source of indigenous marine actinobacteria
(Asnani et al., 2016). Following that report, this
research aimed to analyze the potency of W-5A
isolates as antibacterial and antibiofilm towards
MRSA 2983.
2 MATERIALS AND METHODS
The research was conducted from January to
September 2020 in the Biochemistry Laboratory and
Research Laboratory in UNSOED, Purwokerto.
Streptomyces W-5A was isolated from mangrove
sediment in Segara Anakan Cilacap. The MRSA 2983
is known as a bacterium capable of producing biofilm
matrix. It was isolated from a clinical specimen from
the pus of female patient in Prof. Dr Margono
Soekarjo Hospital, Banyumas regency, Indonesia.
2.1 Cultivation of Isolate W-5A
Streptomyces W-5A was cultivated using continuous
streak on Starch Casein Nitrate (SCN) following the
procedure described by Asnani et al. (2016). Starch
Casein Nitrate (SCN) agar [starch, casein, KNO
3
,
KH
2
PO
4
, MgSO
4
.7H
2
O, NaCl, FeSO
4
.7H
2
O, agar]
was added 1 µL nystatin for every 10 mL of SCN
medium. The cultures were incubated for seven days
at room temperature.
2.2 Production of Anti-MRSA Extract
Production of anti-MRSA extract used SCN broth
following the procedure described by Asnani et al.
(2020). A total of 10 plugs (6 mm diameter) of
Streptomyces W-5A were inoculated into 100 mL of
SCN broth. The culture was incubated at 90 rpm and
room temperature until it reached the exponential
phase for inoculum. Next, 10% of the inoculum was
inoculated into a new SCN broth to produce anti-
MRSA compounds. The cultures were incubated
using an orbital shaker at a speed of 90 rpm. Samples
were taken at incubation times of 0, 3, 6, 9, 12, and
15 days. Each sample was separated by centrifugation
at 4.000 rpm for 10 minutes at 4
o
C, then filtered to
obtain the crude extract. Each extract was tested for
antibacterial activity, inhibition of biofilm formation,
and biofilm degradation.
2.3 Antibacterial Test
The extract's antibacterial activity was evaluated
using the disc paper diffusion method on the Mueller
Hinton Agar (MHA) medium following CLSI (2019).
A total of 30 μL of the extract was added to disc paper
(6 mm), then placed on the MHA medium that had
been inoculated by MRSA 2984 using the spread
plate method. The test culture was incubated at 37
o
C
for 24 hours. A clear zone around the disc paper
indicated a positive result of antibacterial activity
against MRSA. Hence, the parameter observed was
the diameter of the inhibition zone.
2.4 Biofilm Formation Inhibition Test
The potency of the extract to inhibit biofilm
formation was tested using the microtiter plate
method described by Suzuki et al. (2015) with
modification. The MRSA 2983 was inoculated in
Brain Heart Infusion (BHI) medium with 1% glucose
(BHI-Glu) and incubated at 37
o
C for 24 hours. Then,
the culture was adjusted for its turbidity level using a
0.5 McFarland standard and diluted in BHI-Glu with
a ratio of 1:100. The inhibition of biofilm formation
was carried out by adding a total of 10 µL of diluted
MRSA and 100 µL of extract to the microplate and
incubated at 37
o
C for 24 hours. After incubation,
planktonic cells were removed carefully, washed
twice with PBS, stained with 0.1% crystal violet
solution, and incubated for 30 minutes. Then, the
microplate was washed with water to remove excess
crystal violet and dried. HCl in isopropanol (1:20)
was then added, and the optical density (OD) value
was measured using a microplate reader at a
wavelength of 595 nm. The percentage of biofilm
formation inhibition was calculated using the
following formula (Pratiwi et al., 2015).
%Inhibition =
100% (1)
2.5 Biofilm Degradation Test
The potency of extract to degrade biofilms was tested
using the microtiter plate method described by Suzuki
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
110
et al. (2015) with modification. The MRSA 2983 was
prepared as the previous procedure. The biofilm
degradation test was carried out by inoculating
diluted MRSA in each microplate well and incubated
at 37
o
C 24 hours. After incubation, planktonic MRSA
cells were carefully removed, then the extract was
added into each well. The mixtures were incubated at
37
o
C for 24 hours, exposing the extract to biofilm
formation at the bottom of the well. After incubation,
the microplate was treated similarly to the previous
procedure, and the OD value was measured at 595
nm. The percentage of biofilm degradation was be
calculated using the following formula (Pratiwi et al.,
2015).
