
Antibiofilm Activity of Aloe barbadensis Miller Extract against
Staphylococcus aureus
Kanesti Ismirajna
1
a
and Irena Agustiningtyas
1
b
1
Medical Study Program, Faculty of Medicine, Universitas Islam Indonesia, Yogyakarta, Indonesia
2
Department of Microbiology, Faculty of Medicine, Universitas Islam Indonesia, Yogyakarta, Indonesia
Keywords: Biofilm, Staphylococcus aureus, Aloe barbadensis, microtiter plate, antibiofilm activity.
Abstract: Background: Aloe barbadensis Miller is a type of plant that can potentially inhibit the growth of bacteria
causing infections. Improper use of antibiotics can result in the occurrence of bacterial resistance caused by
the formation of biofilms on a living creature. The goal of this study was to investigate the antibiofilm activity
of Aloe barbadensis Miller against the biofilm produced by Staphylococcus aureus. This study is classified as
an experimental in vitro with the post-test only control group design. The Aloe barbadensis Miller rind
macerated with methanol solvent. The extract was divided into concentrations of 20%, 10%, 5%, 2.5%, 1.25%
and 0.63%, tested against biofilm formation by Staphylococcus aureus ATCC 25923. The antibiofilm activity
was tested by microtiter plate biofilm assay method with 3 replications on a 96 well round-bottom microplate.
Then observed by calculating the value of optical density using a microplate reader (λ = 620 nm). Extract
concentrations 20%, 10%, 5%, 2.5%, 1.25% and 0.63% showed a minimum percentage of inhibition of
biofilm concentration were -30.3%; 10.9%; 10.5%; 45.9%; 19.3% and 16.3% respectively. Statistical analysis
using One-Way ANOVA (p = 0.008) followed by post-hoc LSD which showed a concentration of 2.5% had
no significant difference (p > 0.05) with positive control using ciprofloxacin. Although the methanol extract
of Aloe barbadensis Miller rind showed inhibitory activity against S. aureus biofilms was equivalent to
positive control but had a Minimum Biofilm Inhibition Concentration (MBIC50) percentage <50%.
1 INTRODUCTION
Staphylococcus aureus is one of the most common
causes of bacterial infection in humans worldwide
(Singh et al., 2017; Chen et al., 2014; Tong, et al.,
2015). S. aureus can produce an extracellular polymer
matrix consisting of polysaccharides, air, nucleic
acids, proteins, and extracellular DNA (Archer et al.,
2011). This matrix ensures the survival of the biofilm
colony and protects it against the phagocytic activity
of macrophages, the host immune system,
temperature, and pH fluctuations (Leseigneur et al.,
2020). Antibiotic therapy, in general, will only kill
planktonic bacterial cells, while bacteria that are
tightly arranged in biofilm will remain alive so that
they can cause chronic infections that resistant to
antibiotics (Tong et al., 2015; Zaman et al., 2017).
The Indonesian Ministry of Health (2015) states that
from the 2013 WHO data, the death rate due to
a
https://orcid.org/0000- 0003-2009-1029
b
https://orcid.org/0000-0003-4870-9484
resistance is around 700 thousand people per year
(Kemenkes, 2015). It's estimated, by 2050 the death
rate could increase to 10 million per year. The
existence of biofilms has the opportunity to develop
drugs by utilizing the same bioactive plants (Abraham
et al.,2012). Also, Indonesia is mega biodiversity
which is rich in medicinal plants that have the
potential to be developed (Kusmana et al., 2015).
Aloe vera (Aloe barbadensis Miller), is currently
processed as food, drink, and medicine. However,
only part of the gel is used, while the skin of the
leaves becomes waste (Aryanti et a., 2013). The
active ingredients that have been identified in the
extract of the bark of the leaves of Aloe barbadensis
Miller include saponins, sterols, acemannan, and
anthraquinones which are toxic to bacterial cells
(Marimuthu et al., 2012; Benzidia et al., 2018;
Devaraj et al., 2011). Through this research,
hopefully that the incidence of S. aureus resistance
84
Ismirajna, K. and Agustiningtyas, I.
