
Optimization of Immunohistochemical Staining with Anti Protein
Gene Product 9,5 (PGP 9,5) Antibodies to Detecting Intraepidermal
Nerve Fiber
David Pakaya
1a
, Yustina Andwi Ari Sumiwi
2b
, Sri Herwiyanti
2c
, Rina Susilowati
2d
1
Department of Histology, Faculty of Medicine, Universitas Tadulako, Palu, Indonesia
2
Department of Histology and Cell Biology, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada,
Yogyakarta, Indonesia
Keywords: Intraepidermal Nerve Fiber, PGP 9.5, Immunohistochemistry
Abstract: The intraepidermal nerve fibers (INF) are ending branches of the skin sensory nerves. These fibers can be
conceived due to the protein gene product 9.5 (PGP 9.5) as a marker recognized by immunohistochemistry.
Various studies have visualized these INF with anti-PGP 9.5 antibodies. However, this study differs in
immunohistochemical (IHC) staining methods based on the tissue thickness, the antigen retrieval process, and
the antibody product used. This study aimed to find an IHC staining optimizer with an anti-PGP 9.5 antibody
to detect INF from paraffin blocks. The INF was determined by mice skin biopsy stained with IHC anti-PGP
9.5 antibodies. This procedure was altered in the dilution, duration, incubation temperature of primary
antibodies, the tissue's thickness, and the antigen retrieval temperature. We quantitatively analyzed the
staining results. Optimization of IHC stain entail of 1:2000 dilution of the primary antibody, the thickness of
the tissues were 4 µm, overnight incubation, and low temperature of antigen retrieval. However, the results
were inconsistent. The contributing factors that enhance the IHC staining method are thinness of the tissue,
low-temperature antigen retrieval, the ratio of antibodies dilution (1: 2000), and incubation overnight at 21°C.
1 INTRODUCTION
The intraepidermal nerve fibers are the ending
branches of the skin's sensory nerves (Malik et al.,
2011). This fiber is one of the diagnostic parameters
of neuropathy (Chen et al., 2015). Neuropathy is a
clinical problem in the form of unpleasant sensations
caused by peripheral nervous system damage.
Neuropathy is assessed by decreasing the density of
intraepidermal nerve fibers, which can be identified
by immunohistochemical staining.
The data is obtained through the skin tissue
section. Therefore intraepidermal nerve fibers must
be visualized by immunohistochemical staining to
recognize the markers expressed by the nerve fibers,
one of which is protein gene product 9.5 (PGP 9.5)
(Sun et al., 2014). Various studies have visualized
a
https://orcid.org/0000-0002-9791-1200
b
https://orcid.org/0000-0001-8874-3850
c
https://orcid.org/0000-0001-9580-1537
d
https://orcid.org/0000-0003-1694-2054
these intraepidermal nerve fibers with anti-PGP 9.5
antibodies. However, the study differs from the
method of immunohistochemical staining, the
thickness section of the tissue, the antigen retrieval
process, and the antibody product used.
Detection of intraepidermal nerve fibers can use
tissue from paraffin blocks with thin sections (<20
μm). The thin section's advantage is the penetration
of antibodies that are used faster and better and do not
require cutting tools and special microscopes. The
HRP label using to have the advantage of longer
tissue structure to observed than the fluorescent label.
A precise staining technique is needed to obtain a
quantification of intraepidermal nerve fibers. In this
study, we will optimize immunohistochemical
staining with PGP 9.5 antibody (Abcam ab8189) was
performed to detect intraepidermal nerve fibers in a
simple laboratory using tissue from paraffin blocks.
Pakaya, D., Ari Sumiwi, Y., Herwiyanti, S. and Susilowati, R.
Optimization of Immunohistochemical Staining with Anti Protein Gene Product 9,5 (PGP 9,5) Antibodies to Detecting Intraepidermal Nerve Fiber.
DOI: 10.5220/0010487400430046
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 43-46
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
43

Figure 1: Results of phase I optimization, immuno-histochemical staining feature with PGP 9.5 primary antibody on 4 μm
tissue sliced thickness. (A). 1:500 dilution. (B). 1:1000 dilution (C). 1:2000 dilution. 400x magnification.
2 MATERIAL AND METHODS
2.1 Animal Samples
This study type is a quasi-experimental study with a
cross-sectional design. It utilized two male Balb/cJ
mice strain, 8-weeks old, 20-40 g body weight. It
utilized two male Balb/cJ mice strain, 8-weeks old,
20-40 g body weight, using two mice to obtain the
skin samples by applying the principle for animal
laboratory used. This particular study has received its
permit from the ethical commission of integrated
research and development bureau (LPPT) UGM with
its certified number: 00111/04/LPPT/II/2017.