%Degradation =
100% (2)
2.6 Data Analysis
The research data were the diameter of the inhibition
zone, %inhibition, and %degradation. All data were
analyzed using one-factor analysis of variance
(ANOVA) at a 95% confidence level. If the ANOVA
analysis results have a significant effect, it continued
with the Duncan test.
3 RESULTS
3.1 Antibacterial Activity
The extract was tested for antibacterial activity using
the paper disc diffusion method. This method is
expected to determine the sensitivity of a microbe to
various antibiotics. The experimental parameters
were the presence or absence of a clear zone formed
around the disc paper, which showed MRSA's growth
inhibition. The research result indicated that the
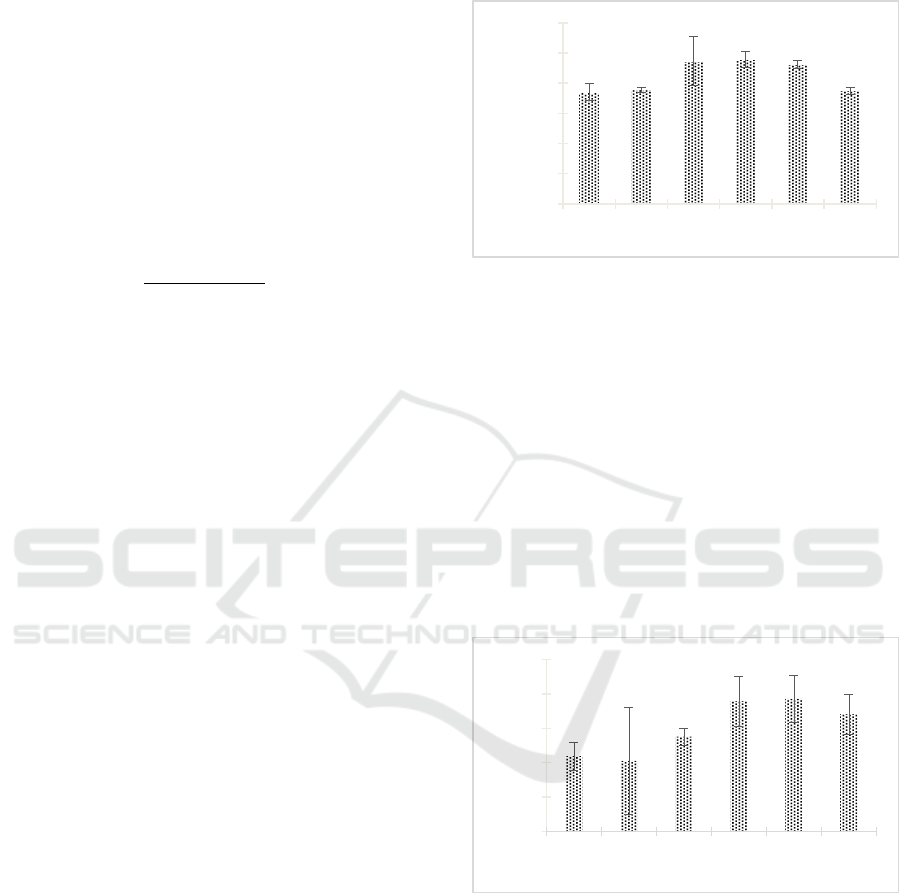
extract has antibacterial activity (Figure 1). The
antibacterial activity of Streptomyces W-5A's extract
increased from day 0 and reached the highest activity
on day 9th with the diameter of the inhibition zone
was 9.589 ± 0.521 mm. The zone of inhibition
showed potent inhibition of extract against MRSA
298.
The one-way ANOVA test analysis results
showed a significant effect (Sig. <0.05) between the
incubation time and the inhibition zone diameter
(Table 1). Further tests using the Duncan test showed
that the 9-day incubation time had an antibacterial
activity significantly different from other incubation
times. This finding indicated that the optimum
incubation time for producing antibacterial
compounds was nine days.
Figure 1: Antibacterial activity of Streptomyces W-5A
extract against MRSA 3983.
