
Low-dose of Acetylsalicylic Acid Upregulates Expression of eNOS
mRNA and Downregulates Interleukin-6 (IL-6) and Transforming
Growth Gactor-β1 (TGF-β1) mRNA in Rat Kidney of Preeclampsia
Model
Yuyun Nailufar
1a
, Rul Afiyah Syarif
2b
, Andi Fitriani Kusuma
3c
, Farmita Chairani
3d
,
Nia Marlina
4e
, Charolina Vivi Vienetta
4f
, M. Hadri Ar-Ridho
4g
, Totok Utoro
5h
, Nur Arfian
1i
1
Department of Anatomy, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta,
Indonesia
2
Department of Pharmacology and Therapy, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada,
Yogyakarta, Indonesia
3
Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
4
Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia
5
Department of Anatomical Pathology, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada,
Yogyakarta, Indonesia
Keywords: Preeclampsia, Inflammation, Kidney Injury, IL-6, TGF-β1, Endothelial Dysfunction
Abstract: Preeclampsia leads to endothelial dysfunction and kidney injury with inflammation and fibrosis. Low-dose
acetylsalicylic acid (ASA) administration may decrease uterine artery resistance; however, its effect on the
kidney has not been elucidated yet. We performed a preeclampsia model in pregnant female Wistar rats (PE
group, n=5, 150-200 grams) using L-NAME 50mg/kg of BW intraperitoneal injection on day 1-18 of
pregnancy. Low doses of ASA with dose 75 (PE+ASA75) and 125 (PE+ASA125) mg/kg body weight were
administered in preeclampsia rats in the day 10-12 day of pregnancy. The Control group was normal pregnant
rats with NaCl treatment (n=5). On day 18, rats were sacrificed; kidneys were harvested, then extracted for
Reverse Transcriptase-PCR (RT-PCR) of eNOS, TGF-β1, and IL-6 mRNA expression measurements.
Proteinuria and rat blood pressure were measured before termination. L-NAME injection-induced
preeclampsia showed significantly higher systolic blood pressure and proteinuria in the PE group than in the
control group (p<0.05). However, there were no changes in podocin and nephrin expression. In conclusion,
the low dose of ASA 125mg/Kg BW ameliorates kidney inflammation and TGF-β1 expression in the rat
preeclampsia model's kidney.
1 INTRODUCTION
The mortality of pregnant women still becomes a
health problem, especially in developing countries.
a
https://orcid.org/0000-0001-8281-4769
b
https://orcid.org/0000-0001-8114-9322
c
https://orcid.org/0000-0002-4803-1434
d
https://orcid.org/0000-0003-1515-7003
e
https://orcid.org/0000-0002-4041-8087
f
https://orcid.org/0000-0001-6082-4448
g
https://orcid.org/0000-0002-9543-3110
h
https://orcid.org/0000-0002-1823-6295
i
https://orcid.org/0000-0003-1694-2054
The major causes of maternal mortality during 2010-
2013 are bleeding, hypertension during pregnancy,
and abortion (Linggardini and Aprilina, 2016). In
recent years preeclampsia and eclampsia are the
leading causes of maternal mortality (Pellicer et al.,
Nailufar, Y., Syarif, R., Kusuma, A., Chairani, F., Marlina, N., Vienetta, C., Ar-Ridho, M., Utoro, T. and Arfian, N.
Low-dose of Acetylsalicylic Acid Upregulates Expression of eNOS mRNA and Downregulates Interleukin-6 (IL-6) and Transforming Growth Gactor-1 (TGF-1) mRNA in Rat Kidney of
Preeclampsia Model.
DOI: 10.5220/0010487800630068
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 63-68
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
63

2011), with the number of preeclampsia in
developing countries ranging from 2-8%.
Preeclampsia is a condition in pregnancy
characterized by hypertension (systolic pressure ≥140
mmHg and diastole ≥90 mmHg) and proteinuria ≥300
mg in24 hours. There is still no effective treatment for
preeclampsia (Kemenkes RI, 2014; Rachmi and
Sulistyono, 2016).
The pathogenesis of preeclampsia is related to
trophoblast invasion of the spiral arteries of the
uterus. This condition causes decreased
uteroplacental blood flow and placental ischemia.
