
Toxicity of Self-nanoemulsifying Drug Delivery System Formulation of
Nigella Sativa L. Seed Oil against Adult Danio rerio
Yanti Tri Utami
1a
and Isnatin Miladiyah
2b
1
Medical student, Faculty of Medicine Universitas Islam Indonesia, Yogyakarta, Indonesia
2
Department of Pharmacology, Faculty of Medicine, Universitas Islam Indonesia, Yogyakarta, Indonesia
Keywords: Self-nanoemulsifying, Drug delivery system, Nigella sativa L., Toxicity, Danio rerio
Abstract: Nigella sativa L. (N. sativa L.) has been used in traditional medicine due to its numerous therapeutic effects,
but its oral form has low solubility and has a minor therapeutic effect. The development of self-
nanoemulsifying drug delivery system (SNEDDS) formulation for N. sativa L may solve this issue. The
pharmacological activity of the SNEDDS formulation of N. sativa L. seed oil extract (NSOE) has been widely
explored but its toxicity remains unknown. This study aimed to determine the lethal concentration of NSOE
SNEDDS formulation. An experimental study was conducted using adult zebrafish, aged 4–6 months,
incubated with NSOE SNEDDS formulation at 500, 250, 125, 62.5, and 31.5 part per million (ppm)
concentrations and with non-SNEDDS formulation at 125, 62.5, 31.25, 15.625, and 7.8125 ppm
concentrations. The mortality was calculated through macroscopic examination after 24, 48, 72, and 96 hours
of exposure. The half-maximal lethal concentration (LC
50
) of the NSOE SNEDDS and non-SNEDDS
formulations was determined using the probit analysis. The LC
50
of NSOE SNEDDS formulations was
154.637 + 75.609 ppm and was not significantly different from that of non-SNEDDS (72.358 + 15.253 ppm)
(p = 0.138). The toxicity of the SNEDDS formulation of NSOE was comparable to that of the non-SNEDDS.
1 INTRODUCTION
Nigella sativa L. (N. sativa L.) is a herbal plant from
the Ranunculaceae family and is widely grown in
Mediterranean countries, the Middle East, Eastern
Europe, and West Asia (Abedi et al., 2017). Several
phytochemical studies of N. sativa L. showed that its
extract contains numerous antioxidant compounds,
including thymoquinone, carvacrol, t-anethole, and 4-
terpineol, which indirectly reduce reactive oxygen
species production and inhibit lipid peroxidation
(Amina, 2016). Thymoquinone has the most powerful
antioxidant properties (Kooti et al., 2016).
Herbal medicines are usually orally administered
since this route of administration is the safest, most
convenient, and most inexpensive (Cherniakov et al.,
2015). However, the low solubility and poor oral
bioavailability of many herbal medicines lead to less
optimal effectiveness. Therefore, researchers have
begun developing oil-based drug formulations in the
form of nanoemulsions that are expected to improve
a
https://orcid.org/0000-0003-4381-0129
b
https://orcid.org/0000-0003-1630-7130
the oral bioavailability and drug solubility of herbal
plant extracts, including the development of a self-
nanoemulsifying drug delivery system (SNEDDS)
(Abdelbary et al., 2013).
The SNEDDS formulation consists of a mixture
of isotropic oils, surfactants, and cosurfactants that
are capable of spontaneously forming oil
nanoemulsions in the gastrointestinal tract by
producing nanometer-sized droplets (<300 nm in
size) when dispersed in liquid media (Christophersen
et al., 2014; Patel et al., 2011) Drug preparations in
SNEDDS formulations have several advantages,
including the ability to maximize absorption and
transportation, modulate drug biodistribution and
disposition, and allow targeted drug delivery to
reduce the side effects. SNEDDS formulations tend
to be more physically and chemically stable for long-
term storage and allow the packaging of drugs in unit
dosage forms, using hydroxypropyl methylcellulose
or both soft and hard gelatin capsules, than other
nanoemulsion systems (Date et al., 2010).
Utami, Y. and Miladiyah, I.
Toxicity of Self-nanoemulsifying Drug Delivery System Formulation of Nigella Sativa L. Seed Oil against Adult Danio rerio.
DOI: 10.5220/0010487300350042
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 35-42
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
35

Previous studies have focused on the
pharmacological effectiveness of SNEDDS
formulations; however, their toxicity in living cells,
including the SNEDDS formulation of N. sativa L.
seed oil extract (NSOE), has not been widely
investigated. Toxic impacts are important and
inseparable from the development of new drugs
(Parasuraman, 2011). Thus, this study aimed to
determine the toxicity of NSOE in a SNEDDS
formulation in comparison with the toxicity of the
non-SNEDDS formulation on adult zebrafish.