2.2 Immunohistochemical Staining
Mice were terminated, and their right foot skin was
necropted using skin punch biopsy (Premier
®
,
PMU9033505, Premier Medical, India) at 5 mm of
diameter. The resultant necropsies were grown in 5%
agarose gel and incubated overnight in
paraformaldehyde 4%; then paraffin blocks were
made. The paraffin blocks section was using
Microtome Leica RM 2235, at 4 and 15 μm thickness
with a fraction of 1/20. Immunohistochemical
staining was performed using anti-PGP 9.5 (Abcam
®
ab8189, Abcam, USA. MA) antibodies. The staining
was performed within several phases and
modifications upon the primary antibody
(comparison of dilution, temperature, and duration of
incubation), tissue thickness, and antigen retrieval.
2.3 Statistical Analysis
The results of the staining were analyzed qualitatively
for the intensity of the nerve fiber color.
3 RESULTS
3.1 Optimizing Immunohistochemical
Staining with Anti PGP 9.5
Antibodies
The first stage optimization is performed to obtain the
best primary antibody dilution. In the thickness of the
4 and 15 μm tissue slices using 1:500 and 1:1000
primary 1-cell antibody dilutions, the resulting
1:2000 dilution of the color with a balanced intensity
and good, but not specific. The immunohistochemical
brown color is seen in most skin epithels so that
intraepidermal nerve fibers cannot be found (Figure
1).
Phase II is carried out at 4 and 15 μ thickness and
lower retrieval antigen temperature. There was one
folded slide with 15 μm thickness. The staining
results were a picture of nerve fibers with good color
intensity, as in Figure 2. In phase III, trials were
conducted on three blocks of samples with 15 μm
thickness using the same phase II method. After the
retrieval antigen process, there were six loose slides
and six folded slides. The results of the staining look
like a picture of less good nerve fibers. In phase, IV
modification is done without antigen retrieval, a
primary antibody with 1: 2000 dilution. The results
appeared in overnight incubation as the medium-
intensity brown color, and no nerve fibers were
found. At incubation of 2 and 3 nights, the results
appear in brown with a concentrated intensity and are
non-specific.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
44
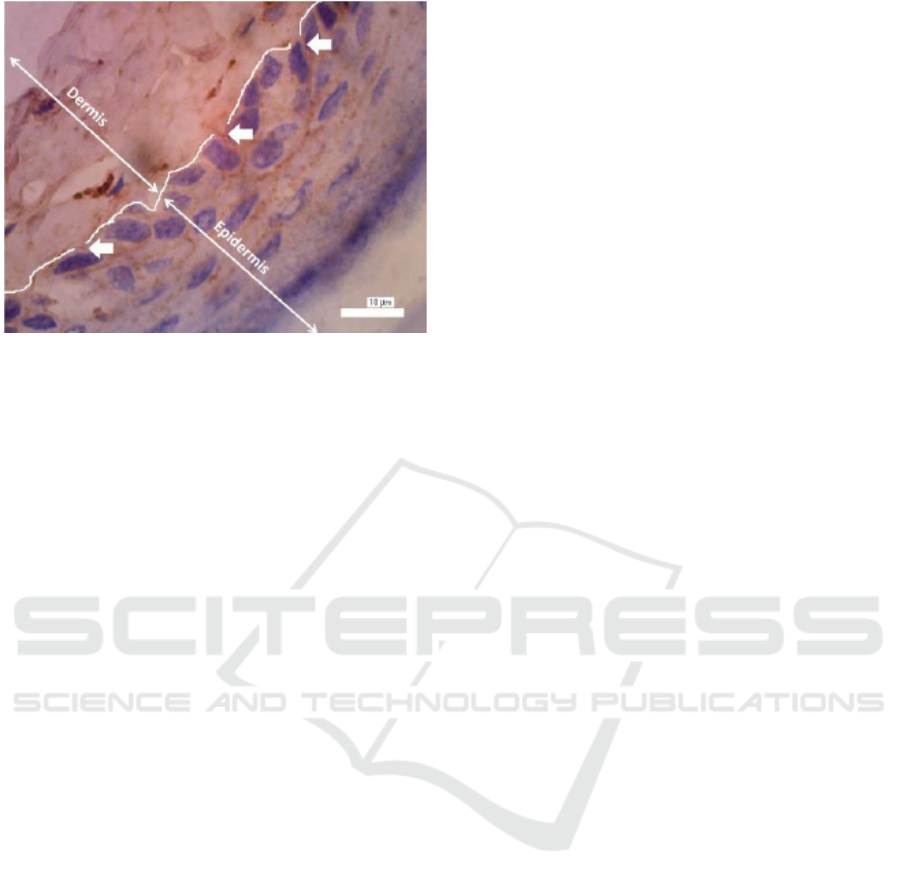
Figure 2: Intraepidermal nerve fibers with anti-PGP 9.5
antibodies are shown as brown perpendicular lines that pass
through the basal membrane (indicated by arrows) with
1000x magnification.