3.2 Inhibition of Biofilm Formation
The inhibition of biofilm formation aimed to
determine the potency of extract to inhibit MRSA
biofilms' formation. Compounds with antibacterial
activity against microorganisms that form biofilms
can be used to inhibit biofilm formation (Memariani
et al., 2019). The tests used a microplate and BHI
medium with glucose. BHI medium is often used to
grow Gram-positive and Gram-negative bacteria,
whereas glucose to the BHI medium aimed to induce
MRSA biofilms formation (You et al., 2014).
Figure 2: The inhibition formation of biofilm MRSA 2983
by Streptomyces W-5A extract.
The research result is presented in Figure 2. The
extract inhibited the formation of biofilm MRSA
2983. The percentage inhibition of biofilm formation
by the extract increased from 0 days until it reached
the optimum incubation time, 12 days, with a per cent
inhibition of 77.806% ± 13.595%.
0
2
4
6
8
10
12
03691215
Diameter of inhibition zone
(mm)
Incubation (Days)
0
20
40
60
80
100
03691215
% Inhibition
Incubation (Days)
The Activities of Streptomyces W-5A as Antibacterial and Antibiofilm towards Methicillin-resistant Staphylococcus aureus 2983
111
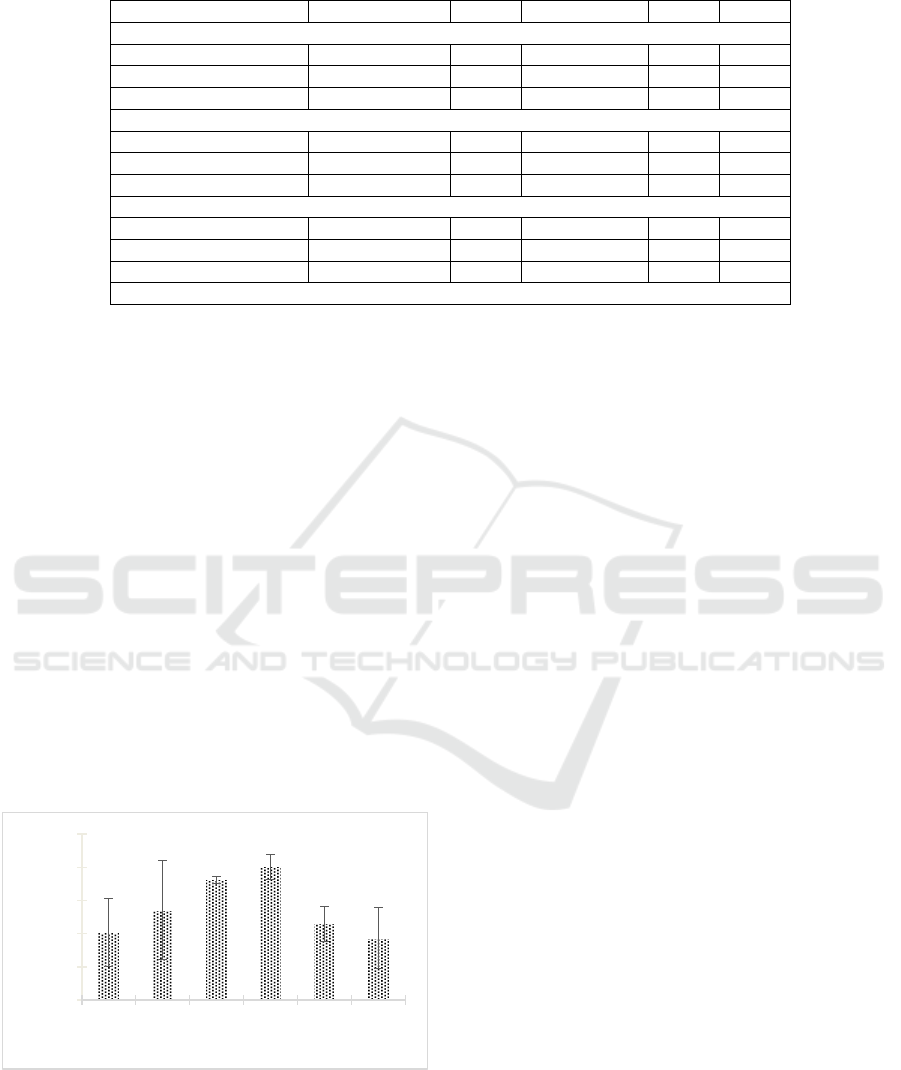
Table 1: Results of one-way ANOVA for antibacterial and antibiofilm activities
Sum of Squares df Mean Square F
*
Sig.