2 MATERIALS AND METHODS
This study was approved by the Medical and Health
Research Ethics Committee of the Faculty of
Medicine of Universitas Islam Indonesia with
protocol Number 37/Ka.Kom.Et/70/KE/V/2019.
2.1 Animal Subjects
Both male and female adult zebrafish (Danio rerio)
were used in the study. The fish were 4–6 months old
and healthy, as characterized by active swimming.
The fishes that died before the research took place
were excluded. Prior to treatment, the fish were
adapted for one week. Zebrafish were identified at the
Biology Research Center, Indonesia Academy of
Science at Bogor, Indonesia.
2.2 Preparation of N. sativa L. Seed Oil
Extract and SNEDDS Formulation
NSOE was prepared following the previous study by
Akrom et al. (Akrom & Fatimah, 2015). N. sativa L.
seeds were dried and mashed to form a powder.
Overall, 1 kg N. sativa L. seeds was soaked in 1 L of
70% ethanol for 48 hours and filtered to separate the
macerated yield from the residue. The product of the
maceration process was collected, and an evaporator
was used to remove the 70% ethanol solvent and form
a thick NSOE. The viscous NSOE was kept upright
until it divided into two phases, the extract and the oil
phases. The oil phase was then used for the SNEDDS
formulation.
In this study, NSOE was produced with the
maceration method using 70% ethyl alcohol. This
method is simple, easy to perform, inexpensive, and
can protect the thermolabile compounds in N. sativa
L. seed from damage (Savitri et al., 2017). Different
concentrations of surfactants and cosurfactants were
added to the oil phase after maceration to prepare the
SNEDDS formulation. The determination of the
optimal NSOE SNEDDS was based on previous
research (Wahyuningsih & Putranti, 2015), in which
the best composition was NSOE 0.154 parts, Tween
80 0.587 parts, and polyethylene glycol 400 (PEG
400) 0.259 parts. Therefore, the formulation prepared
consisted of 0.532 mL of NSOE, 2.047 mL of Tween
80 (surfactant), and 0.258 mL of PEG 400
(cosurfactant) (Wahyuningsih & Putranti, 2015)
2.3 Formulation Stability Test
Stability of SNEDDS formuations was evaluated
using heating-stability, freeze-thaw, and
centrifugation tests (Senapati et al., 2016). The
heating-stability test was conducted by storing
SNEDDS samples in a refrigerator at 4C for 24 hours
and then by transferring them to an incubator at 40C
for 24 hours (48 hours per cycle). The freeze-thaw
test was conducted by storing SNEDDS samples at
temperatures between −21C and 25C for 48 hours.
The centrifugation test was performed by
centrifuging SNEDDS samples at 5000 rpm for 30
minutes. All tests were repeated six times (six cycles);
then, organoleptic observations and instability
parameters (phase separation and precipitation) were
recorded.
2.4 Determination of Globule Size,
Zeta Potential, Polydispersity
Index, and %Transmittance
The NSOE SNEDDS formulation was diluted with
water at a ratio of 1:25 on a magnetic stirrer until a
nanoemulsion was formed. The nanoemulsion was
then put into a cuvette and measured using a Particle
Size Analyzer (SZ 100, HORIBA) to determine
globule size, zeta potential, and polydispersity index
(PDI). The %transmittance was measured by adding
5 mL distilled water to 0.1 mL SNEDDS formulation
of NSOE and then rotating them in a vortex for 30
seconds. The transmittance was read using a UV-Vis
spectrophotometer (UV-Vis UH5300, Hitachi) at a
wavelength of 650 nm with distilled water as the
comparison (Ujilestari et al., 2018).
2.5 Determination of Half-Maximal
Lethal Concentration (LC
50
value)
The toxicity in zebrafish was evaluated for 96 hours
in each test group. Subjects were exposed to five
concentrations of NSOE SNEDDS (500, 250, 125,
62.5, and 31.5 part per million (ppm)) and five
concentrations of NSOE non-SNEDDS (125, 62.5,
31.25, 15.625, and 7.8125 ppm). One subject group
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
36

was given a combination of cosurfactants-surfactants
to ensure that the use of surfactants and cosurfactants
was not toxic to zebrafish. Each observation was
performed in three replications. Zebrafish mortality
was characterized by the absence of movement and
tail response when touched. Dead fish were removed
each day from the test aquarium, and their mortality
was calculated (The Organization for Economic Co-
operation and Development [OECD], 2018). The
half-maximal lethal concentration (lethal
concentration 50% or LC
50
) value was the
concentrations of NSOE SNEDDS and non-
SNEDDS (ppm) that could kill 50% of the zebrafish
in each test group. The LC
50
values were calculated
from the equation for the linear regression line
between the concentration versus the percent
mortality of zebrafish. After 96 hours, the mortality
in each group was calculated in percent.