Antibiofilm Activity of Aloe barbadensis Miller Extract Against Staphylococcus aureus.
DOI: 10.5220/0010488200840090
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 84-90
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

can be overcome with antibiofilm as an alternative
therapy that can reduce the prevalence of infection.
The purpose of this study was to determine the
antibiofilm activity of the methanol extract of the
Aloe barbadensis Miller leaf bark against the biofilm
of Staphylococcus aureus ATCC 25923..
2 MATERIALS AND METHODS
2.1 Research Design
This type of research is experimental in vitro study
with a post-test only control group design. The effect
of Aloe barbadensis Miller in inhibiting biofilm of S.
aureus ATCC 25923 using the Microtiter Plate
Biofilm Assay method(14). S. aureus ATCC 25923
was bred from the Yogyakarta Health Center.
2.2 Time and Place of Research
The process of determining aloe vera plants is carried
out at the Plant Systematics Laboratory of the Faculty
of Biology, Gadjah Mada University. The plant
extraction process is carried out at the Integrated
Laboratory of the Faculty of Mathematics and Natural
Sciences, Islamic University of Indonesia, in the
division of Pharmaceutical Biology. Testing the
potential of Aloe barbadensis Miller leaf bark extract
against Staphylococcus aureus ATCC 25923 biofilm
was conducted at the Microbiology Laboratory of the
Faculty of Medicine, Islamic University of Indonesia.
The research was conducted in June-July 2020.
2.3 Research Tools and Materials
The tools used in this study include a round-bottom
microplate, microplate reader, analytical scale,
refrigerator, oven, autoclave, incubator, Laminar Air
Flow (LAF), bunsen, loop wire, beaker glass,
Erlenmeyer, petri dish, test tube, test tube rack,
cotton, micropipette, micro tip, dropper pipette,
mortar, pestle, vial, microscope, Staphylococcus
aureus ATCC 25923 bacteria, sterile distilled water,
physiological NaCl, Gram stain, McFarland solution,
nutrient agar (NA) media, nutrient broth (NB) media,
catalase, and coagulase reagents.
2.4 Time and Place of Research
The process of determining aloe vera plants is carried
out at the Plant Systematics Laboratory of the Faculty
of Biology, Gadjah Mada University. The plant
extraction process is carried out at the Integrated
Laboratory of the Faculty of Mathematics and Natural
Sciences, Islamic University of Indonesia, in the
division of Pharmaceutical Biology. Testing the
potential of Aloe barbadensis Miller leaf bark extract
against Staphylococcus aureus ATCC 25923 biofilm
was conducted at the Microbiology Laboratory of the
Faculty of Medicine, Islamic University of Indonesia.
The research was conducted in June-July 2020.
2.5 Preparation of Aloe Barbadensis
Miller Leaf Bark Extract and
Secondary Metabolites Test.
Aloe vera leaves are washed with tap water and then
peeled to separate the gel and the skin of the leaves.
The extraction process starts from chopping the
sample, drying the sample in a dry cabinet,
pollinating the alar miller, macerated dry Simplicia
with methanol solvent, filtering, evaporation, and
packaging then testing phytochemicals.
2.6 Purification and Characterization
of Test Bacteria
Purification and characterization of tested bacteria by
inoculating bacteria on NA media with streak plate
techniques, gram staining, catalase test, and catalase
test on glass objects (Kaiser et al., 2016).
2.7 Determination of the Minimum
Inhibitory Level (MIL) and
Minimum Killing Level (MKL).
The dilution series of extract concentrationstested
were 20%, 10%, 5%, 2.5%, 1.25% and 0.63%
compared to positive control (Ciprofloxacin 0.00125
mg/ml), negative control (bacterial suspension). and
media control which was carried out 3 times
replication (Bazargani et al., 2017)
2.8 The Biofilm Formation Test of
Staphylococcus aureus ATCC
25923
Two bacteria were used in the biofilm formation test,
S. aureus ATCC 25923 and S. epidermidis non-
biofilm as a negative control, tested 3 times. The
microplate was washed 3 times with sterile phosphate
buffer saline (PBS) (Teanpasian et al., 2016). Then
stained with 0.1% crystal violet and then rinsed 3-4
times with sterile distilled water (Lestari et al., 2017).