Placental ischemia triggers tissue hypoxia, a release
of oxidative stress, and anti-angiogenic factors'
release into the maternal circulation, such as sFlt1.
Increased levels of sFlt1 can cause a decrease in
vascular endothelial growth factor (VEGF) and
placental growth factor (PIGF) levels. VEGF and
PIGF play a role in maintaining the integrity of
endothelial cells in the body. Decreased VEGF and
PIGF in preeclampsia are believed to be the cause of
systemic endothelial cell dysfunction and
microangiopathy (Oparil et al., 2003).
One genetic predisposition that plays a role in
triggering preeclampsia is an endothelial nitric oxide
synthase (eNOS) gene disorder regulating nitric oxide
activation. Nitric oxide (NO) is an endothelial
vasodilator with functions as antithrombotic and
atheroprotective. Under normal circumstances, the
NO pathway is activated and increases the levels of
NO in vessels. Increased NO levels are responsible
for the maternal vasodilatation required to
accommodate increased circulating volume during
pregnancy without raising blood pressure. However,
in preeclampsia, this process of adaptation is
disrupted, an endothelial disorder occurs. Thus blood
pressure increases, and proteinuria develops. The
decrease in NO production is associated with
polymorphisms in genes that regulate NO production,
namely the endothelial nitric oxide synthase (eNOS)
gene (Suharto et al., 2014).
Endothelial impairment in the kidneys caused by
a decrease in VEGF will lead to glomerular capillary
endothelins and proteinuria. Several studies have
shown that interleukin-6 (IL-6) and transforming
growth factor-β1 (TGF-β1) play a role in renal
disease progression. IL-6 can cause kidney disease by
increasing the tubular epithelial cell signal response
to a pro-fibrotic growth factor such as TGF-β1.
Increased expression of TGF-β1 mRNA may induce
renal fibrosis by producing extracellular matrix
(ECM). Fibrosis can lead to more severe
tubulointerstitial damage resulting in a progressive
decline in the nephron and renal function. The IL-6
and TGF-β1 role, both acutely and chronically in
renal tubular damage in the preeclampsia model, has
not been widely known (Munkhaugen and Vikse,
2009; Jones et al., 2015).
One of the therapeutic management in the case of
preeclampsia is the administration of acetylsalicylic
acid. Low-acetyl-salicylic acid administration may
improve blood vessel circulation and prevent
vasoconstriction, resulting in increased organ
perfusion and preventable kidney damage. Acetyl-
salicylic acid can also stimulate the activation of
eNOS that catalyzes NO synthesis for hypertensive
patients in preeclampsia (Danuyanti et al., 2018).
Therefore, we need to observe the effect of low dose
acetylsalicylic acid administration on renal function
through eNOS, IL-6, TGF-β expression of mRNA on
the preeclampsia model.
2 MATERIALS AND METHODS
2.1 Preparation of Experimental
Animal
This research used Wistar strain female rat obtained
from Integrated Research and Testing Laboratory
(LPPT) Universitas Gadjah Mada with number
00104/04/LPPT/VIII/2017. Wistar strain female rats
with age ± 12 weeks, and bodyweight 150-200 grams
were used for the experiment. The rats were divided
into 4 groups: Control group (normotensive pregnant
rat), PE group (preeclamptic model rat induced with
L-NAME of doses 50 mg/kg BW/day from the first
day to 18th day of pregnancy), PE+ASA75 group
(preeclamptic model and treatment of acetyl-
salicylate acid of doses at 75 mg/Kg BW from the
tenth day until the twelfth day of pregnancy), and
PE+ASA125 group (preeclamptic model rad and
treatment of acetyl-salicylate acid of doses at 125
mg/Kg BW). The doses of ASA was determined by
rat bodyweight that was quantified daily.
2.2 Process of Impregnating Rat
The rats' conception was performed at the Faculty of
Pharmacy Laboratory Unit V by placing one male rat
and two female rats in one cage. The experimental
animals were mixed in the afternoon at around 4 pm
and then check for the vaginal plugs (copulation plug)
on the following morning at 6 am. The presence of
vaginal plugs (copulation plug) in animals was
calculated as the 1st day of pregnancy (Han et al.,
2015; Kaya et al., 2011).