(1) Antibacterial Activit
y
Between Groups 17.209 5 3.442 6.307 0.004
Within Grou
p
s 6.549 12 0.546
Total 23.758 17
(
2
)
Inhibition of biofilm formation
Between Groups 3766.922 5 753.384 2.856 0.630
Within Groups 3165.493 12 263.791
Total 6932.415 17
(
3
)
Biofilm de
g
radation
Between Grou
p
s 4655.138 5 931.028 3.084 0.051
Within Groups 3622.848 12 301.904
Total 8277.986 17
*
Significant value = 0.05
After it reached the optimum incubation time, the rate
of inhibition of biofilm formation decreased.
The analysis results with the one-way ANOVA
test showed no significant effect (Sig. <0.05) between
the incubation time treatment on the percentage of
inhibition of biofilm formation (Table 1). However,
50% inhibition of biofilm formation was achieved
after six days of incubation and reached a maximum
of 12 days of incubation.
3.3 Biofilm Degradation
The research result indicated that the extract could
degrade the biofilm of MRSA 2983 (Figure 3). The
percentage of biofilm degradation increased from day
0 until it reached the optimum incubation time, which
was the 9th day, with the percentage of biofilm
degradation was 80.465% ± 7.586%. After it reached
the optimum incubation time, the rate of biofilm
degradation decreased.
Figure 3: The degradation of biofilm MRSA 2983 by
Streptomyces W-5A extract.
The analysis results using the one-way
ANOVA test showed no significant effect (Sig.
<0.05) between the incubation time treatment on the
percentage of biofilm degradation (Table 1).
However, 50% of biofilm degradation was achieved
after three days incubation and reached a maximum
at nine days incubation.
The tests used the microplate method with BHI-
Glu medium. The clinical MRSA 2983 was added to
a well-filled medium, then incubated for biofilm
formation. After incubation, planktonic cells formed
on the surface, while biofilms formed and stuck
tightly to the well-walls. This adhesion was
accompanied by a build-up of organic materials
covered by an extracellular polymer matrix. This
matrix was a structure of threads crossed with each
other and acted as an adhesive for the biofilm. The
planktonic cells formed were removed by washing,
then the Streptomyces W-5A extract was added to the
well. After incubation, all wells were washed to
remove planktonic bacteria and added crystal violet
to colour the biofilm. Besides using crystal violet,
biofilm quantification can also use resazurin,
safranin, and tryptan blue dyes (Peeters et al., 2008;
Sandasi et al., 2010). In this research, crystal violet
dye was used because it was easy to obtain and
economical in price.
4 DISCUSSION
Actinobacteria have been reported to have
antibacterial activity against MRSA. Bister et al.
(2004) wrote that Abyssomicin C obtained from
actinobacteria has potential as anti-MRSA. León et al.
(2011) reported that the dichloromethane extract from
actinobacteria isolates (I-400A, B1-T61, M10-77)
have high antibacterial activity against MRSA ATCC
43300 and VRE ATCC 51299. Rajan & Kannabiran
(2014) also reported 2,4-dichloro-5-sulfamoyl
benzoic acid (DSBA) extracted from marine
0
20
40
60
80
100
03691215
% Degradation
Incubation (Days)
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
112

Streptomyces sp. VITBRK2 has anti-MRSA activity.
Furthermore, Bhakyashree & Krishnan (2018) wrote
that Streptomyces sp. VITBKA3 is potential as an
anti-MRSA compound against MRSA strains
ATCC43300 and ATCC700699.
Isolate at a particular incubation time that
produces the highest inhibition zone is considered the
optimum time for making antibacterial compounds.
The duration of the production phase of each microbe
varies depending on genetic factors and
environmental conditions. Susilowati et al. (2007)
reported that actinobacteria produced optimal
antibacterial compounds at the optimum incubation
time of 72 hours (isolate A3.5) and 96 hours (isolate
F6.1) against Enteropathogen bacteria. Dhananjeyan
et al. (2012) used an incubation time of five days to
produce antibacterial compounds from actinobacteria
against Escherichia coli MTCC 50, Pseudomonas
aeruginosa MTCC 424, and Bacillus subtilis MTCC
441. Bhakyashree & Krishnan (2018) used an
incubation time of seven days to produce antibacterial
compounds from actinobacteria against MRSA
strains ATCC 43300 and ATCC 700699.