Then add 275 µL of 33% acetic acid to each well to
dissolve the crystal violet and incubate for 10-15
Antibiofilm Activity of Aloe barbadensis Miller Extract Against Staphylococcus aureus
85
minutes. Then OD (Optical Density) was measured
with a microplate reader with a wavelength of 620
nm. OD cut off is calculated by the formula:
OD cut off = O average negative control +
(3x standard deviation OD negative control)
(Singh et al., 2017)
2.9 The Antibiofilm Performance Test
Antibiofilm testing was done using the microdilution
method with a series of dilutions using a 96 well
round-bottom microplate. The group used was the
same as the test group in the antibacterial test but
incubated for 2 days. Washing was done using the
same as in the biofilm formation test. Then entered
into the formula MBIC50 [Inhibitory% = {((OD-
negative control test group) / (OD negative control)}
x 100}] (Singh et al., 2017).
2.10 Data Analysis Method
The data obtained were analyzed using SPSS. The
inhibition of S. aureus ATCC 25923 biofilm
formation was obtained from the Optical Density
(OD) value measured using a microplate reader. The
data were analyzed using Shapiro Wilk and Levene's
test to determine the distribution and homogeneity of
the data. Data that is normally distributed (p> 0.05) is
followed by the One-Way ANOVA test with testing
criteria based on probability, namely H0 is accepted
if p value> 0.05 and H0 is rejected if p-value <0.05.
Then the post hoc test of Least Significant Difference
(LSD) was carried out to determine the MBIC50
(Minimum Biofilm Inhibitory Concentration 50%).
2.11 Research Ethics
This research received permission from the Ethics
Committee for Medical and Health Research, Faculty
of Medicine, Islamic University of Indonesia with
number 4 / Ka.Kom.Et / 70 / KE / XII / 2019.
3 RESULTS
Based on the results of plant determination, it was
proven that the plant used in this study was Aloe vera
(L.) Burm.f. with the synonym Aloe barbadensis
Miller. Then from 900 grams of fresh material,
6.1223 grams of extract were obtained. The extract
was tested for secondary metabolites and it was found
that it contains active compounds of flavonoids,
alkaloids, saponins, and polyphenols. The results of
purification and characterization of S. aureus ATCC
25923 bacteria on nutrient media to obtain the results
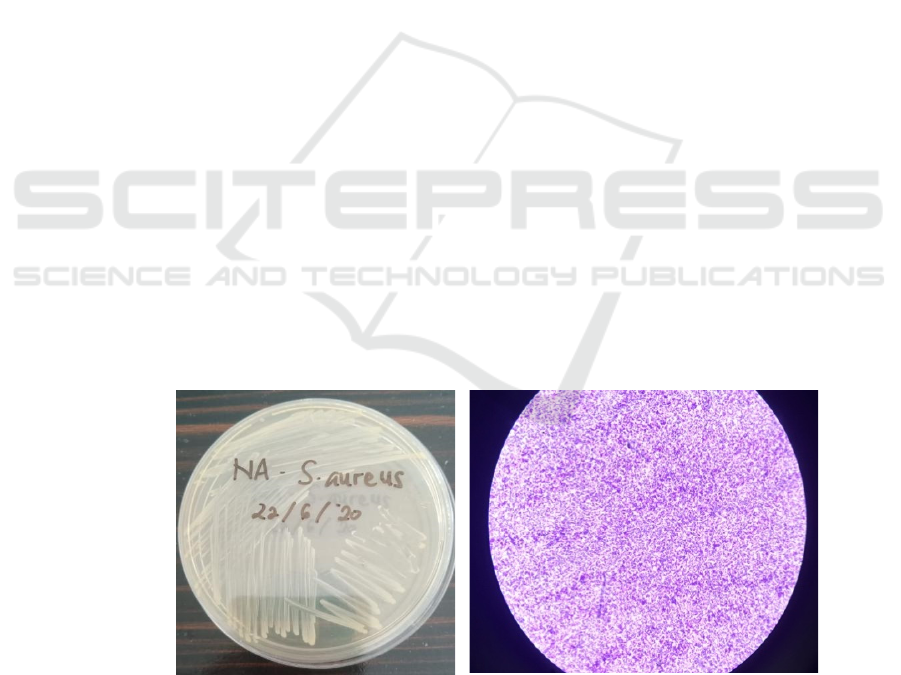
of bacterial colonies that grow yellowish white. Gram
staining obtained the test bacteria showing a purple
colour, cocci-shaped, single or clustered irregularly
like grapes and without spores Figure 1. The catalase
and coagulase
test for bacteria each showed positive results
indicated by the presence of gas bubbles (O2) or foam
and the formation of clots / deposits Figure 2(19)(20).