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
64

2.3 Process of Measuring Blood
Pressure, Proteinuria, and
Preeclampsia Induction
Blood pressure measurement of an experimental
animal was performed using a non-invasive
sphygmomanometer. Blood pressure was measured
five times during the study: a day before mating Day-
0 (D-0), the 5th day of pregnancy (D-5), D-8, D-11,
D-13, and before surgery (D-18). Examination of
proteinuria using uriscan 3 GPH strips. Proteinuria
was measured on the day before mating (D-0), the 6th
day of pregnancy (D-6), D-9, D-12, D-14, and D-19.
Preeclampsia induction was done by dissolving L-
NAME 50 mg/kg BW/day with the gastric probe on
the first day of pregnancy until the 18th day of
pregnancy (Szalai et al., 2015). Each rat weighing
150-200 g was given L-NAME of 1.5 to 2 mL per rat.
2.4 Acetyl-salicylate Acid Therapy and
Proteinuria Examination
Acetyl-salicylate acid therapy was performed by
dissolving 75 mg and 125 mg tablets using aquadest
first. Acetyl-salicylate acid was given orally with the
probe to mice at 10 to 12 days of gestational age. Each
preeclampsia mice with a weight range of 150-200
grams got acetyl-salicylate acid therapy that has been
diluted as much as 1.35 to 1,8 mL/day in 10 to 12 days
of gestational age. Proteinuria examinations using
uriscan 3 GPH strips were performed at: days before
mating (D-0), the 5th day of pregnancy (D-5), D-8,
D-11, D-13 D-18.
2.5 Kidney Harvesting
After being treated for eighteen days, all groups were
terminated 24 hours after the treatment. After
anesthetized using ketamine (dose 60-100 mg/kg
BW), rats were sacrificed with a lethal dose of
ketamine. Rat's abdomen and thorax were opened,
kidneys were harvested. The left kidney was kept in
RNA later® for RNA extraction, and the right kidney
was fixated in 4% PFA in PBS for paraffin making.
2.6 RNA Extraction, cDNA Making,
and Reverse Transcription-PCR
(RT-PCR)
Examination of eNOS, TGF-β1, IL-6, and GAPDH
mRNA expression was done using RT-PCR
(Transcription Reaction of Polymerase Chain
Reactions). Kidney tissue was extracted using Trizol
RNA solution (GENEzolTM; Cat. No. GZR100).
RNA concentrations were quantified using nanodrop.
The cDNA was synthesized using 5xRT-buffer
(Toyobo, TRT-101), random primer (TAKARA®,
3801), dNTP (TAKARA®, 4030), ReverTra-Ace
(Toyobo®; TRT-101).Reverse transcriptase-
polymerase chain reaction (RT-PCR) was carried out
to examine the following cDNAs: eNOS 5 ’ -
GTCCTGCAAACCGTGCAGAG-3’(forward) and
5- TGGGTGCGCAATGTGAGTC-3 ’ (reverse);
TGF- β 1 5-CCGTGGCTTC TAGTGCTGAC-3'
(forward), and 5'-GGCGTTGTTGCG TTAGATAC-
3' (reverse); IL-6 5-GCCCTTCAGGA
ACAGCTATGA-3 ′(forward) and 5 ′-TGTCAA
CAACATCAGTCCCAAAGA-3 ′ (reverse); and
GAPDH 5’-CCCCCAATGTATCCGTTGTG-3’
(forward) and 5'TAGCCCAGGATGCCCTTGAGT-
3'(reverse). Then a 35 cycle PCR was carried out with
a denaturation condition of 94° C for 10 seconds,
annealing at 60° C for 30 seconds and an extension of
72° C for the 1-minute final extension phase ending
with a 72° C condition for 10 minutes.
2.7 Histopathological Examination
The kidney paraffin blocks were cut in 4 μm thickness
for Periodic Acid Schiff (PAS) staining to assess
glomerulosclerosis. The preparation was examined
under a light microscope (Olympus CX22®), an
image was captured using OptiLab software in 400×
magnification with a random area.
2.8 Statistical Analysis
Data were analyzed using a one-way ANOVA test for
normally distributed data and Kruskal-Wallis for data
that were not normally distributed. The value of
p<0.05 was considered statistically significant.