The clinical isolate MRSA 2983 is known as a
biofilm producer. This research used an initial MRSA
concentration of 1.5 × 108 CFU/mL (0.5 McFarland
standard). According to Skogman et al. (2016),
biofilms can be formed with an initial bacterial
concentration of 106-108 CFU/mL. MRSA was
added to the well containing the medium, and then the
extract was added so that the biofilm growth would
coincide with the presence of extract. If the extract
has inhibitory activity, there will be inhibition of
MRSA biofilm formation.
After incubation, the well was washed to remove
planktonic bacteria, then stained with crystal violet.
Crystal violet can bind to proteins and
polysaccharides in the bacterial extracellular matrix
(Peeters et al., 2008). In this research, if, after staining
with crystal violet, a purple colour was formed, the
amount of binding dye was assumed to be the same
as the number of biofilm matrices in the well
(O'Toole, 2011).
Microbes capable of producing biofilms generally
have the potential for resistance to antibiotics.
Bacteria in the biofilm can withstand antibiotics
because the antibiotics fail to penetrate to destroy the
biofilm. Biofilm degradation test is known as an
approach to dealing with biofilms by adding bioactive
compounds that can trigger biofilm destruction
(Belbase et al., 2017; Bjarnsholt et al., 2013; Schierle
et al., 2009). Thus, the biofilm degradation test aims
to determine the potency of extract to degrade biofilm
MRSA.
Bioactive compounds to inhibit biofilm formation
were highly produced at 12 days of incubation in this
research, whereas bioactive compounds to degrade
biofilm were highly made at nine incubation days.
Indeed, the production of anti-MRSA compounds has
various optimum incubation time. Oja et al. (2015)
reported that Streptomyces violaceoruber (DSM-
40701) produced antibiofilm compounds against S.
aureus with an incubation time of four days. Suzuki
et al. (2015) said that Streptomyces sp. strain
MC11024 made antibiofilm compounds against S.
aureus and MRSA N315 with an incubation time of
three days. Balasubramanian et al. (2017) reported
that Streptomyces sp. SBT343 produces antibiofilm
compounds against Staphylococcal bacteria with an
incubation time of ten days.
5 CONCLUSIONS
Streptomyces W-5A isolated from Segara Anakan
Cilacap indicated the potency as anti-MRSA. To our
knowledge, this is the first report of indigenous
Streptomyces with three major anti-MRSA activities,
including antibacterial, biofilm formation inhibition,
and biofilm degradation. This finding highlights
Segara Anakan Cilacap as an essential source of
indigenous microbe for pharmaceutical purposes.
Further research is necessary, particularly for the
isolation and characterization of bioactive
compounds.
ACKNOWLEDGEMENTS
The authors would like to express profound gratitude
for the fundamental research grant contract No.
T/1641/UN23.18/PT.01.03/2020 from the
Directorate of Research and Community Service,
Ministry of Research and Technology/National
Agency for Research and Innovation of the Republic
of Indonesia.
REFERENCES
Archer, N. K., Mazaitis, M. J., Costerton, J. W., Leid, J. G.,
Powers, M. E., & Shirtliff, M. E. (2011).
Staphylococcus aureus biofilms: properties, regulation,
and roles in human disease. Virulence, 2(5), 445–459.
DOI: 10.4161/viru.2.5.17724.
Asnani, A., Ryandini, D., & Suwandri. (2016). Screening
of marine actinomycetes from Segara Anakan for
natural pigment and hydrolytic activities. IOP
The Activities of Streptomyces W-5A as Antibacterial and Antibiofilm towards Methicillin-resistant Staphylococcus aureus 2983
113

Conference Series: Materials Science and Engineering,
107, 12056. DOI: 10.1088/1757-899x/107/1/012056.
Asnani, A., Luviriani, E., & Oedjijono. (2020). Activity of
actinomycetes isolated from mangrove Segara Anakan
Cilacap toward Methicillin-Resistant Staphylococcus
aureus (MRSA). Jurnal Kimia Sains Dan Aplikasi,
23(1), 1–7. DOI: 10.14710/jksa.23.1.1-7.