Figure 1. Purification and characterization of S. aureus bacteria on NA media (left) and microscopic appearance at 100x
magnification (right).
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
86
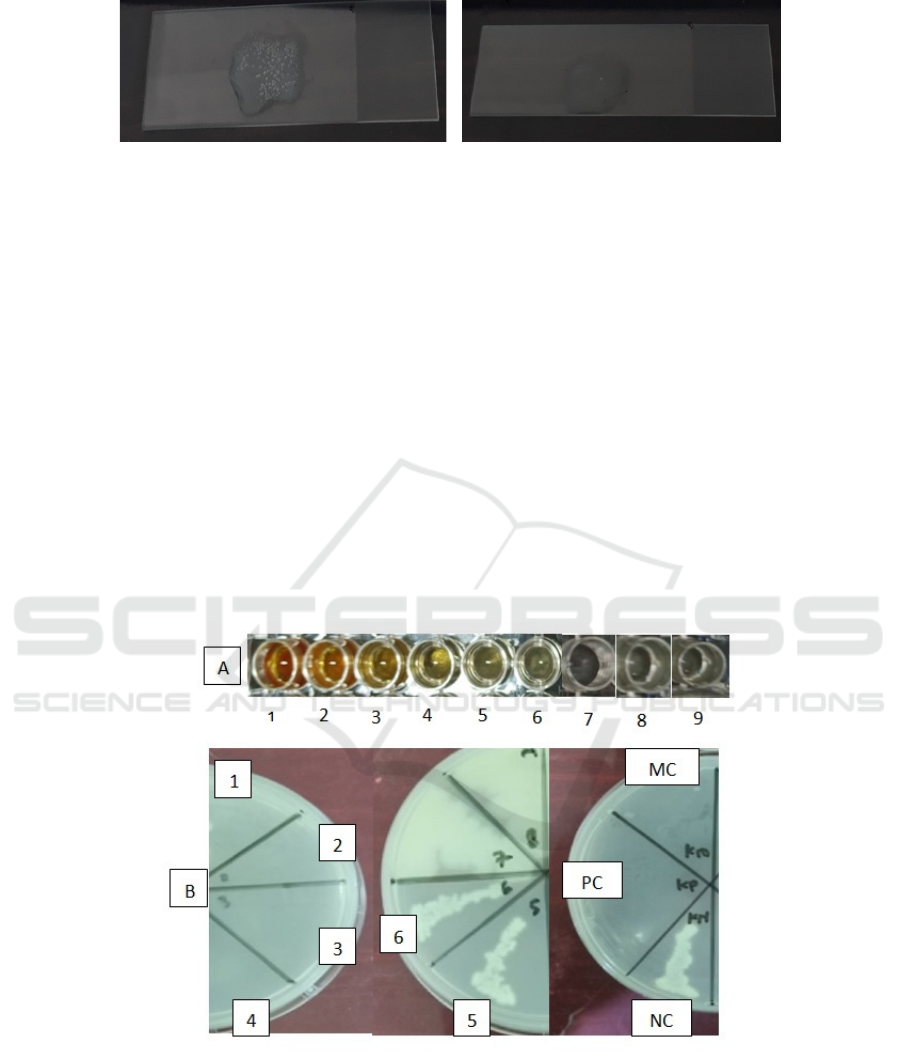
Figure 2. Positive catalase test results, there are air bubbles (left); The coagulase test results are positive, there is clamping
(right).