Statistical analyses were accomplished using SPSS
Software version 23 (SPSS Inc., Chicago).
2.9 PE Condition after L-NAME
Treatment
L-NAME treatment in pregnant rats induced PE
conditions as shown by higher proteinuria scores and
higher systolic blood pressure in the PE group than
the control group (Fig.1). However, both ASA treated
groups did not demonstrate attenuation of blood
pressure and proteinuria score.
Low-dose of Acetylsalicylic Acid Upregulates Expression of eNOS mRNA and Downregulates Interleukin-6 (IL-6) and Transforming
Growth Gactor-1 (TGF-1) mRNA in Rat Kidney of Preeclampsia Model
65
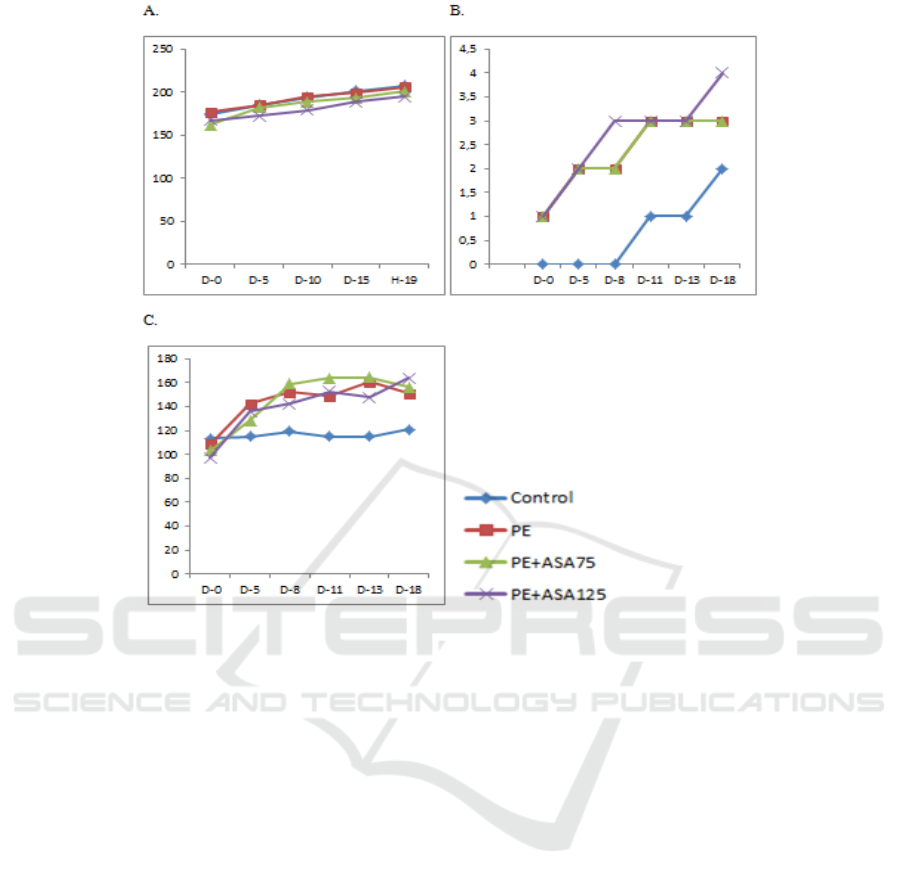
Figure 1: L-Name treatment demonstrated PE condition with increasing body weight, proteinuria, systolic blood pressure.
The ASA treatment could not attenuate PE condition based on proteinuria score and systolic blood pressure. (A)
Representative data of mean body weight from the groups. (B) Mean proteinuria of the groups. (C) Systolic blood pressure
of the groups.
3 RESULTS
Low-dose of acetylsalicylic acid upregulates
expression of eNOS and down-regulates interleukin-
6 (IL-6) and transforming growth factor-β1 (TGF-
β1).
The expression of IL-6 mRNA was described as
the inflammatory condition of the kidney after PE.
Meanwhile, TGF-β1 expression was represented
chronic effect and pro-fibrotic substance. PE
induction led to significantly higher IL-6 and TGF-
β1 mRNA compared to the Control group. RT-PCR
analysis was demonstrated the beneficial effects of
ASA treatment. ASA treated groups was significantly
lower IL-6 and TGF- β1 mRNA expression compared
to the PE group. PE+ASA125 group was showed the
lowest IL-6 and TGF- β1 mRNA expression. This
group was significantly lower than the PE+ASA75
group.