2.6 Statistical Analyses
The death percentage per concentration for each
formulation was analyzed using the probit analysis
(SPSS software version 21) to determine the LC
50
values. The average of IC
50
values of NSOE
SNEDDS and non SNEDDS were compared using t-
test and p value < 0.05 was considered as significant.
3 RESULTS.
Preparation of NSOE and NSOE SNEDDS
Formulation: The extraction process produced
NSOE, which was then formulated into a SNEDDS
preparation. The procedure resulted in a water-
soluble SNEDDS preparation characterized by a
clear, transparent, slightly misty solution.
Formulation Stability Test: The three stability
tests indicated that the NSOE SNEDDS had good
physical stability, characterized by no organoleptic
changes before or after the test, no phase separation,
and no formation of crystal or sediment (Figure 1 and
Table 1).
Globule Size, Zeta Potential, PDI, and
%Transmittance: The particle size was in the
50200 nm range, and the potential zeta value was
lower than 30 mV, demonstrating the good stability
of the nanoemulsion of NSOE. The PDI value was
0.499 and %transmittance was 64.414%, indicating
that the SNEDDS preparation was not fully
monodispersed (Table 2).
LC
50
Value of Formulations: The toxicity of the
test compound was calculated from the zebrafish
mortality rate for 96 hours of treatment (Table 3).
Combinations of the surfactant-cosurfactant (SCS)
showed no subject deaths in all three replications.
This indicated that using surfactants-cosurfactants in
preparing NSOE SNEDDS was safe and nontoxic to
zebrafish. The LC
50
values for the NSOE SNEDDS
and non-SNEDDS formulations calculated using a
probit analysis are listed in Table 4. As shown in
Table 4, although not statistically significant (p value
> 0.05), the toxicity of the NSOE SNEDDS
formulation was lower than in the non-SNEDDS.
Therefore, the toxicities of NSOE SNEDDS and non-
SNEDDS formulations were comparable.
(a) (b) (c)
Figure 1: Appearance of NSOE SNEDDS formulation (left, before the test; right, after the test); (a) before and after the
heating-stability test; (b) before and after the freeze-thaw test; and (c) before and after the centrifugation test.
Table 1: Organoleptic appearance, phase separation, and precipitation before and after stability tests.
Before the test Afte
r
the test
Organoleptic appearance Organoleptic appearance Phase separation Precipitation
Heating-stability test
Brownish-yellow,
clear, smelling typical of
N.sativa L. oil
Brownish-yellow,
clear, smelling typical of
N.sativa L. oil
None None
Freeze-thaw test
Centrifu
g
ation test
Toxicity of Self-nanoemulsifying Drug Delivery System Formulation of Nigella Sativa L. Seed Oil against Adult Danio rerio
37
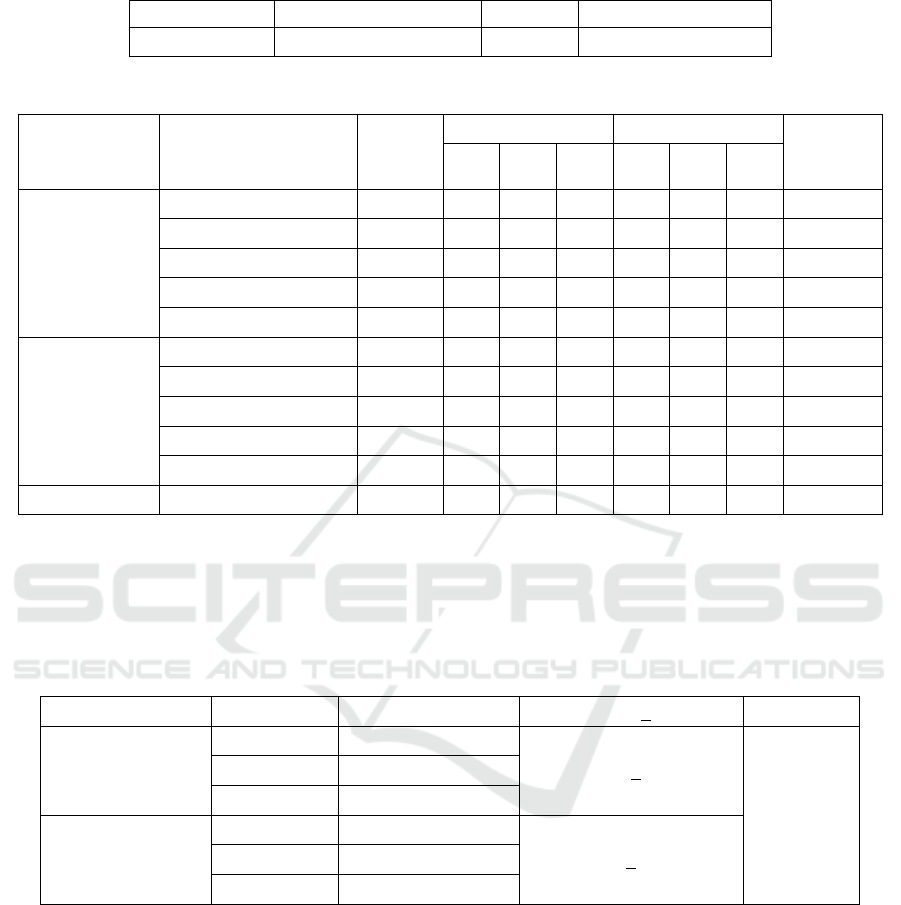
Table 2: Particle size, zeta potential, PDI, and %transmittance of NSOE SNEDDS formulation.