The results of the antibacterial test on NA media
with Petri dish 1 to 4 were clear and no visible
colonies were growing. Meanwhile, in Petri dish 5
and 6, the growth of S. aureus was shown Figure 3.
The results of the S. aureus biofilm formation test
were formed purple biofilm rings on the good walls.
The absorbance / OD values of S. aureus and S.
epidermidis non-biofilm formation tests were
obtained in Table 1 and the antibiofilm test in Table
2.
The absorbance value was analyzed using
statistical tests. The results of the distribution
normality test using the Shapiro-Wilk test, obtained a
p value> 0.05 in each test solution group, meaning
that the data were normally distributed. Levene's test
obtained a p-value <0.05 in each test solution group,
meaning that the data did not have the same variance.
Obtained normal data distribution and variance which
are not the same. One-Way ANOVA test obtained a
p-value of 0.008 which indicates that there is a
significant difference in the test solution group. In the
post-hoc LSD test (Table 3) it was found that some
groups showed significant differences with p-value
<0.05 (significant result) at a concentration of 20%
with a concentration of 2.5%, a concentration of 20%
with 1.25%, a concentration of 20 % with the positive
control, 20% concentration with media control, 10%
concentration with media control, 5% concentration
with media control, 1.25% concentration with media
control, 0.63% concentration with media control,
negative control with the positive control, control
media with a concentration of 5% and control media
with the negative control.
Figure 3. Antibacterial test result in A) microplate and B) nutrient agar.
Information
1. 20% Concentrate
2. 10% Concentrate
3. 5% Concentrate
4. 2,5% Concentrate
5. 1,25% Concentrate
6. 0,625% Concentrate
7. Positive Control (Ciprofloxacin)
8. Negative Control (bacterial suspension
9. Media Control
Antibiofilm Activity of Aloe barbadensis Miller Extract Against Staphylococcus aureus
87
Table 1. Biofilm Inhibition Optical Density Staphylococcus aureus ATCC 25923
Treatment
Absorbance Average %
Inhibition
Re
p
lication 1 Re
p
lication 2 Re
p
lication 3
Extract 20% 0.5495 0.6840 1.6010 0.9448 -30.35
Extract 10% 0.6053 0.5537 0.7781 0.6457 10.92
Extract 5% 0.7637 0.6089 0.5734 0.6487 10.51
Extract 2.5% 0.4006 0.3621 0.4122 0.3916 45.97
Extract 1.25% 0.4969 0.5847 0.6720 0.5845 19.36
Extract 0.625% 0.5231 0.7644 0.5318 0.6064 16.33
Positive Control 0.2443 0.2894 0.3663 0.30 58.61
Negative Control 0.6326 0.8336 0.7083 0.7248
Media Control 0.1214 0.1721 0.1502 0.1479
Table 2.Post-hoc analysis result LSD type
E20% E10% E5% E2.5% E1.25% E0.625% KP KN KM
E20% - 0.097 0.100 0.005 0.049 0.063 0.001 0.214 0.000
E10% 0.097 - 0.986 0.154 0.724 0.821 0.058 0.648 0.009
E5% 0.100 0.198 - 0.149 0.712 0.807 0.056 0.661 0.009
E2.5% 0.005 0.154 0.149 - 0.273 0.224 0.598 0.067 0.170
E1.25% 0.049 0.724 0.712 0.273 - 0.899 0.113 0.422 0.020
E0.625% 0.063 0.821 0.807 0.224 0.899 - 0.089 0.497 0.015
KP 0.001 0.058 0.056 0.598 0.113 0.089 - 0.023 0.385
KN 0.214 0.648 0.661 0.067 0.422 0.497 0.023 - 0.003
KM 0.000 0.009 0.009 0.170 0.020 0.015 0.385 0.003 -
3
Information: E = extract consentration; KP = Positive Control; KN = Negative Control; KM = Media Control.