4 DISCUSSION
This study revealed that a low dose of ASA treatment
did not attenuate blood pressure and proteinuria in the
PE model. However, low doses of ASA treatment had
beneficial effects in inducing eNOS upregulation and
reducing inflammation. ASA125 treatment induced
higher eNOS mRNA expression based on this study.
It seemed that acetylsalicylic acid might increase
eNOS expression in endothelial dysfunction.
Acetylsalicylic acid can stimulate the activation of
eNOS, which can increase NO production for
hypertensive patients in preeclampsia (Suharto et al.,
2014). Nitric oxide can help oxygen transport by
widening the blood vessel wall to facilitate the
transfer of gas from the blood to the tissue. After NOS
synthesizes NO from L-arginine, NO diffuses from
endothelial cells to smooth muscle cells of the blood
vessels and causes an increase in intracellular cyclic
guanosine monophosphate (cGMP). Increased cGMP
will trigger the relaxation of blood vessel muscles to
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
66
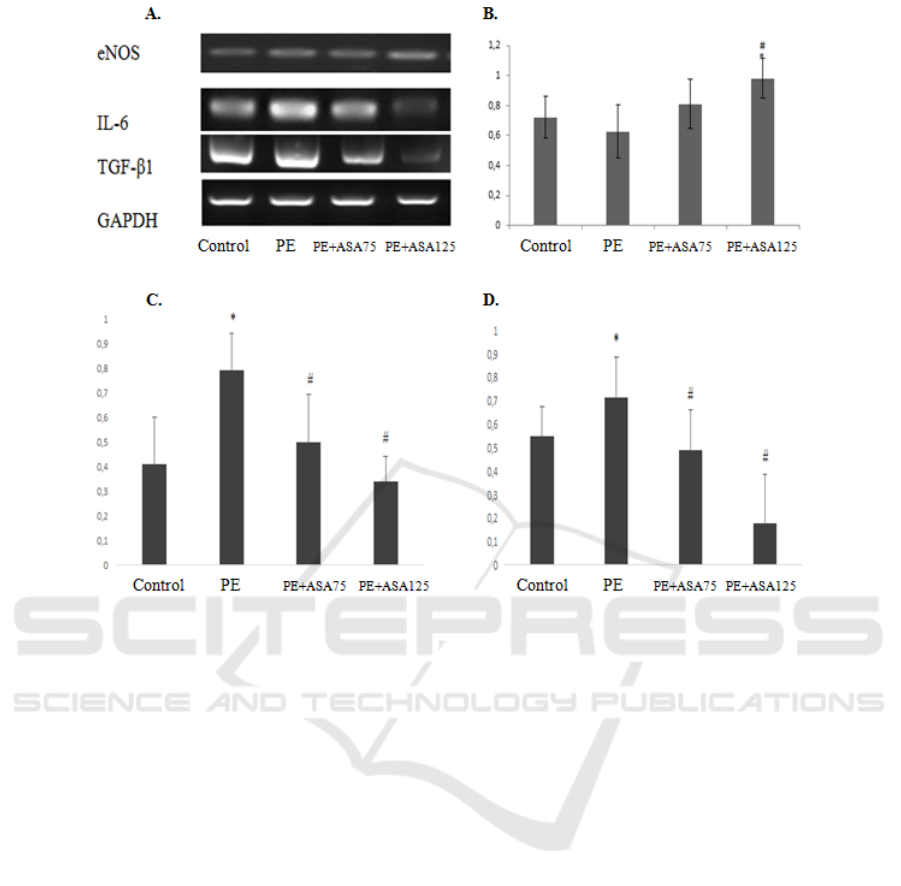
Figure 2. The effect of low-acetyl-salicylic acid on the increased expression of eNOS mRNA and decreased expression of IL-
6 and TGF-β1 mRNA to renal failure in preeclampsia model animals. (A) Representative picture of endothelial function
(eNOS), inflammation marker (IL-6), and fibrosis agent (TGF-β1) expression using RT-PCR. (B) Densitometry analysis of
eNOS mRNA expression from PE kidney model (C) Densitometry analysis of IL-6 mRNA expression from PE kidney model
PE. (D)Densitometry analysis of TGF-β1 mRNA expression from PE kidney model. (E) Representative picture of
glomerulosclerosis based on Periodic Acid-Schiff (PAS) staining in 400× magnification.*:p<0.05 vs control; ##:p<0.01 vs.