Particle size (nm) Zeta potential (mV)
PDI %Transmittance (%)
139.2
59.5
0.499 64.414
Table 3: Deaths of zebrafish in a 96-hour observation of each test group.
Compound Concentration
Number
of
subjects
Number of deaths Mortality (%)
Mortality
average
(
%
)
R1 R2 R3 R1 R2 R3
NSOE
SNEDDS
S1 (500 ppm) 7 7 7 7 100 100 100 100
S2 (250 ppm) 7 4 7 7 57 100 100 86
S3 (125 ppm) 7 0 3 7 0 42 100 47
S4 (62.5 ppm) 7 1 1 0 14 14 0 9
S5 (31.5 ppm) 7 0 1 0 0 14 0 5
NSOE non-
SNEDDS
NS1 (125 ppm) 7 7 7 7 100 100 100 100
NS 2 (62.5 ppm) 7 0 5 0 0 71 71 47
NS 3 (31.25 ppm) 7 0 0 0 0 0 0 0
NS 4 (15.625 ppm) 7 2 0 3 29 0 43 24
NS 5 (7.8125 ppm) 7 0 0 0 0 0 0 0
SCS (control)
125 ppm
7 0 0 0 0 0 0 0
Abbreviations:
S1–S5, SNEDDS concentration 1–5
NS1–NS5, non-SNEDDS concentration 1–5
SCS, surfactant-cosurfactant
R1, replication 1; R2, replication 2; R3, replication 3
Table 4: LC
50
values of NSOE SNEDDS and non-SNEDDS formulations.
Compound Replication LC
50
value* (ppm) Average LC
50
+ SD (ppm) p value **
NSOE SNEDDS
1 237.227
154.637 + 75.609
0.138
2 137.860
3 88.826
NSOE non-
SNEDDS
1 84.037
72.358 + 15.253
2 55.101
3 77.936
Note:
* LC
50
values were obtained from the mortality percentage data at five concentrations of NSOE SNEDDS and non-
SNEDDS formulations and analyzed using a probit analysis to obtain concentrations that caused the death of 50% of
subjects.
** p value was significant at 0.05
4 DISCUSSIONS
This study investigated the toxicity of NSOE
SNEDDS against adult zebra fish compared to the
non SNEDDS. The results showed that the toxicity of
NSOE SNEDDS lower than non SNEDDS, however,
this difference was non statistically significant.
Surfactant selection is a crucial part in the
preparation of SNEDDS formulations. Surfactants
play an important role in forming nanoemulsions and
reducing the surface tension between the two phases
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
38

(oil and water) for good emulsion dispersal (Patel et
al., 2011). Surfactants also stabilize nanoemulsion
preparations by maintaining the physical properties of
the preparation and preventing damage to the
bioactive compounds during processing and storage
(Chuacharoen et al., 2019). Tween 80 is an n-
hexane/water emulsion with non-ionic, nontoxic, and
biocompatible properties that result in a low level of
toxicity, making it safe to use. Tween 80 surfactant is
widely used in the processing of nanoemulsion
preparations in the pharmaceutical industry (Prieto &
Calvo, 2013).
The addition of cosurfactants helps to reduce the
size of nanoemulsion globules compared to the use of
surfactants alone. PEG 400 is a widely used polymer
cosurfactant in drug formulations. Its strong
hydration property allows it to form a stronger
interaction between the polymer and water compared
to the polymer-polymer interaction, thereby
increasing the emulsion mucoadhesion (Chen et al.,
2019). The low toxicity of the Tween 80 and PEG 400
combination has been demonstrated in this study,
characterized by the absence of death (mortality) in
this group.