4 DISCUSSION
Table 1 shows the ODcut value calculated from the
negative control (S. epidermidis non-biofilm) of
0.1341. The average S. aureus biofilm with a value of
0.3991 is a value that is between 2xODcut and
4xODcut. So it can be concluded that the S. aureus
bacteria used in this study were moderate biofilm-
former (Singh et al., 2020).
Based on the microplate reader result in Table 2.
Higher OD score indicates the increasing survived
biofilm microorganism amount (Locke et al., 2012).
From the percentage inhibition formula, it was found
that the higher the absorbance value / OD of the test
solution, the lower the percentage value of inhibition
(inversely proportional). The absorbance/OD value of
Aloe vera leaf extract which was the best in inhibiting
the growth of S. aureus biofilm was produced at a
concentration of 2.5%, namely 0.3918 and had an
inhibition percentage of 45.9% (Teanpaisan et al.,
2017). When compared with a positive control
containing Ciprofloxacin and bacterial suspension, it
had an absorbance / OD value of 0.3000 and an
inhibition percentage of 58.6%. This shows that the
percentage of inhibition in the positive control is
higher than the series test solution for the dilution of
the extract concentration. The percentage value of
inhibition by the series dilution test solution with the
concentration of 2.5% extract did not reach the
MBIC50 requirement, called the minimum inhibitory
concentration of biofilm of 50% (Pirbalouti et al,
2010; Pratiwi et al., 2015).
The results of statistical analysis, the post hoc
LSD test showed that the extract concentration of
2.5% had the best value based on the percentage of
MBIC50 compared to the concentration of other
extracts. The extract concentration of 2.5% had a
significance value of 0.113 against the positive
control group, the antibiotic Ciprofloxacin. This
indicates a meaningless relationship. Thus, the extract
concentration of 2.5% had the same ability as the
positive control in inhibiting S. aureus biofilm. So, it
can be concluded that although the methanol extract
of aloe vera leaf bark (Aloe barbadensis Miller) has
statistically shown inhibitory activity against S.
aureus biofilms is equivalent to that of positive
controls, it has a Minimum Biofilm Inhibition
Concentration (MBIC50) percentage <50%.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
88

Several factors can cause the methanol extract of
the aloe leaf bark (Aloe barbadensis Miller) to have
MBIC50 <50%. First, the methanol solvent was used
in the extraction of the test plants. The methanol has
a polar group that is stronger than its nonpolar group,
this can be seen from the chemical structure of
methanol which contains a hydroxyl group (polar)
and a carbon group (nonpolar). The methanol can
extract a greater amount of phytochemical
compounds so that it can extract more bioactive
components that have higher polarity properties
(Seidel et al., 2012).
According to Seidel (2012), the high polarity
index in methanol solvents can extract secondary
metabolites that have polar properties such as
flavonoids glycosides, tannins, and some alkaloids.
This solvent is also effective for extracting phenolic
compounds with low molecular weight and moderate
polarity levels (Lin et al., 2009) flavonoid aglycones
(Dehkarghanian et al., 2010) anthocyanins,
terpenoids, saponins, flavones, and polyphenol
compounds. Non-polar solvents such as n-hexane
which has a zero polarity index are effective in
dissolving lipophilic compounds, such as alcanas,
waxes, colour pigments, sterols, some terpenoids, and
alkaloids (Romadanu et al., 2014).
Lawrence et al, shows that the ethanol extract of
Aloe vera gel has a wider diameter of inhibition zone
against S. aureus bacteria than the methanol extract
and acetone extract of Aloe vera gel. It is suspected
that the methanol extract of Aloe barbadensis Miller
leaf bark used in this study has not been able to
dissolve other secondary compounds/metabolites that
are lipophilic (Lawrance et al., 2009).
Also, considering the effect produced by the
methanol extract of the Aloe barbadensis Miller is
still the result of the combined work of various
secondary compounds/metabolites that can affect the
mechanism of action of one compound with another.