PE.***:p<.001 vs PE; ##:p<0.01 vs control.
become a vasodilator to suppress hypertension. Nitric
oxide is known to have properties as an inhibitor of
platelet activation (Jones et al., 2015, Burke et al.,
2016)).
The results showed that preeclampsia's condition
tends to increase the expression of IL-6 and TGF-β1
mRNA, which is often associated with inflammation
and fibrosis. The low dose of ASA treatment in this
study had beneficial effects in lowering IL-6 and
TGF-β1 expression. IL-6 is a pleiotropic cytokine
involved in the regulation of the immune response
and inflammation. IL-6 has biological properties such
as activating transducer signals and activation of
transcription factor STAT3 in renal tubular cells,
stimulation of expression of tissue factor, MCP-1,
matrix degeneration enzyme, and low-density
lipoprotein receptor on macrophage (Jones et al.,
2015). The results of this study can be seen that IL-6
expression tends to increase in preeclamptic
condition compared with the control group. This
condition may be due to preeclampsia, the occurrence
of placental ischemia may contribute to maternal
endothelial cell dysfunction by increasing the
synthesis of IL-6, TNF-α, and IL-8 (Creasy et al.,
2004).
An imbalance between vasoconstrictor and
vasodilator might induce kidney injury with
inflammation and fibrosis. We found that
upregulation of eNOS as vasodilator function in this
study after ASA 125 mg/Kg BW treatment associated
with attenuation of kidney inflammation and fibrotic
marker. This phenomenon might relate to the function
of ASA as anti-inflammatory without influencing
vasodilator capacity in the kidney. A low acetyl-
salicylate acid dose positively affects the balance
between PGI2 as a vasodilator, TXA2 as a
vasoconstrictor, and stimulant platelet aggregation.
At a low dose, acetylsalicylic acid can inhibit TXA2
synthesis without affecting PGI2 synthesis in the
vascular endothelium. According to Villa, research
Low-dose of Acetylsalicylic Acid Upregulates Expression of eNOS mRNA and Downregulates Interleukin-6 (IL-6) and Transforming
Growth Gactor-1 (TGF-1) mRNA in Rat Kidney of Preeclampsia Model
67

states that a decrease in TXA2 production without a
decrease in PGI2 production can prevent
vasoconstriction and coagulation problems
characteristic of preeclampsia (Villa et al., 2013).
We further examined the effect of low-dose
acetylsalicylic acid administration on the expression
of TGF-β1 as a pro-fibrotic factor in the renal model
of preeclampsia. TGF-β1 is a pro-fibrotic growth
factor that induces renal fibrosis by producing an
extracellular matrix. In this study's results, it can be
seen that TGF-β1 expression tends to increase in
preeclamptic condition compared with the control
group. This phenomenon may occur because, in
preeclampsia conditions, high urinary protein content
may cause pro-inflammatory and pro-fibrotic effects
that contribute to tubulointerstitial damage and loss of
renal function. The main pro-fibrotic factor involved
in this process is TGF-β1 (Kuusniemi et al., 2005).
Increased expression of TGF-β1 may induce renal
fibrosis.
5 CONCLUSION
In conclusion, low doses of ASA treatment might
attenuate inflammation and fibrosis in the kidney
after PE induction. These effects might be associated
with high eNOS mRNA expression.
REFERENCES
Burke, S.D., Zsengellér, Z.K., Khankin, E.V., Lo, A.S.,
Rajakumar, A., DuPont, J.J., McCurley, A., Moss,
M.E., Zhang, D., Clark, C.D. and Wang, A., 2016.
Soluble fms-like tyrosine kinase 1 promotes
angiotensin II sensitivity in preeclampsia. The Journal
of clinical investigation, 126(7), pp.2561-2574.
Creasy, R.K., Resnik, R., Lockwood, C.J., Greene, M.F.
and Moore, T., 2004. Maternal-fetal medicine:
principles and practice. Gulf Professional Publishing.