Balasubramanian, S., Othman, E. M., Kampik, D., Stopper,
H., Hentschel, U., Ziebuhr, W., Oelschlaeger, T. A., &
Abdelmohsen, U. R. (2017). Marine sponge-derived
Streptomyces sp. SBT343 extract inhibits
Staphylococcal biofilm formation. Frontiers in
Microbiology, 8, 236. DOI:
10.3389/fmicb.2017.00236.
Belbase, A., Pant, N. D., Nepal, K., Neupane, B., Baidhya,
R., Baidya, R., & Lekhak, B. (2017). Antibiotic
resistance and biofilm production among the strains of
Staphylococcus aureus isolated from pus/wound swab
samples in a tertiary care hospital in Nepal. Annals of
Clinical Microbiology and Antimicrobials, 16(1), 15-
19. DOI: 10.1186/s12941-017-0194-0.
Bhakyashree, K., & Krishnan, K. (2018). Actinomycetes
mediated targeting of drug-resistant MRSA pathogens.
Journal of King Saud University - Science, 32(1), 260-
264. DOI: 10.1016/j.jksus.2018.04.034.
Bister, B., Bischoff, D., Ströbele, M., Riedlinger, J., Reicke,
A., Wolter, F., Bull, A. T., Zähner, H., Fiedler, H.-P., &
Süssmuth, R. D. (2004). Abyssomicin C-A polycyclic
antibiotic from a marine Verrucosispora strain as an
inhibitor of the p-aminobenzoic acid/tetrahydrofolate
biosynthesis pathway. Angewandte Chemie, 43(19),
2574–2576. DOI: 10.1002/anie.200353160.
Bjarnsholt, T., Alhede, M., Alhede, M., Eickhardt-
Sørensen, S. R., Moser, C., Kühl, M., Jensen, P. Ø., &
Høiby, N. (2013). The in vivo biofilm. Trends in
Microbiology, 21(9), 466–474. DOI:
10.1016/j.tim.2013.06.002.
Boucher, H. W., & Corey, G. R. (2008). Epidemiology of
methicillin-resistant Staphylococcus aureus. Clinical
Infectious Diseases, 46(5), 344–349. DOI:
10.1086/533590.
CLSI. (2019). Performance standards for antimicrobial
susceptibility testing, 29th Edition. CLSI supplement
M100. CLSI: Wayne, PA.
Deshpande, J., & Joshi, M. (2011). Antimicrobial
resistance: The global public health challenge.
International Journal of Students Research, 1(2), 41-
44. DOI: 10.5549/IJSR.1.2.41-44.
Dhananjeyan, V., Selvan, N., & Dhanapal, K. (2012).
Isolation, characterization, screening and antibiotic
sensitivity of actinomycetes from locally (near MCAS)
collected soil samples. Journal of Biological Sciences,
10(6), 514–519.
Høiby, N., Bjarnsholt, T., Givskov, M., Molin, S., & Ciofu,
O. (2010). Antibiotic resistance of bacterial biofilms.
International Journal of Antimicrobial Agents, 35(4),
322–332. DOI: 10.1016/j.ijantimicag.2009.12.011.
Konai, M. M., & Haldar, J. (2017). Fatty acid comprising
lysine conjugates: anti-MRSA agents that display in
vivo efficacy by disrupting biofilms with no resistance
development. Bioconjugate Chemistry, 28(4), 1194–
1204. DOI: 10.1021/acs.bioconjchem.7b00055.
Lee, J.-H., Park, J.-H., Cho, H. S., Joo, S. W., Cho, M. H.,
& Lee, J. (2013). Anti-biofilm activities of quercetin
and tannic acid against Staphylococcus aureus.
Biofouling, 29(5), 491–499. DOI:
10.1080/08927014.2013. 788692.
León, Q. J., Aponte-Ubillus, J., Rojas, R., Cuadra, D.,
Ayala, N., Tomás, G., & Guerrero, M. (2011). Study of
marine actinomycetes isolated from the central coast of
Peru and their antibacterial activity against methicillin-
resistant Staphylococcus aureus and vancomycin-
resistant Enterococcus faecalis. Revista Peruana de
Medicina Experimental y Salud Publica, 28(2), 237–
283. DOI: 10.1590/S1726-46342011000200010.
Memariani, H., Memariani, M., & Ghasemian, A. (2019).