So for further development, it is necessary to isolate
pure compounds for antibiofilm activity tests.
5 CONCLUSION
The methanol extract of aloe vera leaf bark (Aloe
barbadensis Miller) has an inhibitory activity against
S. aureus biofilms equivalent to positive controls but
has a Minimum Biofilm Inhibition Concentration
(MBIC50) <50%.
ACKNOWLEDGMENTS
Thank you to the author, to the dean of the UII
Medical Faculty who has permitted to research at the
Microbiology Laboratory.
REFERENCES
Singh A, Prakash P, Achra A, Singh G, Das A, Singh R.,
2020. Standardization and classification of in vitro
biofilm formation by clinical isolates of
Staphylococcus aureus. J Glob Infect Dis, [Internet],
9(3), pp. 93–101.
Chen CJ, Huang YC., 2014. New epidemiology of
Staphylococcus aureus infection in Asia. Clin
Microbiol Infect, [online], 20(7), pp. 605–23.
Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler
VG., 2015. Staphylococcus aureus Infections:
Epidemiology, Pathophysiology, Clinical
Manifestations, and Management. Clin Microbiol Rev,
[online], 28(3), pp. 603-61.
Archer NK, Mazaitis MJ, William Costerton J, Leid JG,
Powers ME, Shirtliff ME., 2011. Staphylococcus
aureus biofilms: Properties, regulation and roles in
human disease. Virulance, 2, pp. 445–59.
Leseigneur C, Lê-Bury P, Pizarro-Cerdá J, Dussurget O.,
2020. Emerging Evasion Mechanisms of Macrophage
Defenses by Pathogenic Bacteria. Front Cell Infect
Microbiol, 10, pp. 1-9.
Zaman S Bin, Hussain MA, Nye R, Mehta V, Mamun KT,
Hossain N. A Review on Antibiotic Resistance: Alarm
Bells are Ringing. Cureus, [online], 9(6), pp. e10403.
Kementrian Kesehatan Republik Indonesia., 2015.
Penggunaan Antibiotik Bijak dan Rasional Kurangi
Beban Penyakit Infeksi. [online], cited 2020 Nov 8.
Available from:
https://www.kemkes.go.id/article/view/15081100001/
penggunaan-antibiotik-bijak-dan-rasional-kurangi-
beban-penyakit-infeksi.html
Abraham KP, Srieenivas J, Venkateswarulu TC, Indira M,
Babu DJ, Diwakar T, et al., 2012. Investigation of the
potential anti biofilm activities of plant extracts. Int J
Pharm Pharm Sci, [online] 4(4), pp. 282–5.
Kusmana C, Hikmat A., 2015. The Biodiversity of Flora in
Indonesia. J Nat Resour Environ Manag, [online] 5(2),
pp. 187–98.
Aryanti, N.K., Darmayasa, I.B.G., Sudirga S.K., 2013.
Daya Hambat Ekstrak Kulit Daun Lidah Buaya (Aloe
barbadensis Miller) terhadap pertumbuhan bakteri
staphylococcus aureus ATCC 25923 dan Escherichia
coli ATCC 25922. J Biol, [online] 16(1), pp.1–4.
Marimuthu , A.A., Malar, R, J, J., Beaulah, N., Laju, R.S.,
Anupriya, G., Renola, J. J. E. T., 2012. Anti-Bacterial
and Antifungal Activity of Aloe Vera Gel Extract. Int J
Biomed Adv Res, [online] 3(3), pp. 184–7.
Benzidia, B., Barbouchi, M., Hammouch, H., Belahbib, N.,
Zouarhi, M., Erramli, H., et al., 2018. Chemical
Antibiofilm Activity of Aloe barbadensis Miller Extract Against Staphylococcus aureus
89

composition and antioxidant activity of tannins extract
from green rind of Aloe vera (L.) Burm. F. J King Saud
Univ – Sci,[online] 31(4), pp. 1175–81.