Successful nanoemulsion preparations are
marked by a clear, transparent or slightly foggy
appearance (Handayani et al., 2019), and good
physical stability. Physical stability tests of the
SNEDDS formulation of NSOE demonstrated good
stability and fulfilled the requirements of the
nanoemulsion (Senapati et al., 2016). The presence of
a surfactant and cosurfactant reduced the surface
tension between the oil and water phases of the
emulsion. The greater the reduction of surface
tension, the more stable the nanoemulsion
formulation (Villar et al., 2012).
The mean globule size of the NSOE SNEDDS
formulation was 139.2 nm, which meets the
requirements for nanoemulsion particle size in the
drug delivery system (50–200 nm), making it suitable
for use in both food and drug industries (McClements
& Rao, 2011). The smaller the active substance
particle size in a nanoemulsion formulation, the better
the stability and distribution in dissolution media. The
zeta potential is a parameter for estimating surface
loads to understand the physical stability of
preparations and is important in characterizing
nanoemulsion preparations. The zeta potential value
of the NSOE SNEDDS formulation was 59.5 mV,
which met the criterion of a stable nanoparticle of
more than +30 mV or smaller than30 mV (Kumar
& Dixit, 2017).
The PDI value was 0.499, indicating that the
particles formed in SNEDDS formulations were not
fully monodispersed. The PDI parameter indicates the
globule size distribution; the lower the PDI value, the
b etter the level of monodispersity. In essence, PDI is
a dimensionless particle heterogeneity index. A PDI
of <0.3 meets the monodispersion criterion (Danaei et
al., 2018). Efforts to reduce PDI include prolonging
the ultrasonication process up to 30 minutes
(Mahbubul, 2019). Although the PDI value in this
study was more than 0.3, this was fairly good because
the particle size distribution of a SNEDDS
formulation is deemed heterogeneous (polydispersed
particles) if PDI exceeds 0.7 (Danaei et al., 2018).
The value of %transmittance indicates the level of
clarity of SNEDDS preparations; the closer the
%transmittance to 100%, the smaller the particle size
in nanometers, and the closer the optical clarity is to
water (Khan et al., 2015). SNEDDS preparations with
nearly 100% transmittance appear clearer and more
transparent with a greater possibility of absorption in
the digestive tract (Yen et al., 2017). In this study, the
%transmittance was only 64%, indicating that the
SNEDDS formulation of NSOE was of poor quality.
The %transmittance may be improved by increasing
the surfactant concentrations. A previous study
showed that raising surfactant concentrations (Tween
80) in a SNEDDS preparation containing vitamin D
led to a greatly reduced globular size and an increased
oil-water interface. However, as the surfactant
concentration continues to increase, the globule size
also becomes larger. Thus, to produce the optimal
globule size, the ideal ratio of surfactant/oil is 1:1
(Guttoff et al., 2015). In a different study, an
increased surfactant concentration in a SNEDDS
formulation containing curcumin increased
%transmittance to 92.86%99.51% (Chuacharoen et
al., 2019). These findings suggest that higher
surfactant concentrations improve the trapping of
active compounds in the particles, leading to an
increase in %transmittance to almost 100%.
The acute toxicity test is designed to determine
the toxic effects of a particular dose in a short time.
Usually, acute toxicity is observed from 24 hours up
to 7 days. Such tests aim to evaluate adverse effects
on a test organism after substance exposure within 24
hours by oral, skin, or inhalation routes (Saganuwan,
2017). The test uses the LC
50
to indicate toxicity,
which is determined based on the mortality ratio of
experimental animals (Parasuraman, 2011). The use
of adult zebrafish to test the toxicity of a drug
candidate has currently replaced the use of mammals
to implement the principles of reduction,
replacement, and refinement in the use of animals for
research. Several studies have shown that zebrafish in
both the embryonic and adult forms have an equal
Toxicity of Self-nanoemulsifying Drug Delivery System Formulation of Nigella Sativa L. Seed Oil against Adult Danio rerio
39

sensitiity to chemicals, which is indicated by the
slight difference in their LC
50
values (Kovrižnych et
al., 2013). Embryonic and adult zebrafish also have
comparable sensitivity to cationic and nonionic
surfactants (Vaughan & Van Egmond, 2010).
The toxicity tests of the SNEDDS and non-
SNEDDS formulations of NSOE on zebrafish were
conducted for 96 hours in each test group. For the
SNEDDS formulation, 100% zebrafish mortality
occurred at 500 ppm concentration; at 31.5 ppm, the
zebrafish mortality was zero. As for the non-
SNEDDS group, 100% zebrafish mortality occurred
at 125 ppm concentration and no deaths occurred at
7.8125 ppm. The probit analysis revealed that the
LC
50
value of the SNEDDS formulation of NSOE was
154.637 + 75.609 ppm, whereas the LC
50
of the non-
SNEDDS formulation was 72.358 + 15.253 ppm.