Danuyanti, I.G.A.N., Kristinawati, E. and Resnhaleksmana,
E., 2018. Hubungan Kadar Nitrit Oksida (NO) Dalam
Darah Terhadap Resiko Kejadian Diabetes Mellitus
Tipe 2 Dengan Hipertensi di RSUP NTB. Jurnal
Kesehatan Prima, 8(1), pp.1207-1215.
Han, Y.W., Yang, Z., Ding, X.Y., and Yu, H., 2015.
Differences in liver injury and trophoblastic
mitochondrial damage in different preeclampsia-like
mouse models. Chinese Medical Journal, 128(12),
p.1627.
Jones, S.A., Fraser, D.J., Fielding, C.A. and Jones, G.W.,
2015. Interleukin-6 in renal disease and
therapy. Nephrology Dialysis Transplantation, 30(4),
pp.564-574.
Kaya, A., Boztosun, A., Seckin, H., Guven, A.S.,
Kucukdurmaz, Z., Gulturk, S., and Cevit, O., 2011. The
evaluation of hypoxia-inducible factor 1 in N-nitro-L-
arginine methyl ester preeclampsia model of pregnant
rats. Journal of Investigative Medicine, 59(8), pp.1268-
1272.
Kemenkes RI., 2014. Mothers day: situasi kesehatan ibu.
Available from
http://www.depkes.go.id/download.php?file=downloa
d/pusdatin/infodatin/infod atin-ibu.pdf.
Kuusniemi, A.M., Lapatto, R., Holmberg, C., Karikoski, R.,
Rapola, J., and Jalanko, H., 2005. Kidneys with heavy
proteinuria show fibrosis, inflammation, and oxidative
stress, but no tubular phenotypic change. Kidney
International, 68(1), pp.121-132.
Linggardini, K. and Aprilina, H.D., 2016. Pengaruh
Pendidikan Kesehatan Pada Ibu Hamil Terhadap
Pengetahuan Tentang Preeklamsia di Wilayah Kerja
Puskesmas Sokaraja I. MEDISAINS, 14(2).
Munkhaugen, J. and Vikse, B.E., 2009. New aspects of
preeclampsia: lessons for the nephrologist. Nephrology
Dialysis Transplantation, 24(10), pp.964–2967.
Oparil, S., Zaman, M.A. and Calhoun, D.A., 2003.
Pathogenesis of hypertension. Annals of internal
medicine, 139(9), pp.761-776.
Pellicer, B., Herraiz, S., Leal, A., Simón, C. and Pellicer,
A., 2011. Prenatal brain damage in preeclamptic animal
model induced by gestational nitric oxide synthase
inhibition. Journal of Pregnancy, pp.1-6.
Rachmi, R. and Sulistyono, A., 2016. Aspirin Dosis Rendah
Efektif Menurunan Resistensi Arteri Uterina yang
Abnormal pada Ibu Hamil Usia Kehamilan 16-24
Minggu. Majalah Obstetri & Ginekologi, 24(1), pp.25-
30.
Suharto, F.F., Saleh, M.I. and Subandrate, S., 2014.
Identifikasi Polimorfisme Glu298Asp Gen eNOS pada
Penderita Preeklampsia di Rumah Sakit Dr.
Mohammad Hoesin Palembang. Jurnal Kedokteran
dan Kesehatan, 1(1), p.61-66.
Szalai, G., Romero, R., Chaiworapongsa, T., Xu, Y., Wang,
B., Ahn, H., Xu, Z., Chiang, P.J., Sundell, B., Wang, R.
and Jiang, Y., 2015. Full-length human placental sFlt-
1-e15a isoform induces distinct maternal phenotypes of
preeclampsia in mice. PloS one, 10(4), p.e0119547.
Villa, P.M., Kajantie, E., Räikkönen, K., Pesonen, A.K.,
Hämäläinen, E., Vainio, M., Taipale, P., Laivuori, H.
and Predo Study group, 2013. Aspirin in the prevention
of preeclampsia in high‐risk women: a randomised
placebo‐controlled PREDO Trial and a meta‐analysis
of randomised trials. BJOG: An International Journal
of Obstetrics & Gynaecology, 120(1), pp.64-74.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
68