Devaraj, a., Karpagam, T., 2011. Evaluation of anti-
inflammatory activity and analgesic effect of aloe vera
leaf extract in rats. Int Res J Pharm, [online] 2(3), pp.
103–10.
O’Toole, G.A., 2010. Microtiter dish Biofilm formation
assay. J Vis Exp, [online] 47, pp. 10–1.
, G., 2016. Lab 15: Isolation and Identification of
Staphylococci - Biology LibreTexts. LibreTexts,
[online] 1–12.
Mohammadi-Bazargani, M., Rohloff, J., Atapour, M.,
2017. Comparative Analysis of Essential Oil and
Different Extracts of Ajuga chamaecistus ssp. scoparia
on Antibacterial Activity. J Essent Oil-Bearing Plants,
[online], 20(4), pp. 1117–24.
Teanpaisan, R., Kawsud, P., Pahumunto, N.,
Puripattanavong, J., 2016. Screening for antibacterial
and antibiofilm activity in Thai medicinal plant extracts
against oral microorganisms. J Tradit Complement
Med, [online] 7(2), pp. 172–7.
Lestari, D.R.S., Soegianto, L., Hermanu, L.S., Potensi
Antibakteri dan Antibiofilm Ekstrak Etanol Bunga
Bintaro ( Cerbera odollam ) terhadap Staphylococcus
aureus ATCC 6538. J Pharm Sci. 2017;4(1):30–5.
Gillaspy, A.F., Iandolo, J.J., Tang, Y.W., Stratton, C.W.,
2019. Staphylococcus. In: Encyclopedia of
Microbiology. Elsevier. p. 309–20.
Locke, T., Keat, S., Walker, A., Mackinnon, R., Read, R.C.,
2012. Microbiology and infectious diseases on the
move. Microbiology and Infectious Diseases on the
Move. CRC Press, [online]. pp. 1–242 p.
Rollando, R., Susilo, Y.P.A., Sitepu, R., 2015. Uji
Antimikroba Minyak Atsiri Masoyi ( Massoia
aromatica ) Terhadap Bakteri Streptococcus mutans.
Maj Farm dan Farmakol, [online] 23(2), pp. 52–7.
Pirbalouti, A.G., Moosavi, S.H., Momtaz H., Rahimi, E.,
Hamedi, B., 2010. Antibacterial activities of the
essential oils of some Iranian herbs against
Campylobacter jejuni and Campylobacter coli. Adv
Food Sci, [online] 32(1), pp. 30–4.
Pratiwi, S.U.T., Lagendijk, E.L., de Weert, S., Idroes, R.,
Hertiani, T., Van den Hondel, C., Effect of
Cinnamomum burmannii Nees ex Bl. and Massoia
aromatica Becc. Essential oils on planktonic growth
and biofilm formation of Pseudomonas aeruginosa and
Staphylococcus aureus In Vitro. Int J Appl Res Nat
Prod; [online], 8(2), pp. 1–13.
Seidel, V., 2012. Initial and bulk extraction of natural
products isolation. Methods Mol Biol, [online], 864, pp.
27–41.
Lin, H.Y., Kuo, Y.H., Lin, Y.L., Chiang, W., 2009.
Antioxidative Effect and Active Components from
Leaves of Lotus (Nelumbo nucifera). J Agric Food
Chem, [online], 57 (15), pp. 6623–9.
Dehkharghanian, M., Adenier, H., Vijayalakshmi, M.A.,
2010. Study of flavonoids in aqueous spinach extract
using positive electrospray ionisation tandem
quadrupole mass spectrometry. Food Chem, [online]
121(3):863–70.
Romadanu, R., Hanggita, S., Lestari, S., 2014. Pengujian
Aktivitas Antioksidan Ekstrak Bunga Lotus (Nelumbo
nucifera). J FishtecH, [online] 3(1), pp. 1–7.
Lawrence, R., Tripathi, P., Jeyakumar, E., 2009. Isolation,
purification and evaluation of antibacterial agents from
Aloe Vera. Brazilian J Microbiol, [online], 40(4), pp.
906–15.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
90