These LC
50
values showed that the SNEDDS
preparation of NSOE was less toxic than the non-
SNEDDS preparation; however, this difference was
not significant (p = 0.138).
This result is in agreement with previous studies
that showed that SNEDDS formulations were safer
than their non-SNEDDS preparations. The toxicity of
a SNEDDS formulation of Ipomoea reptans against
Vero cells using a 3-(4,5-dimethylthiazol-2-yl)-2,5-
diphenyl-tetrazolium bromide (MTT) assay did not
cause Vero cell death; instead, the cell culture growth
improved
(Chabib et al., 2019). Another study
showed that the preparation of arteether SNEDDS as
an antimalarial in Plasmodium yoelii nigeriensis-
infected mice showed a better bioavailability with
minimal toxicity (Dwivedi et al., 2014). In another
acute toxicity test, the SNEDDS formulation of bay
leaf chloroform extract also gave a very high LC
50
value; in the subchronic toxicity test, the preparation
had minimal effects on the pancreas, kidneys, and
liver at low to moderate doses. However, organ
damage was directly proportional to the increasing
dosage
(Prihapsara et al., 2018).
This study results might have implications for
nanoparticle research and might recommend against
the use of best combinations of surfactant and co-
surfactant in preparation of SNEDDS formulations.
However, further ongoing research is required to
ensure the safety of NSOE SNEDDS formulation for
oral drug delivery in animals and modification of this
formulation to guarantee their future application.
One limitation of this study was the composition
of the SNEDDS formulation of NSOE, which was
based on previous research. SNEDDS formulation
preparation using this composition resulted in good
stability as evidenced by the stability tests and
measurements of globules and zeta potential, but its
PDI and %transmittance was not optimal. Another
limitation is that only the toxicity was tested, not the
efficacy or pharmacological activity. The SNEDDS
formulation of NSOE is nontoxic, but the efficacy
remains to be determined. Thus, an investigation into
the efficacy and toxicity in one study is
recommended.
5 CONCLUSIONS
The LC
50
value of the SNEDDS formulation of NSOE
(154.637 ± 75.609) ppm was better than its non-
SNEDDS form (72.358 ± 15.253 ppm), but this
difference was not statistically different. Thus, the
toxicities of SNEDDS and non-SNEDDS
formulations of NSOE were comparable. The
SNEDDS preparation did not reach optimal
conditions, as indicated by good globule size and zeta
potential values but non-optimal PDI and
%transmittance values. Thus, the toxicity of the
SNEDDS formulation may improve with
optimization.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Sufi Desrini for
suggestions during the preparation of the study, and
Mr. Haryanto, Mr. Angga, and Mr. Marno for their
help during the data collection
REFERENCES
Abdelbary, G., Amin, M., & Salah, S. (2013). Self nano-
emulsifying simvastatin based tablets: Design and in
vitro/in vivo evaluation. Pharmaceutical Development
and Technology, 18(6), 1294–1304.
https://doi.org/10.3109/10837450.2012.672989
Abedi, A. S., Rismanchi, M., Shahdoostkhany, M.,
Mohammadi, A., & Mortazavian, A. M. (2017).
Microwave-assisted extraction of Nigella sativa L.
essential oil and evaluation of its antioxidant activity.
Journal of Food Science and Technology, 54(12),
3779–3790. https://doi.org/10.1007/s13197-017-2718-
1
Akrom, A., & Fatimah, F. (2015). Ekstrak heksan biji jintan
hitam (Nigella sativa L.) meningkatkan aktivitas
fagositosis makrofag tikus betina galur SD (Sprague
Dawley) yang diinduksi DMBA (7,12-
Dimetilbenz(α)antrasen) secara in vitro. Pharmaciana,
5(1). https://doi.org/10.12928/pharmaciana.v5i1.2288
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
40

Amina, B. (2016). Toxicity and anti-oxidant activity of the
essential oil of Nigella sativa. Der Pharmacia Lettre,
8(15), 245–249.
Chabib, L., Hayati, F., Awaluddin, R., & Pangestu, M. I.
(2019). Pengujian Toksisitas Seluler SNEDDS Ekstrak
Daun Kangkung (Ipomoea reptans, Poir) Terstandar.