Phase V was performed at 15 μm thickness,
dilution of primary antibody at 1: 2000 with
modification of the antigen retrieval temperature and
the primary antibody's incubation temperature. The
result showed several loose or folded slides, and the
staining results appear as a brown stain with moderate
intensity, and no nerve fibers are found. Stage VI was
performed at a thickness of 4 μm, with 1: 2000
dilution of primary antibody and the modification of
lower retrieval antigen temperature. The result
showed no slide folding or losing, and there was a
picture of nerve fibers.
4 DISCUSSION
Optimization is important to make a good quality of
tissue staining. We will optimize
immunohistochemical staining with PGP 9.5
antibody to detect intraepidermal nerve fibers using
skin tissue from paraffin blocks to diagnose
neuropathy. The immunohistochemical optimizing at
phase I, a 1:2000 dilution was obtained with its best
stain production and balanced and good intensity. In
phase II, the nerve fibers appear well at 15 μm
thickness. In another study, nerve fibers appeared
well on 5 μm slice thickness of paraffin blocks
(Thomsen et al., 2009; Ventura et al., 2011), 50-100
μm thicknesses with frozen section (Stavniichuk et
al., 2011) and 2 μm thickness with a confocal
microscope (Periquet et al., 1999). To quantify the
number of nerve fibers with specific antibody
markers, it's is recommended to use thin slices
(Beiswenger et al., 2008). In phase III, the same
thickness and method do not produce a consistent
picture. This phenomenon is influenced by other
processes such as antigen retrieval temperature and
the incubation of primary antibodies.
Good nerve fibers appear after antigen retrieval
was conducted with low temperatures. However,
these low-temperature modifications remain
inconsistent, just like the results in phase V. In the
case of axon injury, the image of nerve fibers is also
obtained after low-temperature antigen retrieval
(Stone et al., 2009). This study's obstacles were the
thickness of the skin foot sample (15 μm) with the
cornification at risk of loose or folded after antigen
retrieval. In phase IV, the retrieval antigen process is
not performed, but the results are not specific. The
phase VI optimization was carried out at 4 μm
thickness with the same retrieval antigen temperature
as stage V, obtained a picture of nerve fibers with
balanced color intensity. To get a good picture of the
nerve fibers needs to be preceded by the retrieval
antigen at low temperatures. The open epitope will be
very good to bind to the incubated antibody. A good
description of the nerve fibers was obtained in the
second stage of optimization with 15 μm slice
thickness and stage VI with 4 μm slice thickness. The
thickness of 4 μm slices is the most likely to be done
because at 15 μm, the thickness is risky to lose or fold
after retrieval antigen.
To quantitate the intraepidermal nerve fibers
density, the stereology principle was used. The
samples were randomly and systematically obtained
from the paraffin block slices that have the same
thickness. With this method, we will get some slices
to be quantification according to the specified
fraction. The entire cross-section must look good to
be quantified, so when using a 15 μm thickness, it will
have difficulty reporting the results. Skin tissue is not
isotropic, i.e., uniformity in parameter values in all
directions, so it takes orientation or determination of
the direction of network cutting to avoid bias (Witgen
et al., 2006). However, manual quantification still
possesses the possibility of bias, so that software
usage is recommended. The software will generate
randomly oriented virtual isotropic fields in a 3D
virtual field containing parallel lines so that any nerve
fibers tangent to the line can be quantified (Karlsson
et al., 2013; Karlsson et al., 2016). Inconsistent
optimization results and the shortcoming of manual
quantification may cause difficulty in conducting the
study.