An overview on anti-biofilm properties of quercetin
against bacterial pathogens. World Journal of
Microbiology and Biotechnology, 35(143). DOI:
10.1007/s11274-019-2719-5.
O'Toole, G. A. (2011). Microtiter dish biofilm formation
assay. Journal of Visualized Experiments, (47). DOI:
10.3791/2437.
Oja, T., Galindo, P. S. M., Taguchi, T., Manner, S.,
Vuorela, P. M., Ichinose, K., Metsä-Ketelä, M., &
Fallarero, A. (2015). Effective antibiofilm polyketides
against Staphylococcus aureus from the
pyranonaphthoquinone biosynthetic pathways of
Streptomyces species. Antimicrobial Agents and
Chemotherapy, 59(10), 6046-6052. DOI:
10.1128/AAC. 00991-15.
Peeters, E., Nelis, H., & Coenye, T. (2008). Resistance of
planktonic and biofilm-grown Burkholderia cepacia
complex isolates to the transition metal gallium. The
Journal of Antimicrobial Chemotherapy, 61(5), 1062–
1065. DOI: 10.1093/jac/dkn072.
Pratiwi, S. U. T., Lagendijk, E., Weert, S., Idroes, R.,
Hertiani, T., & Hondel, C. (2015). Effect of
Cinnamomum burmannii Nees ex Bl. and Massoia
aromatica Becc. essential oils on planktonic growth
and biofilm formation of Pseudomonas aeruginosa and
Staphylococcus aureus in vitro. International Journal
of Applied Research in Natural Products, 8(2), 1–13.
Rajan, B. M., & Kannabiran, K. (2014). Extraction and
identification of antibacterial secondary metabolites
from marine Streptomyces sp. VITBRK2. International
Journal of Molecular and Cellular Medicine, 3(3), 130-
137.
Sandasi, M., Leonard, C. M., & Viljoen, A. M. (2010). The
in vitro antibiofilm activity of selected culinary herbs
and medicinal plants against Listeria monocytogenes.
Letters in Applied Microbiology, 50(1), 30–35. DOI:
10.1111/j.1472-765X.2009.02747.x.
Schierle, C. F., De la Garza, M., Mustoe, T. A., & Galiano,
R. D. (2009). Staphylococcal biofilms impair wound
healing by delaying reepithelialization in a murine
cutaneous wound model. Wound Repair and
Regeneration, 17(3), 354–359. DOI: 10.1111/j.1524-
475X.2009.00489.x.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
114

Skogman, M. E., Vuorela, P. M., & Fallarero, A. (2016). A
platform of anti-biofilm assays suited to the exploration
of natural compound libraries. Journal of Visualized
Experiments, (118), 54829. DOI: 10.3791/54829.
Susilowati, D. N., Hastuti, R. D., & Yuniarti, E. (2007).
Isolasi dan karakterisasi aktinomisetes penghasil
antibakteri Enteropatogen Escherichia coli K1.1,
Pseudomonas pseudomallei 02 05, dan Listeria
monocytogenes 5407. Jurnal AgroBiogen, 3(1), 15–23.
DOI: 10.21082/jbio.v3n1. 2007.p15-23.
Suzuki, N., Ohtaguro, N., Yoshida, Y., Hirai, M., Matsuo,
H., Yamada, Y., Imamura, N., & Tsuchiya, T. (2015).
A compound inhibits biofilm formation of
Staphylococcus aureus from Streptomyces. Biological
and Pharmaceutical Bulletin, 38(6), 889-892. DOI:
10.1248/bpb.b15-00053.
Tarver, T. (2009). Biofilms: A threat to food safety. In Food
Technology, 5(63).
You, Y., Xue, T., Cao, L., Zhao, L., Sun, H., & Sun, B.
(2014). Staphylococcus aureus glucose-induced
biofilm accessory proteins, GbaAB, influence biofilm
formation in a PIA-dependent manner. International
Journal of Medical Microbiology 304(5–6), 603–612.
DOI: 10.1016/j.ijmm.2014.04.003.
The Activities of Streptomyces W-5A as Antibacterial and Antibiofilm towards Methicillin-resistant Staphylococcus aureus 2983
115