Jurnal Pharmascience, 6(2), 48.
https://doi.org/10.20527/jps.v6i2.7350
Chen, Y. S., Chiu, Y. H., Li, Y. S., Lin, E. Y., Hsieh, D. K.,
Lee, C. H., Huang, M. H., Chuang, H. M., Lin, S. Z.,
Harn, H. J., & Chiou, T. W. (2019). Integration of PEG
400 into a self-nanoemulsifying drug delivery system
improves drug loading capacity and nasal mucosa
permeability and prolongs the survival of rats with
malignant brain tumors. International Journal of
Nanomedicine, 14, 3601–3613.
https://doi.org/10.2147/IJN.S193617
Cherniakov, I., Domb, A. J., & Hoffman, A. (2015). Self-
nano-emulsifying drug delivery systems: an update of
the biopharmaceutical aspects. Expert Opinion on Drug
Delivery, 12(7), 1121–1133.
https://doi.org/10.1517/17425247.2015.999038
Christophersen, P. C., Christiansen, M. L., Holm, R.,
Kristensen, J., Jacobsen, J., Abrahamsson, B., &
Müllertz, A. (2014). Fed and fasted state gastro-
intestinal in vitro lipolysis: In vitro in vivo relations of
a conventional tablet, a SNEDDS and a solidified
SNEDDS. European Journal of Pharmaceutical
Sciences, 57(1), 232–239.
https://doi.org/10.1016/j.ejps.2013.09.007
Chuacharoen, T., Prasongsuk, S., & Sabliov, C. M. (2019).
Effect of surfactant concentrations on physicochemical
properties and functionality of curcumin
nanoemulsions under conditions relevant to
commercial utilization. In Molecules (Vol. 24, Issue
2744). https://doi.org/10.3390/molecules24152744
Danaei, M., Dehghankhold, M., Ataei, S., Hasanzadeh
Davarani, F., Javanmard, R., Dokhani, A., Khorasani,
S., & Mozafari, M. R. (2018). Impact of particle size
and polydispersity index on the clinical applications of
lipidic nanocarrier systems. Pharmaceutics, 10(2), 1–
17. https://doi.org/10.3390/pharmaceutics10020057
Date, A. A., Desai, N., Dixit, R., & Nagarsenker, M. (2010).
Self-nanoemulsifying drug delivery systems:
Formulation insights, applications and advances.
Nanomedicine, 5(10), 1595–1616.
https://doi.org/10.2217/nnm.10.126
Dwivedi, P., Khatik, R., Khandelwal, K., Srivastava, R.,
Taneja, I., Rama Raju, K. S., Dwivedi, H., Shukla, P.,
Gupta, P., Singh, S., Tripathi, R., Paliwal, S. K.,
Wahajuddin, Dwivedi, A. K., & Mishra, P. R. (2014).
Self-nanoemulsifying drug delivery systems
(SNEDDS) for oral delivery of arteether:
Pharmacokinetics, toxicity and antimalarial activity in
mice. RSC Advances, 4(110), 64905–64918.
https://doi.org/10.1039/c4ra09267h
Guttoff, M., Saberi, A. H., & Mcclements, D. J. (2015).
Formation of vitamin D nanoemulsion-based delivery
systems by spontaneous emulsification: Factors
affecting particle size and stability.
Food Chemistry,
171, 117–122.
https://doi.org/10.1016/j.foodchem.2014.08.087
Handayani, F. S., Nugroho, B. H., & Munawiroh, S. Z.
(2019). Optimization of low energy nanoemulsion of
Grape seed oil formulation using D-Optimal Mixture
Design (DMD). Jurnal Ilmiah Farmasi, 14(1), 17–34.
Khan, A. W., Kotta, S., Ansari, S. H., Sharma, R. K., & Ali,
J. (2015). Self-nanoemulsifying drug delivery system
(SNEDDS) of the poorly water-soluble grapefruit
flavonoid Naringenin: Design, characterization, in vitro
and in vivo evaluation. Drug Delivery, 22(4), 552–561.
https://doi.org/10.3109/10717544.2013.878003
Kooti, W., Hasanzadeh-Noohi, Z., Sharafi-Ahvazi, N.,
Asadi-Samani, M., & Ashtary-Larky, D. (2016).
Chinese Journal of Natural Medicines Phytochemistry,
pharmacology, and therapeutic uses of black seed
(Nigella sativa). Chinese Journal of Natural Medicines,
14(10), 732–745. https://doi.org/10.1016/S1875-
5364(16)30088-7
Kovrižnych, J. A., Sotńikóva, R., Zeljenková, D.,
Rollerová, E., Szabová, E., & Wimmerov
́
a, S. (2013).
Acute toxicity of 31 different nanoparticles to zebrafish
(Danio rerio) tested in adulthood and in early life stages
- Comparative study. Interdisciplinary Toxicology,
6(2), 67–73. https://doi.org/10.2478/intox-2013-0012
Kumar, A., & Dixit, C. K. (2017). Methods for
characterization of nanoparticles. In S. Nimesh, R.