Optimization of Immunohistochemical Staining with Anti Protein Gene Product 9,5 (PGP 9,5) Antibodies to Detecting Intraepidermal
Nerve Fiber
45

5 CONCLUSION
The immunohistochemical staining method with the
most optimal anti-PGP 9.5 antibodies was performed
on thin, with low-temperature antigen retrieval and 1:
2000 antibody dilution incubated overnight at 21°C.
The results of this study can be directed to become
a diagnostic method of neuropathy.
ACKNOWLEDGEMENTS
The author would like to express gratitude to BPPDN
Dikti 2015 (906.II/E4.4/2015). Mrs. Wiwiet
Setyowati, Histology, and Cell Biology Laboratory of
UGM Medical School.
REFERENCES
Beiswenger, K.K., Calcutt, N.A. and Mizisin, A.P., 2008.
Epidermal nerve fiber quantification in the assessment
of diabetic neuropathy. Acta histochemica, 110(5),
pp.351-362.
Chen, X., Graham, J., Dabbah, M.A., Petropoulos, I.N.,
Ponirakis, G., Asghar, O., Alam, U., Marshall, A.,
Fadavi, H., Ferdousi, M. and Azmi, S., 2015. Small
nerve fiber quantification in the diagnosis of diabetic
sensorimotor polyneuropathy: comparing corneal
confocal microscopy with intraepidermal nerve fiber
density. Diabetes care, 38(6), pp.1138-1144.
Karlsson, P., Haroutounian, S., Polydefkis, M., Nyengaard,
J.R. and Jensen, T.S., 2016. Structural and functional
characterization of nerve fibres in polyneuropathy and
healthy subjects. Scandinavian Journal of Pain, 10,
pp.28-35.
Karlsson, P., Porretta‐Serapiglia, C., Lombardi, R., Jensen,
T.S. and Lauria, G., 2013. Dermal innervation in
healthy subjects and small fiber neuropathy patients: a
stereological reappraisal. Journal of the Peripheral
Nervous System, 18(1), pp.48-53.
Malik, R.A., Veves, A., Tesfaye, S.A., Smith, G., Cameron,
N., Zochodne, D., Lauria, G. and Toronto Consensus
Panel on Diabetic Neuropathy, 2011. Small fibre
neuropathy: role in the diagnosis of diabetic
sensorimotor polyneuropathy. Diabetes/metabolism
research and reviews, 27(7), pp.678-684.
Periquet, M.I., Novak, V., Collins, M.P., Nagaraja, H.N.,
Erdem, S., Nash, S.M., Freimer, M.L., Sahenk, Z.,
Kissel, J.T. and Mendell, J.R., 1999. Painful sensory
neuropathy: prospective evaluation using skin biopsy.
Neurology, 53(8), pp.1641-1641.
Stavniichuk, R., Drel, V.R., Shevalye, H., Maksimchyk, Y.,
Kuchmerovska, T.M., Nadler, J.L. and Obrosova, I.G.,
2011. Baicalein alleviates diabetic peripheral
neuropathy through inhibition of oxidative–nitrosative
stress and p38 MAPK activation. Experimental
neurology, 230(1), pp.106-113.
Stone, J.R., Walker, S.A. and Povlishock, J.T., 1999. The
visualization of a new class of traumatically injured
axons through the use of a modified method of
microwave antigen retrieval. Acta neuropathologica,
97(4), pp.335-345.
Sun, Y., Zhu, L., Huang, X., Zhou, C. and Zhang, X., 2014.
Immunohistochemical localization of nerve fibers in
the pseudocapsule of fibroids. European journal of
histochemistry: 8;58(2):2249.
Thomsen, N.O.B., Englund, E., Thrainsdottir, S., Rosén, I.
and Dahlin, L.B., 2009. Intraepidermal nerve fibre
density at wrist level in diabetic and non‐diabetic
patients. Diabetic medicine, 26(11), pp.1120-1126.
Ventura-Sobrevilla, J., Boone-Villa, V.D., Aguilar, C.N.,
Román-Ramos, R., Vega-Avila, E., Campos-
Sepúlveda, E. and Alarcón-Aguilar, F., 2011. Effect of
varying dose and administration of streptozotocin on
blood sugar in male CD1 mice. In Proc West
Pharmacol Soc (Vol. 54, pp. 5-9).
Witgen, B.M., Grady, M.S., Nyengaard, J.R. and
Gundersen, H.J.G., 2006. A new fractionator principle
with varying sampling fractions: exemplified by
estimation of synapse number using electron
microscopy. Journal of microscopy, 222(3), pp.251-
255.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
46