Chandra, & N. Gupta (Eds.), Advances in
Nanomedicine for the Delivery of Therapeutic Nucleic
Acids (pp. 44–58). Woodhead Publishing.
https://doi.org/10.1016/B978-0-08-100557-6.00003-1
Mahbubul, I. M. (2019). Stability and Dispersion
Characterization of Nanofluid. In Preparation,
Characterization, Properties and Application of
Nanofluid (pp. 47–112). William Andrew Publishing.
https://doi.org/10.1016/b978-0-12-813245-6.00003-4
McClements, D. ., & Rao, J. (2011). Edible nanoemulsions:
Fabrication, properties, and functional performance.
Soft Matter, 7(6), 2297–2316.
https://doi.org/10.1039/c0sm00549e
Parasuraman, S. (2011). Toxicological screening. JJ
Pharmacol Pharmacother, 2(2), 74–79.
https://doi.org/10.4103/0976-500X.81895
Patel, J., Patel, A., Raval, M., & Sheth, N. (2011).
Formulation and development of a self-
nanoemulsifying drug delivery system of irbesartan. J
Advanced Pharmaceutic Technol Res, 2(1), 9–16.
https://doi.org/10.4103/2231-4040.79799
Prieto, C., & Calvo, L. (2013). Performance of the
Biocompatible Surfactant Tween 80, for the Formation
of Microemulsions Suitable for New Pharmaceutical
Processing. J Appl Chem, 2013, 1–10.
https://doi.org/10.1155/2013/930356
Prihapsara, F., Mufidah, Artanti, A. N., & Harini, M.
(2018). Acute and Subchronic Toxicity of Self
Nanoemulsifying Drug Delivery Systems (SNEDDS)
from Chloroform Bay Leaf Extract (Eugenia Polyantha
W.) with Palm Kernel Oil as A Carrier. IOP Conference
Series: Materials Science and Engineering, 333(1).
https://doi.org/10.1088/1757-899X/333/1/012066
Toxicity of Self-nanoemulsifying Drug Delivery System Formulation of Nigella Sativa L. Seed Oil against Adult Danio rerio
41

Saganuwan, S. A. (2017). Toxicity studies of drugs and
chemicals in animals: An overview. Bulg. J. Vet. Med,
20(4), 291–318. https://doi.org/10.15547/bjvm.983
Savitri, I., Suhendra, L., & Wartini, N. M. (2017). Pengaruh
jenis pelarut pada metode maserasi terhadap
karakteristik ekstrak Sargassum polycystum. Rekayasa
Dan Manajemen Agroindustri, 5(3), 93–101.
Senapati, P. C., Sahoo, S. K., & Sahu, A. N. (2016). Mixed
surfactant based (SNEDDS) self-nanoemulsifying drug
delivery system presenting efavirenz for enhancement
of oral bioavailability. Biomedicine and
Pharmacotherapy, 80, 42–51.
https://doi.org/10.1016/j.biopha.2016.02.039
The Organization for Economic Co-operation and
Development [OECD]. (2018). OECD Guideline for
testing of chemicals (Draft revised version): Vol. 11th
versi (Issue July 2018).
Ujilestari, T., Martien, R., Ariyadi, B., Dono, N. D., &
Zuprizal. (2018). Self-nanoemulsifying drug delivery
system (SNEDDS) of Amomum compactum essential
oil: Design, formulation, and characterization. Journal
of Applied Pharmaceutical Science, 8(6), 14–21.
https://doi.org/10.7324/JAPS.2018.8603
Vaughan, M., & Van Egmond, R. (2010). The use of the
zebrafish (Danio rerio) embryo for the acute toxicity
testing of surfactants, as a possible alternative to the
acute fish test. ATLA Alternatives to Laboratory
Animals, 38(3), 231–238.
https://doi.org/10.1177/026119291003800310
Villar, A. M. S., Naveros, B. C., Campmany, A. C. C.,
Trenchs, M. A., Rocabert, C. B., & Bellowa, L. H.
(2012). Design and optimization of self-
nanoemulsifying drug delivery systems (SNEDDS) for
enhanced dissolution of gemfibrozil. Int J
Pharmaceutics, 431(1–2), 161–175.
https://doi.org/10.1016/j.ijpharm.2012.04.001
Wahyuningsih, I., & Putranti, W. (2015). Optimasi
Perbandingan Tween 80 dan Polietilenglikol 400 Pada
Formula Self Nanoemulsifying Drug Delivery System
(SNEDDS) Minyak Biji Jinten Hitam. Pharmacy,
12(02), 223–241.
Yen, C. C., Chang, C. W., Hsu, M. C., & Wu, Y. T. (2017).
Self-Nanoemulsifying drug delivery system for
resveratrol: Enhanced oral bioavailability and reduced
physical fatigue in rats. International Journal of
Molecular Sciences, 18(9).
https://doi.org/10.3390/ijms18091853
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
42
